Abstract
Exogenous application of plant hormone abscisic acid (ABA) can inhibit root growth. We recently reported that the proline-rich extensin-like receptor kinase 4 (PERK4) functions at an early stage of ABA signaling to inhibit primary root cell elongation by perturbing Ca2+ homeostasis.1 Transcription analysis indicated that PERK4 modulates the expression of the genes related to cell elongation and ABA signaling in root growth, such as polygalacturonases, AtExt1, AtMYC2 and ABR1. Under ABA treatment, the transcript level of ZAT10, a Ca2+-responsive gene, increased in perk4 plants compared to that of wild-type. Based on both present data and the previous evidence, we propose a probable model for PERK4-mediated ABA-regulated primary root cell growth.
Key words: abscisic acid, PERK4, root cell elongation, polygalacturonases, extensin, Ca2+, Arabidopsis
Although the intrinsic genetic program determines the development pattern of the organs, a sophisticated developmental plasticity allows plants to optimize the size and shape of their new organs in order to shrink away from unfavorable conditions or grow near nutrients or light to maximize resource acquisition.2,3 For example, the architecture of the root system was strongly influenced by environmental and endogenous signals including soil type, moisture and nutrients and phytohormones,3–6 which result in highly variable numbers, placement and direction of growth of each root in the morphologies, even among genetically identical plants. Morphological changes in the root system as an adaptive response result from complicated development pathways, that require plants to both recognize constantly changing environmental signals and to convert such signals into appropriate actions.3
The plant hormone abscisic acid (ABA) has multiple functions in regulating plant development and stress responses.7,8 For example, ABA inhibits the well-watered seedlings primary root growth and the formation of lateral roots and root hairs.9–12 Therefore, the diversity of ABA responses may require multiple sites of ABA perception.13 Two G-protein-coupled receptors (GTG1 and GTG2) have been implicated in ABA response and can directly bind ABA in vitro.14 Two other soluble ABA receptors, PYRABACTIN RESISTANCE 1 (PYR1) and PYR1-LIKE 9 (PYL9, RCAR1), have been identified and both interact with ABI1 in an ABA dependent manner and inhibit its activity.15,16 We recently demonstrated a member of the proline-rich extensin-like receptor kinase family, PERK4, functions at an early stage of the ABA signaling pathway to inhibit root growth through intracellular calcium signaling.1 The decreased sensitivity to ABA in primary root tip growth of perk4 mutant was due to enhanced cell elongation.
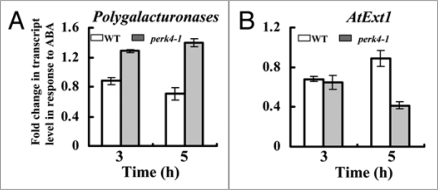
To understand how PERK4 modulates the ABA response at the gene expression level, we investigated the expression patterns of the genes related to ABA signaling in root growth in wild-type and perk4 mutant by quantitative real-time polymerase chain reaction (qRT-PCR). We first examine the transcription levels of genes encoding cell wall hydrolytic enzymes which loosen the structure of the wall and control the process of cell growth. These enzymes mainly include polygalacturonases (pectinase), xyloglucan endo-transglycosylases (XTHs) and expansins.17–20 Extensins are a family of hydroxyproline-rich glycoproteins (HRGPs) found in the cell walls of higher plants,21 which have been implicated in nearly all aspects of plant growth and development including the cessation of cell elongation.22,23 Transcript levels of one or two genes of these protein families were tested. The qRT-PCR data showed that the expression levels of a polygalacturonases gene (At3g06770) decreased in the wild-type, while increased in the mutant after ABA treatment (Fig. 1A). Approximately 2-fold expression in the perk4 plants was observed compared to wild-type plants. After treatment with ABA for 5 h, the level of AtExt1 transcripts was slightly affected in the wild-type, but significantly decreased in the mutant (Fig. 1B). However, no significant differences were found in the transcript levels of the selected XTH and expansin genes. As mentioned above, pectinase and extensins could loosen the structure of the wall and control the process of cell growth.17,18,20 Our result suggested that these two genes might have some role in the PERK4-mediated ABA response. Physiological substrates of the PERK4 kinase are not known. The similar expression patterns of the selected genes encoded XTHs or expansins in perk4-1 and wild-type plants did not rule out expansins or XTHs would be the PERK4 target. Further work is needed to determine which the direct target of the PERK4 kinase is and what event exactly occurs mediated by PERK4 in response to ABA.
Figure 1.
