Abstract
Combined nitrogen (N) sources are known to strongly affect initiation, development and functioning of Nitrogen-Fixing-Nodules whose formation is triggered by lipochitin-oligosaccharide signals secreted in the rhizospere by the Rhizobium partner. The rapid effects of N supply on nodule initiation have been mainly described when N sources are present at the moment of Rhizobium inoculation or purified Nod Factors addition. We recently reported that high ammonium nitrate growth conditions might also strongly affect the nodulation competence of Lotus japonicus plants, prior to the Rhizobium inoculation. This is a long-term effect, which suggests a change of the general nutritional status as the signal controlling the reduced nodulation capacities. The mechanisms underlying these inhibitory pathways are apparently different and the identification of the molecular actors involved may provide new insights into the linkage between N environmental changes and root organogenesis programs.
Key words: legume symbiosis, N-signalling, nodule organogenesis, rhizobium, nod factors
Plants have evolved an amazing capacity to respond quickly and in a very effective way to the environment commitments by modulating their root and shoot developmental processes. The symbiosis is an excellent example of how the plants adapt to the changing environment, as nodulation does not occur when fixed nitrogen is readily available in the soil (Fig. 1). The mechanisms underlying this environmental control are not elucidated yet although the first observations on the inhibitory effect of different N sources were reported long time ago. The effect of nitrate has been extensively studied and the inhibitory action on nodule initiation seems to act locally,1,2 whereas both a systemic and local nitrate action was reported for nodules growth and N fixation activity.3,4 However, it must be taken into account that different N sources might have different effects on nodule organogenesis. We previously reported discrimination between nitrate and ammonium effects on the Nod factor dependent transduction pathway.5 Both N sources affect early stages of the cascade of events leading to nodule formation but their actions were positioned in different points on the transduction pathway. In particular, ammonium action takes place earlier than nitrate by affecting even root hair deformation events.5
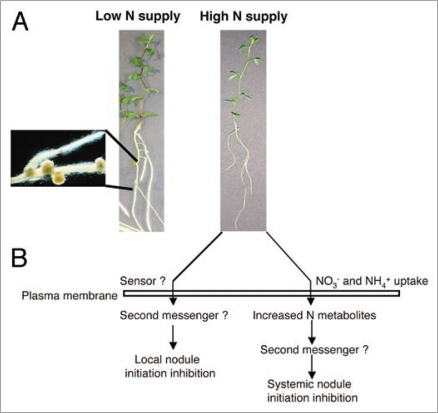
Figure 1.
(A) Lotus japonicus plants inoculated with Mesorhizobium loti in a N-free condition (left) and in the presence of 10 mM ammonium nitrate (right). (B) Scheme of the dual N dependent regulation of the nodulation program.
The analysis reported in Omrane et al.6 indicates that N supply can also modulate the early steps leading to nodule initiation by predisposing Lotus japonicus plants to a successful/unsuccessful interaction with Rhizobium, prior to the inoculation. The inhibitory effect of 10 mM NH4NO3 on the legumes nodulation competence is maintained for at least six days after transfer in permissive, low N concentration, suggesting the existence of a negative feedback that is controlled by the general nutritional status of the plant. Our preliminary data obtained with a split-root experimental system where two root sides are exposed to different N regimes seem to confirm this hypothesis. Of course a systemic effect controlled by the general nutritional status of the plant should not change with different N sources such as ammonium and nitrate. Nevertheless, plants might reveal N sources preferentiality and this could affect the external concentration required for the systemic inhibitory effect to occur.
