Abstract
Carnivorous pitcher plants of the genus Nepenthes capture prey with a pitfall trap that relies on a micro-structured, slippery surface. The upper pitcher rim (peristome) is fully wettable and causes insects to slip by aquaplaning on a thin water film. The high wettability of the peristome is probably achieved by a combination of hydrophilic surface chemistry, surface roughness and the presence of hygroscopic nectar. Insect foot attachment could be prevented by the delayed drainage of the thin water film between the adhesive pad and the surface. Drainage should be faster for insects with a hairy adhesive system; however, they slip equally on the wet peristome. Therefore the stability of the water film against dewetting appears to be the key factor for aquaplaning. New experimental techniques may help to clarify the detailed function of the pitcher plant peristome and to explore its potential for biomimetic applications.
Key words: carnivorous plants, insect aquaplaning, superhydrophilic leaves, Nepenthes, peristome
Introduction
The paleotropic genus Nepenthes comprises approximately 90 species of carnivorous plants,1 all of which use highly specialized pitcher-shaped leaves to capture mainly insect prey.2–5 The bottom part of each pitcher is filled with a digestive fluid in which the captured prey drowns and subsequently decomposes. The released nutrients are absorbed through multicellular glands on the inner pitcher wall.6 The utilization of this additional nutrient source enables pitcher plants to colonize extremely nutrient-poor habitats where other plants struggle to survive.7
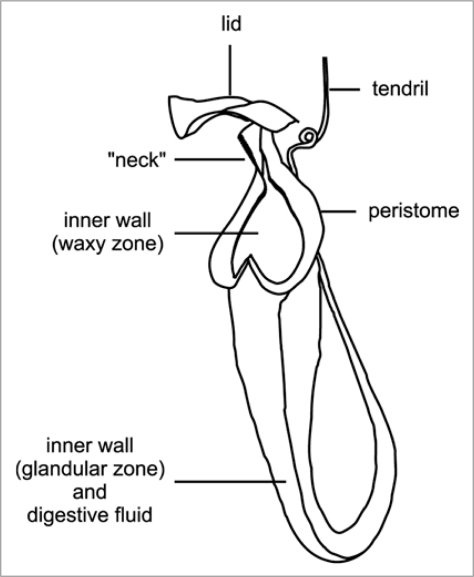
The pitcher trap consists of several specialized structures (Fig. 1). The inner pitcher wall is divided into a lower glandular and an upper waxy zone (absent in some species). The pitcher rim (pe-ri-stome) is often conspicuously colored and characterized by a regular pattern of radial ridges. Its inner edge overhangs the pitcher and is densely packed with extrafloral nectaries. The pitcher rim is often elongated upwards to the pitcher lid, forming a distinct “neck”. In most species, the lid covers the pitcher opening and thus shelters it from heavy rain, but in some species it is reduced or bent backward.
Figure 1.
Schematic illustration of a Nepenthes pitcher.
Like insect-pollinated flowers, pitchers attract visitors by presenting visual and olfactory signals and offering food rewards. For example, upper pitchers of N. rafflesiana exhibit distinctive UV reflection patterns8 and exude a strong sweet scent.3,9,10 A significant part of the prey spectrum consists of anthophilous flying insects, indicating that these pitchers successfully mimic flowers.3 Unlike most flowers, however, pitchers attract large numbers of ants. This is achieved by extrafloral nectaries located on the tendril, the outer pitcher wall, the underside of the lid and the inner margin of the peristome.4,11 Studies on N. bicalcarata have shown that the secretion of these nectaries changes during pitcher development: the nectaries on the tendril and outer wall are mainly active in developing, unopened pitchers while in mature pitchers most nectar is secreted under the lid and on the peristome.11,12 Attraction of ants prior to pitcher opening not only provides protection against herbivory for the developing pitchers11,13 but also establishes foraging trails of ant visitors which can later fall prey to the pitcher. The subsequent shift of nectar production from the tendril and outer pitcher surface to the lid and the peristome lures ants into more perilous positions on the pitcher. Our recent study shows that the peristome nectaries of N. rafflesiana only start secreting after the pitcher has opened.10 This ensures that no nectar is wasted before pitcher opening.
