Abstract
We have shown that, unexpectedly, AtTSPO (Arabidopsis thaliana TSPO-related protein) is an endoplasmic reticulum and Golgi-localized membrane protein in plant cells.1 This localization contrasts with that of mammalian 18-kDa translocator protein (at least for the mostly studied isoform, 18-kDa TSPO), a mitochondrial outer membrane protein (reviewed in ref. 2). Whereas the potential functions of 18-kDa TSPO are well documented, involved mainly in mitochondrial physiology,2 and its interest as a drug target is being explored,3 the roles of TSPO-related proteins in plant growth and development are yet to be specified. AtTSPO is expressed in dry seeds and can be induced in vegetative tissues by osmotic and salt stress or abscisic acid (ABA) treatment. Moreover, it was shown that the ABA-dependent induction is transient, and that boosting tetrapyrroles biosynthesis through 5-aminolevulinic acid (ALA) feeding enhanced downregulation of AtTSPO, suggesting an inherent post-translational regulation mechanism also involving ABA and likely porphyrins. We present additional evidence that ABA can help stabilize constitutively expressed AtTSPO and that ALA feeding to knockout mutant seeds, induces substantial germination delay. Here we discuss the possible link between ABA and tetrapyrroles in AtTSPO expression and posttranslational regulation.
Key words: plant TSPO, subcellular localization, regulation, abscisic acid, porphyrins
TspO/MBR Domain-Containing Proteins Family
Tryptophan-rich sensory protein/mitochondrial benzodiazepine receptor (TspO/MBR) domain-containing proteins are found in Archaea to vertebrate, with few exceptions such as Saccharomyces cerevisiae. Vertebrates' 18-kDa TSPO, a conserved cholesterol and drug-binding mitochondrial outer membrane protein, is expressed in almost every tissue type analysed so far, but is particularly enriched in steroidogenic cells and some cancer cells.2 However, the structure/function of this protein may vary in some cell type.4 18-kDa TSPO appears to be an essential gene since knockout or knockdown mutants can be embryo-lethal.5,6
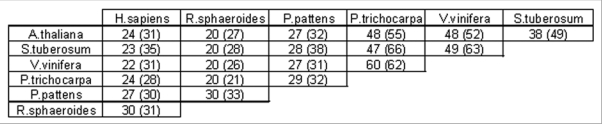
Although highly expressed in dry seeds, it was shown that AtTSPO is almost undetectable in plantlets and leaves, but ABA treatment could induce the protein in these same tissues.1 Knockout Arabidopsis lines for At2g47770 are viable with no obvious phenotype, as compared to wild type plants, when grown under normal conditions. These fundamental and contrasting features between plants and vertebrates TSPO proteins indicate a possible functional evolution of members of this fairly conserved protein family (Fig. 1). Primary sequence comparison revealed that plant TSPO-related proteins have an amino-terminal extension of 40–50 amino acids not found in prokaryotic or other eukaryotic members of this family. Although the identity percentiles were higher when only the putative TspO/MBR domains were compared (Fig. 1, values in parentheses), even the plant proteins were still relatively divergent (less than 70% identity), suggesting that the TspO/MBR may be a structural domain conferring instead a conserved fold to these proteins.
Figure 1.
Aminoacids identity comparisons between AtTSPO and related proteins from different species. ClustalW algorithm was used to align and compare residues identity between Arabidopsis AtTSPO (accession # AAL16286), potato StTSPO (accession # CAH10765), grapevine VvTSPO (accession # CAO39983), moss PptSPO1 (accession # ABG37902), Rhodobacter TspO and human HstSPO (accession # AAQ75703) full length proteins. Values in parentheses are for the predicted TspO/mBr domains alone.
TspO/MBR domain-containing proteins are thought to bind in vivo and in vitro a structurally diverse array of ligands. The isoquinoline carboxamide PK11195 [1-(2-chlorophenyl) -N-methyl-N-(1-methylpropyl)-3-isoquinoline-carboxamide] is an antagonist of mammalian 18-kDa TSPO, and the benzodiazepine Ro5-4864 [7-chloro-5-(4-chlorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one] an agonist. 18-kDa TSPO is essential for cholesterol translocation from the cytosol to the mitochondrial interior.2 Other endogenous ligands of TspO/MBR domain-containing proteins identified so far are the 10 kDa acyl-CoA binding protein (DBI, diazepam binding inhibitor) and porphyrins. Mammalian and bacterial TSPO are thought to be involved in porphyrin transport and heme biosynthesis.8,9 Whether a plant TSPO-related protein can use the same complement of ligands (in vivo and/or in vitro) as 18-kDa TSPO await experimental evidence.
