Abstract
Much is already known about the function and functioning of the three genes that make up the SOS (Salt-Overly-Sensitive) pathway in plants, but recent studies indicate that the linkage between external increases in salinity and stress protection provided by genes SOS1, SOS2 and SOS3 is more complex than previously appreciated. It has recently been shown that the engineered reduced expression of the sodium/proton antiporter SOS1 affected several pathways indicating a role for SOS1 that exceeds its known function as an antiporter. Interference with expression of SOS1, characterized as a sodium/proton antiporter in the halophyte Thellungiella salsuginea converted Thellungiella into an essentially glycophytic species.
Key words: SOS1 function, Thellungiella salsuginea, halophytism
The SOS1 gene product encodes a protein specifying a plant plasma membrane Na+/H+-antiporter that is characterized by a N-terminal domain with 12-transmembrane helices that constitutes the antiporter and an intracellular domain of largely unknown function(s). When knocked out or mutationally inactivated in Arabidopsis thaliana, SOS1 has been identified to generate a most severe salt stress-sensitive phenotype.1,2 The protein is unusually large, larger than other cation/proton antiporters contained in the Arabidopsis genome, owing to the C-terminal region that comprises more than 60% of the entire coding region.3 Further genetic and biochemical studies revealed SOS1 to be part of a pathway, in which it is activated by a protein kinase, SOS2, in a complex with SOS3, a calmodulin-binding calcium sensor.4–6 It has been speculated that an increase of Na+ is recorded by a yet unidentified sensor/receptor, leading to an increase in intracellular Ca2+ that in turn activates the SOS pathway resulting in the extrusion of Na+ ions from the cytosol.7–9 Measurements of ion contents in plant tissues suggested that SOS1 is indeed a part of the a pathway that functions in extruding Na+ ions under salt stress conditions.10 Ectopic expression of the protein confers some salt stress tolerance in Arabidopsis and yeast cells.4,11,12
Although much work has been devoted to outlining the working of the pathway in Arabidopsis, and to modeling the sodium extrusion activity in yeast,4 several tantalizing questions remain. The gaps are both mechanistic and conceptual. Such an ion-exclusion strategy may be sufficient for relieving ionic stress in unicellular organisms. However, explaining how a single membrane protein might dramatically alter the tolerance in a multicellular context is less easily explained. A major lack of understanding concerns the necessity and function of the long C-terminal extension that has, apparently, no relationship to sodium transport as other Na+/H+-antiporters of NHX family lack this C-terminus although the N-terminal domains are conserved.3 Interestingly, NHX8, most closely related to SOS1 at the N-terminus but lacking the C-terminal region, encodes a Li+/H+ antiporter.13 In the category of unknown facts is how SOS1-dependent removal of Na+ from the cytosol of one cell would have to be orchestrated with both vacuolar, sequentially apoplastic, and external sodium partitioning and transport mechanisms, so as not to increase stress for the entire organism. Considering the characterization of sodium extrusion based on SOS1 in the highly sodium-sensitive, glycophytic Arabidopsis, it remained to be studied whether the SOS pathway, and particularly SOS1, had any function and relevance for the sodium tolerance of halophytic species. We have now characterized the function of SOS1 in the Arabidopsis-relative Thellungiella salsuginea, an extremely salt-tolerant species14–17,27 with special emphasis on intracellular processes that might be altered by the inhibition of SOS1 expression. The study identified SOS1 as an intrinsic part of the halophytic nature of this species because the downregulation of SOS1 transcript expression converted Thellungiella into a glycophytic species.18
SOS1 is critical for the halophytic nature of Thellungiella by protecting predominantly cells of the root under salt stress in the zone of maximum cell expansion. In wild type plants, root cell elongation slowed by application of NaCl to the medium while cell death is not observed but, rather, the inhibition of cell expansion is gradually overcome leading to slower but normal expansion growth. Cells newly emerging from the division zone, which is not affected by sub-lethal salt stress, accumulated sodium in vacuoles indicating successful adaptation. In contrast, the reduction of SOS1 transcript amounts to less than 50% by RNAi expression in Thellungiella precipitated several intra-root and intracellular responses not observed in wild type plants. Sodium ions accumulated under salt stress to a much higher degree first in vacuoles and over time also in the cytosol. Prolonged exposure to NaCl changed the shape of the vacuoles, eventually leading to fragmentation. Also, endocytosis became increasingly inhibited, culminating in the rupture of plasma membranes and cell death beginning in a segment of the root adjacent to the cell division zone.18
The observations suggested a novel function for SOS1, extending beyond its role as an antiporter. However, caution is advised for distinguishing SOS1 functions from pleiotropic consequences of the lack of SOS1 under stress condition. Mutations of the SOS pathway in Arabidopsis are already known to affect proton flux in root cells, both under normal condition and in the presence of Na+.19 Disruption of intracellular pH homeostasis by a mutation of AVP1 inhibited endocytosis and auxin transport,20 hence the observed inhibition of endocytosis in thsos1–4 under salt stress18 might be a result of disrupted pH homeostasis.