The mechanisms and factors involved in both local and systemic N supply inhibitions as well as the potential targets of their action on the pathways leading to nodule initiation are almost completely unknown (Fig. 1). Some insights into the elucidation of these signaling pathways could come from the information obtained on the effects of nitrate on root architecture in Arabidopsis thaliana.7 Both local and systemic nitrate effects on the secondary root elongation and early stages of development have been described.8,9 The effect of local nitrate supply was not observed in the axr4 auxin-resistant mutant, suggesting an overlap between the nitrate and auxin response pathways,9 whereas the systemic effect was modulated by the nitrate accumulation in the shoot.9,10 In the latter case the long-distance signal could be a shoot-derived auxin signal that is modulated by the nitrate status of the shoot.11 Although legume nodules and lateral roots are structurally and developmentally distinct, several analogies can be identified. For example, actinorhizal nodules and nodules formed on the roots of Parasponia are considered to be modified lateral roots;12 both non-legume nodules and lateral roots are characterized by a single vascular bundle that traverses the nodule. Furthermore, peanut nodules share with lateral roots the pericyclic site origin and structures elicited by Rhizobium meliloti mutants appearing to resemble roots more closely than nodules were described.13 A physiological relationship also, seems to be present between lateral roots and nodules as nodulated clover seedlings have fewer lateral roots than un-inoculated plants.12 The involvement of plant hormones as a secondary signal for nodule morphogenesis has been extensively discussed12,14–16 and a cytokinin receptor with a crucial role in the Nod factor transduction pathway has been recently identified.17–19 Gresshoff in 1993,20 proposed the so-called auxin-burst-control (ABC) hypothesis to explain nitrate inhibition and other aspects of nodule initiation in legumes (e.g., autoregulation of the nodules number). The hypothesis suggests that Nod-factor perception alters axial and radial auxin transport, allowing the initiation of cortical cell divisions through a shift in the local auxin-to-cytokinin ratio. Such changes alter pericycle and epidermis responses, leading to nodule formation. Once an increased amount of nodule initiation has occurred, the shoot responds through an increase in translocation of auxins, leading to an auxin burst, which in turns is inhibitory for further nodule initiation. The ABC hypothesis predicts that nitrate increases the auxin sensitivity of root cortical cells and thus in the presence of nitrate, cortical cells are strongly prevented from sensing the Nod-factor-related auxin decrease. Experimental data reported that a 45% increase of IAA content was actually observed in inoculated soybean plants grown 1 mM nitrate, whereas no apparent increase in root auxin content was observed in the presence of high nitrate.21
The transcriptomic approach described in Omrane et al.6 potentially permitted the identification of molecular actors of the regulatory circuit linking the accumulation of N metabolites in the roots to inhibition of nodule formation (Fig. 1). The analysis of the regulated sequences might be also performed at the light of the recent findings revealing an association between plant nutrients and regulation mediated by miRNAs.22–24 A recent paper describes in Arabidopsis the involvement of microRNA167 (miRNA167) and one of its targets, Auxin Responsive Factor 8 (ARF8) in the early arrest of lateral root development under high rates of N supply.25
In conclusion, legumes represent an excellent experimental system to investigate different organogenesis programs modeling general root architecture and nodulation. These processes are both strongly modulated in response to external N supply and possible overlapping can be found between regulatory mechanisms and factors involved in the response to different N sources and concentrations.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9735
References
- 1.Carroll BJ, Gresshoff PM. Isolation and initial characterization of constitutive nitrate reductase-deficient mutants NR328 and NR345 of soybean (Glycine max) Plant Physiol. 1986;81:572–586. doi: 10.1104/pp.81.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho M, Harper JE. Effect of localized nitrate application on isoflavonoid concentration and nodulation in split-root systems of wild-type and nodulation-mutant soybeans plants. Plant Physiol. 1991;95:1106–1112. doi: 10.1104/pp.95.4.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caetano-Anollès G, Gresshoff PM. Plant genetic control of nodulation. Ann Rev Microbiol. 1991;45:345–382. doi: 10.1146/annurev.mi.45.100191.002021. [DOI] [PubMed] [Google Scholar]