Prey is captured by a pitfall mechanism; no moving plant parts are involved in the trapping process.7 Insects lose their footing on the specialized, anti-adhesive surfaces of the peristome14 and/or the inner pitcher wall.15–18 Many Nepenthes species possess epicuticular wax crystals on the upper part of the inner pitcher wall. The platelet-shaped crystals project perpendicularly from the surface. As a result, the crystals break off very easily, thus contaminating and disabling the insects' adhesive pads.15,19 In addition, the micro-rough crystal surface reduces the contact area for insect adhesive pads. The slipperiness of the inner wall surface is further aided by downward-pointing epidermal cells that provide no foothold for insect claws while climbing upwards.20 The anti-adhesive surface on the inner pitcher wall may be more important for the effective retention of captured prey than for the initial trapping.14 In addition, arthropod prey is also prevented from escaping by the digestive fluid.16,21
The Peristome: Surface Structure, Wettability and Trapping Function
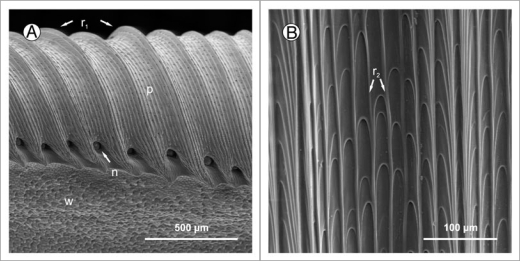
Pitchers of most Nepenthes species have a well-developed peristome, the surface of which is characterized by a highly regular microstructure composed of first and second order radial ridges (Fig. 2). The larger first order ridges vary between species in height, shape and spacing. The much smaller second order ridges consist of straight rows of overlapping epidermal cells which form a series of steps towards the pitcher inside. The second order ridges are more uniform among species (Bauer U, unpublished results). This may be a consequence of constraints in cell development and architecture but it could also mean that their dimension is advantageous for the function of the peristome. The surface of each epidermal cell is smooth and free of epicuticular wax crystals.
Figure 2.
(A) Peristome surface (p) of Nepenthes alata, structured by first (r1) and second order radial ridges. In between the tooth-like projections at the inner edge of the peristome the pores of large extrafloral nectaries (n) can be seen. Below the peristome is the wax-covered inner wall surface (w). (B) The second order ridges (r2) are formed by straight rows of overlapping epidermal cells.
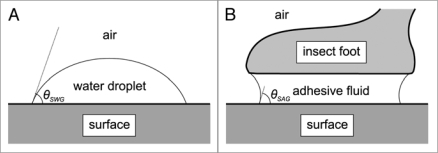
The trapping function of the peristome has long been overlooked, probably because of its dependence on wetness.14,22 The dry peristome surface provides a secure foothold for insects.14,16,22,23 Under humid conditions, however, the peristome becomes extremely slippery, and experimentally obtained capture rates of ants reached more than 80%. A key factor for the slipperiness of the peristome is that its surface is completely wettable.14,22 Wettability is determined by the combination of adhesive and cohesive forces in the interaction between water (W), the solid surface (S) and the surrounding gas (G). The contact angle θSWG of a water droplet on a surface (Fig. 3A) provides a measure of its wettability; according to Young's equation, it is determined by the interfacial tensions γSG, γSW and γWG, denoting the energy per unit area of the solid/gas, solid/water and water/gas interfaces:
| 1 |
Figure 3.
(A) The contact angle θSWG of a water droplet on a surface provides a measure of the wettability of the surface. (B) Simplified model of the contact of an insect adhesive pad with a surface, mediated by an adhesive fluid.