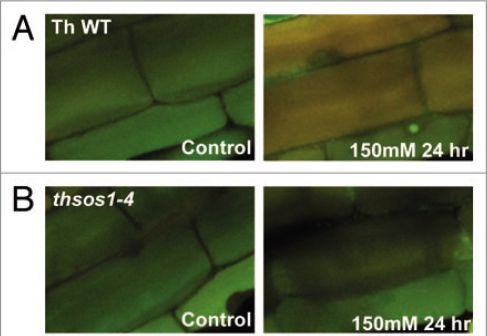
Observations from transcript profiling that compared wild type and sos1-RNAi lines have provided indications for the disturbance of expression of several ion channels, including Ca2+/H+ antiporters, CNGCs, NHXs, and many proton-ATPases, by SOS1 knockdown.21 Activities of CAX1 and CAX2, the vacuolar Ca2+/H+-antiporters are regulated by pH22 suggesting that changes inta a intracellular pH could be converted to Ca2+ signal. Additional observations revealed changes of intracellular pH under salt stress (Fig. 1). In wild type, the root cell vacuole became alkalinized as Na+ accumulated (Fig. 1A). The vacuolar alkalization upon salt stress was observed also in Arabidopsis wild type,23 but demolished in both thsos1–4, a representative transgenic Thellungiella line expressing the ThSOS1-RNAi (Fig. 1B) and atsos1-1, an Arabidopsis SOS1 knockout mutant (Oh DH, unpublished). A function of SOS1 in signaling even in the absence of Na+ ions has recently been suggested. SOS1 might be activated by different proteins in shoot and root.24 Different from other Na+/H+ antiporters (NHX family), the long cytosolic C-terminal domain of SOS1 includes a cyclic nucleotide (cNMP) binding domain.21 In this respect, the different regulation of CNGCs in the Thellungiella sos1-RNAI line might be significant.21 Members of the CNGC family are involved in different pathways that affect ion homeostasis, defense and developmental pathways.25
Figure 1.
Compromised vacuolar alkalization in the root cortex cells under salt stress by inhibition of SOS1 expression in Thellungiella salsuginea. Root cells were loaded with carboxyl-SNARF, a fluorescence pH indicator whose emission spectrum shifts from 580 nm (green) to 640 nm (red) as the pH increases. Confocal images were collected at 580 nm and 640 nm separately and merged. Seedlings of five day-old Thellungiella wild type (A) and the thsos1-4 line (B) were subjected to 150 mM NaCl. Root cortex cells are shown.
Unlike inhibition of endocytosis,18 alkalization of the vacuoles was observed only when SOS1 expression was intact, but not in Thellungiella RNAi lines and the Arabidopsis KO-line. This could suggest a more direct involvement of SOS1 in the control of the vacuolar pH. Consequently, studies are required aimed at identifying whether SOS1 is directly involved in membrane trafficking, for example, by regulating the pH of endosomes as has been observed in some animal Na+/H+-antiporters.26 Also, it must be ascertained whether the observed inhibition of endocytosis is a manifestation of imbalanced ion homeostasis caused by SOS1 deficiency or whether SOS1 has a role in the process. As well, SOS1 could function as the speculative plasma membrane Na+ sensor7–9 by either directly or indirectly, via regulation of other H+ pumps,19 changing the intracellular pH in the presence of Na+ ions, which in turn induce Ca2+ signals and activate the protein itself by the pathway involving SOS2/SOS3 complex.