Expressed AtTSPO Can be Stabilized by ABA
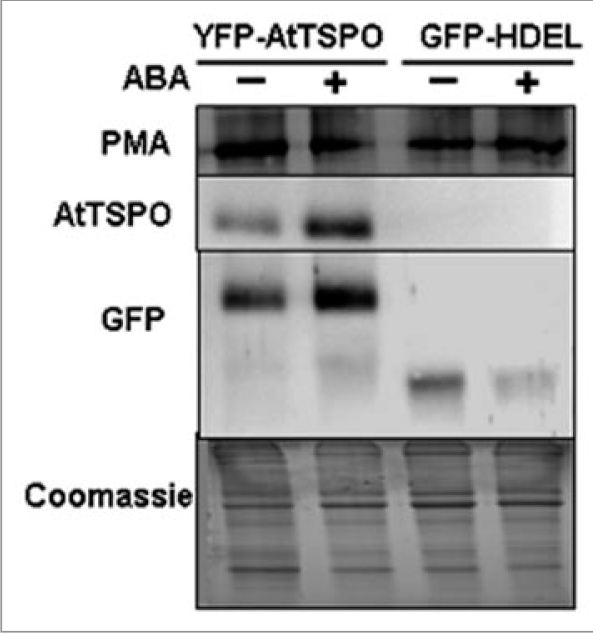
It was shown that the ABA-dependent induction of AtTSPO in seedlings was transient, with an optimum of induction after about 24 hours, AtTSPO levels decreasing afterward to becoming undetectable after 72 hours of incubation in presence of the phytohormone (incubation in 50 µM ABA).1 Transcriptional regulation of plant TSPO-related genes appears to be dependent upon the stress hormone ABA. The putative promoter region of Arabidopsis At2g47770, encoding AtTSPO, is enriched in ABA responsive cis regulatory elements (ABRE-like) and the ABI4 (ABA insensitive 4) cognate CCAC motif responsible for binding and ABI4-dependent downregulation of nuclear-encoded chloroplastic proteins.7 Expression of AtTSPO seems to be developmentally regulated. For example the protein is detected in maturing and dry seeds but not in ten-day-old seedlings, in mature pollen grains but not at earlier developmental stages, in senescent leaf but not in rosette green leaf. Constitutive expression of AtTSPO increased plant sensitivity to ABA (ABA-induced growth inhibition) and to salinity stress, suggesting that either ABA perception or signaling is affected by ectopic expression of AtTSPO. Constitutive expression of fluorescent protein-tagged AtTSPO (YFP-AtTSPO) showed variable fluorescent intensity depending on the cell type and growth stage of the transgenic plant. In leaves of tobacco and Arabidopsis transgenic plants expressing YFP-AtTSPO, consistently we observed that YFP fluorescent was restricted to epidermal cells with no or little fluorescence in mesophyll cells. These observations suggest that AtTSPO may be a short-lived protein and probably more so in photosynthetic active cells. In support of this interpretation, we posit that incubating transgenic tissues expressing YFP-AtTSPO in presence of ABA may at least transiently, stabilize the protein. Figure 2 shows that YFP-AtTSPO (and not the control GFP-HDEL) levels increased after 24 hours incubation in presence of 10 µM ABA. ABA may not only induce AtTSPO expression but may also be involved in post-translational regulation of the protein. The 5′-untranslated region of At2g47770 contains light regulatory cis elements (IBOX and GBOX). It is possible that at steady state, the turnover of expressed AtTSPO is efficient to preclude its detection in some tissues or growth conditions. An increase of ABA (during water deficit for example or in seed) will transiently modify the balance between synthesis and degradation of AtTSPO. The implications for this transient increase in AtTSPO in cell physiology under stressful conditions and the degradation mechanisms are not yet clear.