A surface is wettable if θ < 90° and non-wettable if θ > 90°. With regard to water, wettable surfaces are often termed hydrophilic (superhydrophilic if θ < 10°) and non-wettable ones hydrophobic. The interfacial tensions γSW, γWG and γSG determine the spreading coefficient:
| 2 |
If SSWG ≥ 0, a droplet will spread and completely wet the surface; the higher the value of SSWG, the stronger the tendency of the droplet to spread. Most hydrophilic surfaces are also well wettable by non-polar liquids, a phenomenon sometimes referred to as amphiphilicity. A study on N. alata has confirmed amphiphilic properties for the peristome; however, contact angles were found to be lower for water than for non-polar liquids.24
Superhydrophilic leaf surfaces have evolved independently in several groups of plants and are often associated with specialized functions such as absorption of water from mist (e.g., Bromeliaceae, some Cactaceae) or underwater growth.25 Wettable leaves are characterized by an absence of epicuticular wax crystals.26 A recent study on superhydrophilicity in the tropical herb Ruellia devosiana suggested that in this species high wettability is facilitated by the secretion and spreading of a surfactant from specialized glands.27 It is still unclear whether such a detergent is also present in the peristome nectar of Nepenthes; however, high sugar content renders the nectar hygroscopic. The presence of nectar on the peristome has been shown to significantly enhance surface wetting by condensation.22
Microscopic roughness acts as an enhancer of the general wetting properties of a given surface.28–31 In other words, microroughness increases the wettability of a hydrophilic surface (e.g., in R. devosiana27) but decreases the wettability of a hydrophobic surface (e.g., in Lotus leaves26). However, Nepenthes peristomes are not simply micro-rough; their surface pattern of microscopic ridges and grooves is highly organized and directional (Fig. 2). Water droplets, when placed on a peristome, rapidly spread along these grooves even against the force of gravity, but little or no spreading occurs perpendicularly across the first-order ridges14,32 (our own observations). This suggests that, in addition to the absence of wax crystals and presence of hygroscopic nectar, Nepenthes peristomes make use of micro-topography and capillary forces to facilitate complete wetting. As a result, the pe-ri-stome is covered with a continuous thin water film under humid conditions.
Effect of Peristome Water Films on Insect Adhesion
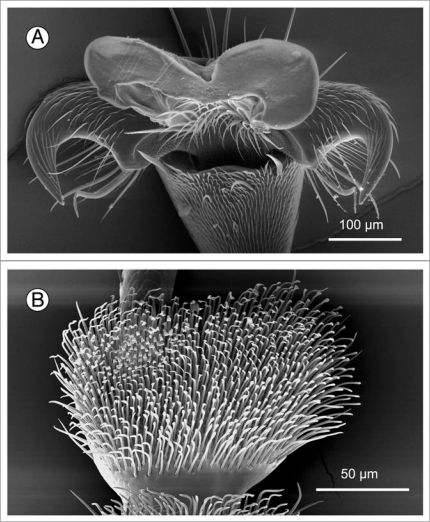
It has been shown that water films on the peristome disable insect adhesive pads while the anisotropic surface topography prevents interlocking of claws when an insect slips into the pitcher.14 The exact mechanism of how the water films disrupt pad attachment is still unclear; however, some predictions can be made from what is known about insect adhesion. Insects cling to (dry) smooth surfaces by means of either smooth or hairy adhesive pads (Fig. 4). The contact between the pad and the surface is mediated by an adhesive fluid33–35 (Fig. 3B). Adhesive pads with a watery secretion fail when they are submerged in water, as experiments with frogs have shown.36 This may be due to the loss of capillary bridges between the foot and the surface when the watery toe pad mucus fuses with the surrounding water. In insects, however, the main component of the adhesive fluid is a water-in-oil emulsion which is likely to be stable under water.34
Figure 4.
Two different designs of adhesive pads are found in insects. (A) Smooth pad of an ant (Oecophylla smaragdina). (B) Hairy pad of a beetle (Gastrophysa viridula).
When an insect steps onto the wet peristome, its adhesive pads need to make contact with the surface in order to avoid slipping or falling. This process can be split up into three phases: (1) penetrating the water surface, (2) bringing the pad close to the surface by squeezing out the subjacent water layer and (3) dewetting and establishing the contact. Phase (1) is dependent on the wettability of the insect foot which is determined by the interfacial tension between the water film on the peristome and the oily adhesive fluid covering the insect's adhesive pad. The interfacial tension of vegetable oil has been shown to be in the same order of magnitude against water and air:37
| 3 |
Hence, the contact angle θAWG of a water droplet (W) on the surface of the adhesive pad (A) should be approximately 90°:
| 4 |
Equation 4 suggests that a foot pad should easily penetrate the water surface, because attractive or repulsive forces scale with cos θAWG. However, this conclusion may be less reliable for insects with hairy adhesive pads where the general wetting properties are enhanced by topography so that small differences between γAG and γAW could lead to significant forces.