PERK4 regulates the expression of genes related to cell wall loosening. Total RNA was isolated from the 8-d-old mutant and wild-type seedling roots with or without 100 µM ABA treatment with the TRIzolR reagent (Invitrogen, Carlsbad, CA, USA). qRT-PCR was performed on a Rotor-Gene 3000 (Corbett Research, Australia). Reaction contained 10 µL SYBR Premix Ex Taq (TaKaRa), 0.6 µL primer (to 0.3 µM), 2 µL cDNA, and 6.8 µL deionized water to make a total volume of 20 µL. The gene-specific primers were 5′-TAG CCT CAC CAG CCA CCT CAC TC-3′ and 5′-GCA ATT CCA TAT TCA TCC CAA CC-3′ for the polygalacturonases gene (At3g06770, A), 5′-CCG CAA ATT ACT TCT ACT CTT CC-3′ and 5′-GTA TTC GTA GTG CTT CTT GGG TG-3′ for AtExt1 (B). Actin2 gene was used as a standard control. For relative quantification the method of Pfaffl (2001)27 was used to determine the relative expression ratio. Means ± SD are shown.
We also examined the transcription levels of other well-known genes regulated by ABA in wild-type and perk4 mutant. These genes include a basic helix-loop-helix (bHLH) transcription factor gene (AtMYC2) and an APETALA2 (AP2)-domain protein (ABR1). Among these, the transcription level of MYC2 was reduced 8.3-fold after 5 h ABA treatment in perk4 mutants, less induced than that in wild-type after ABA treatment (Fig. 2). By contrast, two-fold more expression of ABR1 in the perk4 plants was observed compared to wild-type plants (e.g., approximately 19-fold induction by ABA in perk4 mutants compared approximately nine-fold induction in wild-type). Since ABR1 is strongly responsive to ABA and functions as a negative transcription factor of ABA responses, disruption of ABR1 led to hypersensitive response to ABA in root growth assay.24 The result here coincides with the fact that root growth of perk4 mutants is less sensitive to ABA compared to that of wild-type plants. Interestingly, under ABA treatments, expression levels of other stress-responsive genes, such as Alcohol Dehydrogenase 1 (ADH1), Dehydration-Responsive 22 (RD22), ABA-Responsive Element Binding Protein 1 (AREB1) and ABI2, were found at similar levels in the wild-type and perk4 plants (Fig. 2).
Figure 2.
Expression of ABA- and stress-responsive genes in wild-type and perk4 mutants. qRT-PCR was performed in the same conditions as in Figure 1.
Both ZAT10 and TCH2 were Ca2+-responsive upregulated genes by which ABA regulates the physiological responses.25,26 For ZAT10 gene, the transcript level in perk4 plants was increased for nine-fold after ABA treatment, while only 1.5-fold induction by ABA was observed in wild-type plants (Fig. 2). However, transcripts of a potential Ca2+ sensor gene TCH2 were found at similar levels in the wild-type and perk4 mutant plants. The observation that PERK4 activity appears to be increased by ABA or Ca2+ in vitro implies a possibility of direct interaction with ABA.1 That PERK4 might be (part of) an ABA receptor is very exciting, because alternative pathways provide flexibility for evolutionary changes and are an important mechanism to prevent undesirable mutations causing plant death.
Based on both present data and the previous findings that PERK4 localized to the plasma membrane and is bound to the pectin by the pectinase digestion of the cell wall,1 we present a simplified model describing the ABA signal transduction pathway in root morphological changes associated with PERK4 (Fig. 3). This model provides new insights into root development plasticity mediated by ABA. In the presence of ABA, PERK4 as a positive regulator of ABA signaling is activated, which lead to activation of Ca2+ channel and stimulation expression of genes related with cell growth. Calcium functions as a downstream messenger that is essential for ABA responses inhibiting root cell elongation. And through a positive feedback loop, Ca2+ regulated the kinase activity of PERK4. Finally, the wild-type root tip growth was inhibited. PERK4 protein-mediated event would thereby represent a kind of ABA perceiving step, and PERK4 imposes a positive role toward the ABA response.
Figure 3.
A simplified model showing ABA-regulated root cell elongation through PERK4 in the wild-type (WT) and perk4-1 seedlings. Arrows indicate positive interactions; line with a bar at the end denotes negative effect.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30625005, 90817106 and 30771095).