- 4.Fujikake H, Yamazaki A, Ohtake N, Sueyoshi K, Matsuhashi S, Ito T, et al. Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J Exp Bot. 2003;54:1379–1388. doi: 10.1093/jxb/erg147. [DOI] [PubMed] [Google Scholar]
- 5.Barbulova A, Rogato A, D'Apuzzo E, Omrane S, Chiurazzi M. Differential effects of combined N sources on early steps of the Nod factor-dependent transduction pathway in Lotus japonicus. Mol PlantMicrob Interact. 2007;20:994–1003. doi: 10.1094/MPMI-20-8-0994. [DOI] [PubMed] [Google Scholar]
- 6.Omrane S, Ferrarini A, D'Apuzzo E, Rogato A, Delledonne M, Chiurazzi M. Symbiotic competence in Lotus japonicus is affected by plant nitrogen status: transcriptomic identification of genes affected by a new signalling pathway. New Phytol. 2009;183:380–394. doi: 10.1111/j.1469-8137.2009.02873.x. [DOI] [PubMed] [Google Scholar]
- 7.Walch-Liu P, Ivanov II, Filleur S, Gan Y, Remans T. Nitrogen regulation of root branching. Ann Bot. 2006;97:875–881. doi: 10.1093/aob/mcj601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HM, Forde BG. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- 9.Zhang HM, Jennings A, Barlow PW, Forde BG. Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA. 1999;96:6529–6534. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheible WR, Gonzales-Fontes A, Lauerer M, Muller-Rober B, Caboche M, Stitt M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell. 1997;9:973–978. doi: 10.1105/tpc.9.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forde BG. The role of long-distance signalling in plant responses to nitrate and other nutrients. Annu Rev Plant Biol. 2002;53:39–43. [PubMed] [Google Scholar]
- 12.Hirsch AM. Developmental biology of legume nodulation. Plant Physiol. 1992;122:211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x. [DOI] [PubMed] [Google Scholar]
- 13.Dudley ME, Jacobs TW, Long SR. Microscopic studies of cell divisions induced in alfalfa roots by Rhizobium meliloti. Planta. 1987;171:289–301. doi: 10.1007/BF00398674. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch AM, Fang Y. Plant hormones and nodulation: what's the connection? Plant Mol Biol. 1994;26:5–9. doi: 10.1007/BF00039514. [DOI] [PubMed] [Google Scholar]
- 15.Long SR. Rhizobium symbiosis: Nod factors in perspective. Plant Cell. 1996;8:1885–1898. doi: 10.1105/tpc.8.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathesius U, Schlaman HRM, Spaink HP, Sautter C, Rolfe BG, Djordjevic MA. Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J. 1998;14:23–34. doi: 10.1046/j.1365-313X.1998.00090.x. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Rizzo S, Crespi M, Frugier F. The Medicago truncatula CREI cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell. 2006;18:2680–2693. doi: 10.1105/tpc.106.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray DJ, Karas BJ, Sato S, Tabata S, Amyot L, Scczyglowski K. A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science. 2007;315:101–104. doi: 10.1126/science.1132514. [DOI] [PubMed] [Google Scholar]
- 19.Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, et al. A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science. 2007;315:104–107. doi: 10.1126/science.1132397. [DOI] [PubMed] [Google Scholar]
- 20.Gresshoff PM. Molecular genetic analysis of nodulation genes in soybean. Plant Breed Rev. 1993;11:275–318. [Google Scholar]
- 21.Caba JM, Centeno ML, Fernandez B, Gresshoff PM, Ligero F. Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a super-nodulating mutant and the wild type. Planta. 2000;211:98–104. doi: 10.1007/s004250000265. [DOI] [PubMed] [Google Scholar]
- 22.Aung K, Lin SI, Wu CC, Huang YT, Su C, Chiou TJ. pho2, a phosphate overaccumulator, is caused by a non-sense mutation in a microRNA 399 target gene. Plant Physiol. 2006;141:1000–1011. doi: 10.1104/pp.106.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bari R, Datt PB, Stitt M, Scheible WR. PHO2, microRNA399 and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol. 2006;141:988–999. doi: 10.1104/pp.106.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiou TJ. The role of microRNAs in sensing nutrient stress. Plant Cell Environm. 2007;30:323–332. doi: 10.1111/j.1365-3040.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 25.Gifford ML, Dean A, Gutierrez RA, Coruzzi GM, Birnbaum KD. Cell-specific nitrogen responses mediate developmental plasticity. Proc Natl Acad Sci USA. 2008;105:803–808. doi: 10.1073/pnas.0709559105. [DOI] [PMC free article] [PubMed] [Google Scholar]