Phase (2) is strongly influenced by the load on the foot which depends on the insect's body mass, geometry and motion, and the slope of the peristome. The time to squeeze out the water film underneath the pad depends on its thickness and viscosity. Some Nepenthes species secrete copious amounts of concentrated nectar from their peristome nectaries.10,11 As sugar solutions have a higher viscosity than water, the nectar could inhibit drainage and thus assist insect aquaplaning.38 On the other hand, the ridges on the peristome surface form channels that should enhance drainage.39
Once the water film between the pad and the surface has become very thin (∼0.1 µm), complete removal of the water by dewetting becomes possible.40,41 To make direct contact, the adhesive fluid covering the foot needs to displace the thin layer of water. For simplification, we treat the adhesive pad as a piece of solid material with the same surface energy as the adhesive fluid. Spontaneous dewetting will only occur if the spreading coefficient SSWA for water in between the pad and the surface is negative. From equation 2 it follows that
Since γWA ≈ γAG (equation 3) and SSAG = γSG − (γAG + γAS)
| 5 |
where SSAG is the spreading coefficient of the adhesive fluid on a dry peristome surface. The wettability of the peristome for water appears to be higher than that for hydrophobic oils,24 i.e., SSWG − SSAG > 0. Therefore, and since the surface tension of water is likely to be at least twice as high as that of the hydrophobic pad secretion (γWG ≈ >2γAG), SSWA is likely to be positive. This suggests that no dewetting should occur between the insect pad and the peristome and that the lubricating water film should remain stable.
We tested this prediction and the relative importance of water drainage versus surface wettability by comparing the performance of ants (smooth adhesive pads) and beetles (hairy adhesive pads) on a wet peristome. If drainage was the limiting factor, beetles should adhere better since their hairy pads should drain more easily than smooth pads (diameter of individual hair contacts only ca. 5 µm42). We placed individual insects on the outer surface of a pitcher and observed one single visit of the wet peristome for each insect. Table 1 shows that both beetles and ants were captured efficiently, suggesting that not drainage but the difficulty of de-wetting is the main barrier for adhesion, consistent with the above prediction.
Table 1.
Running performance of ants and beetles on a wet Nepenthes sp. peristome.
| Insect species | n | Captured | Not captured |
| Smooth adhesive pads: | |||
| Carpenter ant (Camponotus sp.) | 20 | 18 | 2 |
| Hairy adhesive pads: | |||
| Dock beetle (Gastrophysa viridula) | 13 | 13 | 0 |
| Harlequin ladybird (Harmonia axyridis) | 16 | 16 | 0 |
Conclusions and Outlook
The high wettability of the Nepenthes peristome is achieved by a combination of hydrophilicity, surface micro-topography and secretion of hygroscopic nectar. The stability of water films on the peristome is probably the key factor that prevents insect adhesion. To reach a quantitative understanding of the function of the peristome, more detailed data on insect adhesion and on the surface chemistry of the peristome are needed. The fabrication of high-resolution surface replicas43 might make it possible to separate experimentally between the effects of surface chemistry and topography. Moreover, modern micro/nanofabrication techniques provide a tool to create artificial surfaces that mimic the topography of the peristome. Similar to biomimetic substrates inspired by the superhydrophobic surface of the Lotus leaf,26 these “peristome mimics” could be used for a variety of applications, such as water-lubricated surfaces or anti-fogging coatings on mirrors, lenses, windows and screens that prevent droplet formation.44
Acknowledgements
Our research is financially supported by an external research studentship of Trinity College Cambridge to U.B. and a research grant of The Leverhulme Trust to W.F.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9664