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9739
References
- 1.Bai L, Zhang GZ, Zhou Y, Zhang ZP, Wang W, Du YY, et al. Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca2+ signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant J. 2009;60:314–327. doi: 10.1111/j.1365-313X.2009.03956.x. [DOI] [PubMed] [Google Scholar]
- 2.Callahan HS, Pigliucci M, Schlichting CD. Developmental phenotypic plasticity: Where ecology and evolution meet molecular biology. Bioessays. 1997;19:519–525. doi: 10.1002/bies.950190611. [DOI] [PubMed] [Google Scholar]
- 3.Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005;28:67–77. doi: 10.1111/j.1365-3040.2005.01306.x. [DOI] [PubMed] [Google Scholar]
- 4.Fitter AH, Stickland TR. Architectural analysis of plant root systems 2. Influence of nutrient supply on architecture in contrasting plant species. New Phytol. 1991;118:383–389. [Google Scholar]
- 5.López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhang H, Rong H, Pilbeam D. Signalling mechanisms underlying the morphological responses of the root system to nitrogen in Arabidopsis thaliana. J Exp Bot. 2007;58:2329–2338. doi: 10.1093/jxb/erm114. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein RR, Gampala SSL, Rock CD. Abscisic acid signaling in seeds and seedlings. Plant Cell. 2002;14:15–45. doi: 10.1105/tpc.010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- 9.Tucker DJ, Manisfield TA. Apical dominance in Xanthium strumarium. A discussion in relation to current hypotheses of correlative inhibition. J Exp Bot. 1973;24:731–740. [Google Scholar]
- 10.Bellandi DM, Dorffling K. Effect of abscisic acid and other plant hormones on growth of apical and lateral buds of seedlings. Physiol Plant. 1974;32:369–372. [Google Scholar]
- 11.Sharp RE, Wu Y, Voetberg GS, Saab IN, LeNoble ME. Confirmation that abscisic acid accumulation is required for maize primary root elongation at low water potentials. J Exp Bot. 1994;45:1743–1751. [Google Scholar]
- 12.Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, et al. Root growth maintenance during water deficits: physiology to functional genomics. J Exp Bot. 2004;55:2343–2351. doi: 10.1093/jxb/erh276. [DOI] [PubMed] [Google Scholar]
- 13.McCourt P, Creelman R. The ABA receptors-we report, you decide. Curr Opin Plant Biol. 2008;11:474–478. doi: 10.1016/j.pbi.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Pandey S, Nelson DC, Assmann SM. Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science. 2009;324:1068–1071. doi: 10.1126/science.1173041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, et al. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science. 2009;324:1064–1068. doi: 10.1126/science.1172408. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrove DJ. Loosening of plant walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Jones L, McQueen-Mason S. Expansins and cell growth. Current Opinion in Plant Biology. 2003;6:603–610. doi: 10.1016/j.pbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Vissenberg K, Fry SC, Pauly M, Hofte H, Verbelen JP. XTH acts at the microfibril-matrix interface during cell elongation. J Exp Bot. 2005;56:673–683. doi: 10.1093/jxb/eri048. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Shiu SH, Thoma S, Li WH, Patterson SE. Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biology. 2006;7:87. doi: 10.1186/gb-2006-7-9-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Showalter AM. Structure and function of plant cell wall proteins. Plant Cell. 1993;5:9–23. doi: 10.1105/tpc.5.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleland R, Karlsnes A. A possible role for hydroxyprolinecontaining proteins in the cessation of cell elongation. Plant Physiology. 1967;42:669–671. doi: 10.1104/pp.42.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadava D, Chrispeels MJ. Hydroxyproline-rich cell wall protein extensin: role in the cessation of elongation in excised pea epicotyls. Developmental Biology. 1973;30:49–55. doi: 10.1016/0012-1606(73)90047-x. [DOI] [PubMed] [Google Scholar]
- 24.Girdhar KP, John JG, Yong HC, Beom GK, Legong L, Sheng L. ABR1, an APETALA2-domain transcription factor that gunctions as a repressor of ABA response in Arabidopsis. Plant Physiol. 2005;139:1185–1193. doi: 10.1104/pp.105.066324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan B, Davydov O, Knight HR, Galon Y, Knight MR, Fluhr R, et al. Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell. 2006;18:2733–2748. doi: 10.1105/tpc.106.042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikki AD, Keith AJ, Naweed IC, Janet B. CML24, regulated in expression by diverse stimuli, encodes a potential Ca2+ sensor that functions in responses to abscisic acid, daylength and ion stress. Plant Physiol. 2005;139:240–253. doi: 10.1104/pp.105.062612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]