Figure 2.
Exogenous ABA can stabilize AtTSPO-containing fusion protein. Leaf from transgenic tobacco expressing YFP-AtTSPO or GFP-HDEL (control), was incubated for 24 hours in water containing 10 µm ABA. Anti-GFP and anti-AtTSPO were used to monitor the relative level of the protein by western blot. The plasma membrane proton pump ATPase (PMA) was used as internal control. Coomassie blue stained replicate of the SDS-PAGE gel is also shown.
Delayed Germination after ALA Feeding of AtTSPO-Null Mutant Seeds
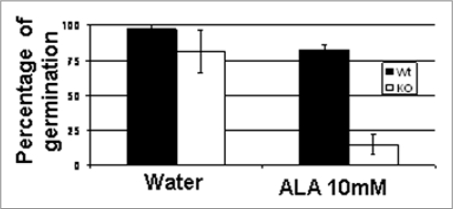
Porphyrins are prosthetic groups mediating critical cellular functions such as electron transfer, oxygen transport and photosynthetic energy transduction. Oxidized porphyrins are important intermediates in the catalytic cycles of heme proteins and in photosynthetic processes. All the porphyrins are derived from a common monopyrrolic precursor, δ-aminolevulinic acid (ALA). In contrast with the situation in animal, all the biochemically important porphyrins in plant cell (heme, bilirubin, chlorophylls…) are synthesized in the plastids and transported to other subcellular compartments when needed. We reported that boosting the porphyrins pathway by feeding transgenic seedling overexpressing AtTSPO or wild type seeds with ALA can enhance AtTSPO degradation.1 Heme and some intermediates in this pathway are thought to be involved in chloroplast-to-nucleus retrograde signaling although the situation is not yet clear in angiosperms.10–13 However, free porphyrins are powerful potential toxic molecules and boosting their biosynthesis is not without physiological consequences. As shown in Figure 3, incubating wild type seeds in ALA had just a marginal effect on seeds germination. In contrast, germination (after 2 days) of AtTSPO null mutant seeds was drastically reduced after ALA feeding. The mutant seeds were not irreversibly damaged by the ALA treatment, but reached the control germination rate with a delay of 3–4 days. This result suggests that AtTSPO may contribute in protecting seed germination from potential toxic effects of free tetrapyrroles. Downregulation of AtTSPO seems concomitant to acquisition of autotrophic properties by the seedling. The identity of the tetrapyrroles(s) involved and the underlying mechanism are under investigation.
Figure 3.
ALA feeding affects the germination of AtTSPO-null mutant seeds. Wild type (WT) and knockout (KO) arabidopsis thaliana seeds were imbibed in distilled water containing 0.1% agarose with or without 10 mm ALA. After 24 hours imbibition the seeds were allowed to germinate on water humidified Whatman paper. Germination (radicle protrusion) was scored in triplicate. Standard deviations represent the average of two independent experiments.
Conclusions
Plant TSPO-related proteins are localized in organelles of the early secretory pathway (endoplasmic reticulum and Golgi). This may not be specific to the angiosperm clade of this fairly conserved protein family. A recently described avian divergent TSPO isoform (TSPO214) seems to be specific to hematopoietic tissues in which the mitochondrial isoform is not expressed. Interestingly, this hematopoietic cells-specific isoform is targeted to the endoplasmic reticulum and the nuclear membrane, and still involved in intracellular cholesterol redistribution in relation to erythrocyte maturation.14 Whether AtTSPO can bind plant specific sterols has not yet been investigated but the C-terminal cholesterol binding motif in 18-kDa TSPO, the socalled CRAC domain,2 is not conserved in AtTSPO.
Transcriptional regulation of AtTSPO involves ABA, through the ABRE-like elements present in the 5′-untranslated region of the gene. It is also likely that ABI4 regulates the expression of this gene. Interestingly, the AP2/ERF transcriptional regulator ABI4 is important in a variety of physiological conditions including sugar responses, seed germination and chloroplast-to-nucleus retrograde signaling.7 Ectopic expression of AtTSPO can be detrimental to plant cell, thus a post-translational regulatory mechanism involving again ABA. It would be interesting to see how these pathways (induction and turnover) may be affected in known ABA signaling and biosynthetic mutant background.