References
- 1.Ellison AM, Gotelli NJ. Energetics and the evolution of carnivorous plants—Darwin's ‘most wonderful plants in the world’. J Exp Bot. 2009;60:19–42. doi: 10.1093/jxb/ern179. [DOI] [PubMed] [Google Scholar]
- 2.Kato M, Hotta M, Tamin R, Itino T. Inter- and intra-specific variation in prey assemblages and inhabitant communities in Nepenthes pitchers in Sumatra. Trop Zool. 1993;6:11–25. [Google Scholar]
- 3.Moran JA. Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. J Ecol. 1996;84:515–525. [Google Scholar]
- 4.Clarke CM. The effects of pitcher dimorphism on the metazoan community of the carnivorous plant Nepenthes bicalcarata Hook. f. Malay Nat J. 1997;50:149–157. [Google Scholar]
- 5.Adam JH. Prey spectra of Bornean Nepenthes species (Nepenthaceae) in relation to their habitat. Pertanika J Trop Agric Sci. 1997;20:121–134. [Google Scholar]
- 6.Owen TP, Lennon KA, Santo MJ, Anderson AN. Pathways for nutrient transport in the pitchers of the carnivorous plant Nepenthes alata. Ann Bot. 1999;84:459–466. [Google Scholar]
- 7.Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London, San Diego: Academic Press; 1989. [Google Scholar]
- 8.Moran JA, Booth WE, Charles JK. Aspects of pitcher morphology and spectral characteristics of six Bornean Nepenthes pitcher plant species: Implications for prey capture. Ann Bot. 1999;83:521–528. [Google Scholar]
- 9.Di Giusto B, Grosbois V, Fargeas E, Marshall DJ, Gaume L. Contribution of pitcher fragrance and fluid viscosity to high prey diversity in a Nepenthes carnivorous plant from Borneo. J Biosci. 2008;33:121–136. doi: 10.1007/s12038-008-0028-5. [DOI] [PubMed] [Google Scholar]
- 10.Bauer U, Willmes C, Federle W. Effect of pitcher age on trapping efficiency and natural prey capture in carnivorous Nepenthes rafflesiana plants. Ann Bot. 2009;103:1219–1226. doi: 10.1093/aob/mcp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merbach MA, Zizka G, Fiala B, Maschwitz U, Booth WE. Patterns of nectar secretion in five Nepenthes species from Brunei Darussalam, Northwest Borneo, and implications for ant-plant relationships. Flora. 2001;196:153–160. [Google Scholar]
- 12.Clarke CM. Initial colonisation and prey capture in Nepenthes bicalcarata (Nepenthaceae) pitchers in Brunei. Sandakania. 1998;12:27–36. [Google Scholar]
- 13.Merbach MA, Zizka G, Fiala B, Merbach D, Booth WE, Maschwitz U. Why a carnivorous plant cooperates with an ant—selective defense against pitcher-destroying weevils in the myrmecophytic pitcher plant Nepenthes bicalcarata Hook. f. Ecotropica. 2007;13:45–56. [Google Scholar]
- 14.Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc Natl Acad Sci USA. 2004;101:14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juniper BE, Burras JK. How pitcher plants trap insects. New Sci. 1962;13:75–77. [Google Scholar]
- 16.Gaume L, Gorb S, Rowe N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytol. 2002;156:479–489. doi: 10.1046/j.1469-8137.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- 17.Riedel M, Eichner A, Jetter R. Slippery surfaces of carnivorous plants: composition of epicuticular wax crystals in Nepenthes alata Blanco pitchers. Planta. 2003;218:87–97. doi: 10.1007/s00425-003-1075-7. [DOI] [PubMed] [Google Scholar]
- 18.Gaume L, Perret P, Gorb E, Gorb S, Labat J-J, Rowe N. How do plant waxes cause flies to slide? Experimental tests of wax-based trapping mechanisms in three pitfall carnivorous plants. Arth Struct & Dev. 2004;33:103–111. doi: 10.1016/j.asd.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Gorb E, Haas K, Henrich A, Enders S, Barbakadze N, Gorb S. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. J Exp Biol. 2005;208:4651–4662. doi: 10.1242/jeb.01939. [DOI] [PubMed] [Google Scholar]
- 20.Gorb E, Kastner V, Peressadko A, Arzt E, Gaume L, Rowe N, et al. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. J Exp Biol. 2004;207:2947–2963. doi: 10.1242/jeb.01128. [DOI] [PubMed] [Google Scholar]