Acknowledgements
The work has been supported by the World Class University Program (grant no. R32-10148) and the Biogreen 21 project of the Rural Development Administration (grant no. 20070301034030) of Korea.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9786
References
- 1.Liu J, Zhu JK. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc Natl Acad Sci USA. 1997;94:14960–14964. doi: 10.1073/pnas.94.26.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi H, Ishitani M, Kim C, Zhu JK. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc Natl Acad Sci USA. 2002;99:9061–9066. doi: 10.1073/pnas.132092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halfter U, Ishitani M, Zhu JK. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc Natl Acad Sci USA. 2000;97:3735–3740. doi: 10.1073/pnas.040577697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA. 2002;99:8436–8441. doi: 10.1073/pnas.122224699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu JK. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 2000;124:941–948. doi: 10.1104/pp.124.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu JK. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol. 2003;6:441–445. doi: 10.1016/s1369-5266(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 9.Chinnusamy V, Zhu J, Zhu JK. Salt stress signaling and mechanisms of plant salt tolerance. Genet Eng (NY) 2006;27:141–177. doi: 10.1007/0-387-25856-6_9. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Quintero FJ, Pardo JM, Zhu JK. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi H, Lee BH, Wu SJ, Zhu JK. Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol. 2003;21:81–85. doi: 10.1038/nbt766. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, et al. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007;143:1001–1012. doi: 10.1104/pp.106.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An R, Chen QJ, Chai MF, Lu PL, Su Z, Qin ZX, et al. AtNHX8, a member of the monovalent cation: proton antiporter-1 family in Arabidopsis thaliana, encodes a putative Li/H antiporter. Plant J. 2007;49:718–728. doi: 10.1111/j.1365-313X.2006.02990.x. [DOI] [PubMed] [Google Scholar]
- 14.Bressan RA, Zhang C, Zhang H, Hasegawa PM, Bohnert HJ, Zhu JK. Learning from the Arabidopsis experience. The next gene search paradigm. Plant Physiol. 2001;127:1354–1360. [PMC free article] [PubMed] [Google Scholar]
- 15.Gong Q, Li P, Ma S, Indu Rupassara S, Bohnert HJ. Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J. 2005;44:826–839. doi: 10.1111/j.1365-313X.2005.02587.x. [DOI] [PubMed] [Google Scholar]
- 16.Amtmann A. Learning from Evolution: Thellungiella Generates New Knowledge on Essential and Critical Components of Abiotic Stress Tolerance in Plants. Molecular Plant. 2009;2:3–12. doi: 10.1093/mp/ssn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong CE, Li Y, Labbe A, Guevara D, Nuin P, Whitty B, et al. Transcriptional profiling implicates novel interactions between abiotic stress and hormonal responses in Thellungiella, a close relative of Arabidopsis. Plant Physiol. 2006;140:1437–1450. doi: 10.1104/pp.105.070508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh DH, Leidi E, Zhang Q, Hwang SM, Li Y, Quintero FJ, et al. Loss of halophytism by interference with SOS1 expression. Plant Physiol. 2009;151:210–222. doi: 10.1104/pp.109.137802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shabala L, Cuin TA, Newman IA, Shabala S. Salinity-induced ion flux patterns from the excised roots of Arabidopsis sos mutants. Planta. 2005;222:1041–1050. doi: 10.1007/s00425-005-0074-2. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Yang H, Peer WA, Richter G, Blakeslee J, Bandyopadhyay A, et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- 21.Oh DH, Gong Q, Ulanov A, et al. Sodium Stress in the Halophyte Thellungiella halophila and Transcriptional Changes in a thsos1-RNA Interference Line. J Integr Plant Biol. 2007;49:1484–1496. [Google Scholar]
- 22.Pittman JK, Shigaki T, Hirschi KD. Evidence of differential pH regulation of the Arabidopsis vacuolar Ca2+/H+ antiporters CAX1 and CAX2. FEBS Lett. 2005;579:2648–2656. doi: 10.1016/j.febslet.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 23.Leshem Y, Melamed-Book N, Cagnac O, Ronen G, Nishri Y, Solomon M, et al. Suppression of Arabidopsis vesicle-SNARE expression inhibited fusion of H2O2-containing vesicles with tonoplast and increased salt tolerance. Proc Natl Acad Sci USA. 2006;103:18008–18013. doi: 10.1073/pnas.0604421103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin H, Yang Y, Quan R, et al. Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell. 2009;21:1607–1619. doi: 10.1105/tpc.109.066217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan B, Sherman T, Fromm H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007;581:2237–2246. doi: 10.1016/j.febslet.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Orlowski J, Grinstein S. Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr Opin Cell Biol. 2007;19:483–492. doi: 10.1016/j.ceb.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinocur B, Altman A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr Opin Biotechnol. 2005;16:123–132. doi: 10.1016/j.copbio.2005.02.001. [DOI] [PubMed] [Google Scholar]