The putative promoter region of AtTSPO contains two consensus motif of the recently described plastid response element,15 acting as enhancer for Chlamydomonas HSP70A induction by defined porphyrins. It seems that boosting porphyrins biosynthetic pathway enhances AtTSPO turnover. It is likely that ABA can modify the cellular levels and/or distribution of porphyrins, and ABA catabolism requires the hemoprotein ABA 8′-hydroxylase. The possible involvement of AtTSPO in ABA perception and/or signaling is an interesting working hypothesis.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9796
References
- 1.Guillaumot D, Guillon S, Déplanque T, Vanhee C, Gumy C, Masquelier D, et al. The Arabidopsis TSPO-related protein is a stress and abscisic acidregulated, endoplasmic reticulum-Golgi-localized membrane protein. Plant J. 2009;60:242–256. doi: 10.1111/j.1365-313X.2009.03950.x. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Rupprecht R, Rammes G, Eser D, Baghai TC, Schule C, Nothdurfter C, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- 4.Favreau F, Rossard L, Zhang K, Desurmont T, Manguy E, Belliard A, et al. Expression and modulation of translocator protein and its partners by hypoxia re-oxygenation or ischemia and reperfusion in porcine renal models. Am J Physiol Renal Physiol. 2009;297:F177–F190. doi: 10.1152/ajprenal.90422.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacapere JJ, Delavoie F, Li H, Peranzi G, Maccario J, Papadopoulos V, et al. Structural and functional study of reconstituted peripheral benzodiazepine receptor. Biochem Biophys Res Commun. 2001;284:536–541. doi: 10.1006/bbrc.2001.4975. [DOI] [PubMed] [Google Scholar]
- 6.Delavoie F, Li H, Hardwick M, Robert JC, Giatzakis C, Peranzi G, et al. In vivo and in vitro peripheraltype benzodiazepine receptor polymerization: functional significance in drug ligand and cholesterol binding. Biochemistry. 2003;42:4506–4519. doi: 10.1021/bi0267487. [DOI] [PubMed] [Google Scholar]
- 7.Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:715–719. [PubMed] [Google Scholar]
- 8.Taketani S, Kohno H, Furukawa T, Tokunaga R. Involvement of peripheral-type benzodiazepine receptors in the intracellular transport of heme and porphyrins. J Biochem. 1995;117:875–880. doi: 10.1093/oxfordjournals.jbchem.a124790. [DOI] [PubMed] [Google Scholar]
- 9.Yeliseev AA, Kaplan SA. Novel mechanism for the regulation of photosynthesis gene expression by the TspO outer membrane protein of Rhodobacter sphaeroides 2.4.1. J Biol Chem. 1999;274:21234–21243. doi: 10.1074/jbc.274.30.21234. [DOI] [PubMed] [Google Scholar]
- 10.von Gromoff ED, Alawady A, Meinecke L, Grimm B, Beck CF. Heme, a plastid-derived regulator of nuclear gene expression in Chlamydomonas. Plant Cell. 2008;20:552–567. doi: 10.1105/tpc.107.054650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strand A, Asami T, Alonso J, Ecker JR, Chory J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature. 2003;421:79–83. doi: 10.1038/nature01204. [DOI] [PubMed] [Google Scholar]
- 12.Moulin M, McCormac AC, Terry MJ, Smith AG. Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc Natl Acad Sci USA. 2008;105:15178–15183. doi: 10.1073/pnas.0803054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mochizuki N, Tanaka A, Masuda T, Nagatani A. The steady state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc Natl Acad Sci USA. 2008;105:15184–15189. doi: 10.1073/pnas.0803245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan J, Rone MB, Papadopoulos V. Translocator protein 2 is involved in cholesterol redistribution during erythropoiesis. J. Biol. Chem. 2009 doi: 10.1074/jbc.M109.029876. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Gromoff ED, Schroda M, Oster UI, Beck CF. Identification of a plastid response element that acts as an enhancer within the Chlamydomonas HSP70A promoter. Nucl Acids Res. 2006;34:4767–4779. doi: 10.1093/nar/gkl602. [DOI] [PMC free article] [PubMed] [Google Scholar]