- 21.Gaume L, Forterre Y. A viscoelastic deadly fluid in carnivorous pitcher plants. PLoS ONE. 2007;2:1185. doi: 10.1371/journal.pone.0001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer U, Bohn HF, Federle W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proc R Soc B. 2008;275:259–265. doi: 10.1098/rspb.2007.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd FE. The carnivorous plants. Waltham: Chronica Botanica; 1942. [Google Scholar]
- 24.Gorb EV, Gorb SN. Physicochemical properties of functional surfaces in pitchers of the carnivorous plant Nepenthes alata Blanco (Nepenthaceae) Plant Biol. 2006;8:841–848. doi: 10.1055/s-2006-923929. [DOI] [PubMed] [Google Scholar]
- 25.Koch K, Barthlott W. Superhydrophobic and superhydrophilic plant surfaces: an inspiration for biomimetic materials. Philos T Roy Soc A. 2009;367:1487–1509. doi: 10.1098/rsta.2009.0022. [DOI] [PubMed] [Google Scholar]
- 26.Barthlott W, Neinhuis C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta. 1997;202:1–8. [Google Scholar]
- 27.Koch K, Blecher IC, König G, Kehraus S, Barthlott W. The superhydrophilic and superoleophilic leaf surface of Ruellia devosiana (Acanthaceae): a biological model for spreading of water and oil on surfaces. Funct Plant Biol. 2009;36:339–350. doi: 10.1071/FP08295. [DOI] [PubMed] [Google Scholar]
- 28.Wenzel RN. Resistance of solid surfaces to wetting by water. Ind Eng Chem. 1936;28:988–994. [Google Scholar]
- 29.Herminghaus S. Roughness-induced non-wetting. Europhys Lett. 2000;52:165. [Google Scholar]
- 30.Bico J, Thiele U, Quere D. Wetting of textured surfaces. Colloid Surface A. 2002;206:41–46. [Google Scholar]
- 31.Bhushan B, Jung YC. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces. J Phys: Condens Matter. 2008;20:225010. [Google Scholar]
- 32.Gorb SN, Voigt D, Gorb EV. Visualisation of small fluid droplets on biological and artificial surfaces using the cryo-SEM approach. In: Méndez-Vilas A, Diaz J, editors. Modern research and educational topics in microscopy. Badajoz: Formatex; 2007. pp. 812–819. [Google Scholar]
- 33.Gorb S. Attachment devices of insect cuticle. Dordrecht: Kluwer Academic Publishers; 2001. [Google Scholar]
- 34.Federle W, Riehle M, Curtis ASG, Full RJ. An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integr Comp Biol. 2002;42:1100–1106. doi: 10.1093/icb/42.6.1100. [DOI] [PubMed] [Google Scholar]
- 35.Federle W, Brainerd EL, McMahon TA, Hölldobler B. Biomechanics of the movable pretarsal adhesive organ in ants and bees. Proc Natl Acad Sci USA. 2001;98:6215–6220. doi: 10.1073/pnas.111139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emerson SB, Diehl D. Toe pad morphology and mechanisms of sticking in frogs. Biol J Linn Soc. 1980;13:199–216. [Google Scholar]
- 37.Fisher LR, Mitchell EE, Parker NS. Interfacial tensions of commercial vegetable oils with water. J Food Sci. 1985;50:1201–1202. [Google Scholar]
- 38.Wolf AV, Brown MG, Prentiss PG. Concentrative properties of aqueous solutions: conversion tables. In: Weast RC, editor. Handbook of Chemistry and Physics. Boca Raton: CRC Press; 1984. pp. 223–272. [Google Scholar]
- 39.Persson BNJ. Wet adhesion with application to tree frog adhesive toe pads and tires. J Phys—Condens Mat. 2007;19:376110. [Google Scholar]
- 40.Sharma A, Ruckenstein E. Dewetting of solids by the formation of holes in macroscopic liquid films. J Colloid Interf Sci. 1989;133:358–368. [Google Scholar]
- 41.Martin P, Brochard-Wyart F. Dewetting at soft interfaces. Phys Rev Lett. 1998;80:3296–3299. [Google Scholar]
- 42.Bullock JMR, Federle W. Division of labour and sex differences between fibrillar, tarsal adhesive pads in beetles: effective elastic modulus and attachment performance. J Exp Biol. 2009;212:1876–1888. doi: 10.1242/jeb.030551. [DOI] [PubMed] [Google Scholar]
- 43.Koch K, Schulte AJ, Fischer A, Gorb SN, Barthlott W. A fast, precise and low-cost replication technique for nano- and high-aspect-ratio structures of biological and artificial surfaces. Bioinspir Biomim. 2008;3:046002. doi: 10.1088/1748-3182/3/4/046002. [DOI] [PubMed] [Google Scholar]
- 44.Gould P. Smart, clean surfaces. Mater Today. 2003;6:44–48. [Google Scholar]