Abstract
High soil boron (B) concentrations lead to the accumulation of B in leaves, causing the development of necrotic regions in leaf tips and margins, gradually extending back along the leaf. Plants vary considerably in their tolerance to B toxicity, and it was recently discovered that one of the tolerance mechanisms involved extrusion of B from the root. Expression of a gene encoding a root B efflux transporter was shown to be much higher in tolerant cultivars. In our current research we have shown that the same gene is also upregulated in leaves. However, unlike in the root, the increased activity of the B efflux transporter in the leaves cannot reduce the tissue B concentration. Instead, we have shown that in tolerant cultivars, these transporters redistribute B from the intracellular phase where it is toxic, into the apoplast which is much less sensitive to B. These results provide an explanation of why different cultivars with the same leaf B concentrations can show markedly different toxicity symptoms. We have also shown that rain can remove a large proportion of leaf B, leading to significant improvements of growth of both leaves and roots.
Key words: Bor genes, boron tolerance, boron toxicity, efflux pumping, leaf necrosis, membrane transport
B-toxic soils are widespread throughout agricultural areas of the world where they cause significant and often substantial reductions in crop quality and yield. The mechanism by which B is toxic to plants is not well understood1 but toxicity symptoms include reduced root growth which affects uptake of water and nutrients, and the development of necrotic patches on leaves which impairs photosynthesis. Tolerance to B toxicity has been recognized in a number of crops, notably in cereals. In most cases, tolerance is achieved by reduced uptake of B into the root, which then leads to reduced uptake into the shoot. Genetic studies established that in barley, a locus associated with reduced tissue B occurred on chromosome 4 and that this locus could be transferred to other barley cultivars with desirable agronomic traits.2
Hayes and Reid3 made a careful study of the characteristics of B uptake in a highly tolerant landrace barley cultivar Sahara, and found that although B was highly permeable, the root B concentration in this cultivar could be maintained at only half that in the external medium, whereas in sensitive cultivars, B was the same in both intracellular and extracellular phases. It was concluded that tolerant cultivars must have a membrane active transporter that exports B from the root. A B exporter, AtBor1 had previously been discovered in Arabidopsis where it was involved in B loading into the xylem4 but it was later found to be degraded under high B conditions5 and therefore would not be useful in B tolerance.
However, other Bor1 homologues were subsequently discovered in Arabidopsis and in rice. Based on homology with rice, Reid6 cloned genes from barley and from wheat (HvBor2 and Tabor2 respectively) which were shown to be strongly upregulated in roots of tolerant cultivars, and virtually undetectable in sensitive cultivars. Thus, a simple mechanism to explain tolerance was established; efflux of B from the root reduced the intracellular concentration of B in the root cells, thereby reducing toxicity and improving root growth. At the same time, the lower root content meant that less B was transferred to the shoot, resulting in lower shoot toxicity.
Yet there remained several unanswered questions regarding B toxicity. Firstly, it was commonly observed that toxicity symptoms were not reliably correlated with leaf B concentration, and that often after rain, toxicity symptoms became less severe. Nable et al.7 had investigated the effect of rain on shoot B concentrations and concluded that although rain did reduce the B concentration in leaves, it did not affect growth and yield. Secondly, field trials with cultivars in which the B tolerance traits were expressed, did not show the improvements in growth and yield that could be observed in glasshouse trials.8,9
Our recent work10 has provided new insights into these phenomena. Sensitive and tolerant cultivars of both wheat and barley were grown in varying levels of B. Then, ignoring the level of B in the growth solution, leaves of the different cultivars that displayed the same degree of leaf necrosis were selected. This revealed that in the tolerant cultivars, necrosis began to appear at leaf B levels that were two-to five-fold higher than in sensitive cultivars. Since no internal tolerance mechanism had been reported, it was hypothesised that in the tolerant cultivars, internal toxicity was reduced by pumping B from the cytoplasm into the cell wall where B is much less toxic. To prove this hypothesis three types of experiment were conducted. Firstly protoplasts were isolated from leaves of tolerant and sensitive cultivars of barley, and it was shown that when incubated in the same concentration of B, the tolerant cultivar was able to reduce the intracellular B concentration to approximately half that of the sensitive cultivar. Secondly, it was reasoned that if more B was accumulated in the apoplast of the tolerant cultivar, then it should be more quickly released by washing of the leaf; this was confirmed. Thirdly, it was shown that the same efflux transporters that were responsible for B export from the root were also highly expressed in leaves of tolerant cultivars of wheat and barley. The combination of these three experiments provided compelling evidence that redistribution of B in the leaf was a significant factor in B tolerance.
The elution experiment also highlighted the fact that because B is highly soluble and has high membrane permeability, it can easily be washed from leaves. Obviously in the field B could be removed from leaves by rain, but no positive effect of this on growth had been quantified. In our experiments, we simulated the average rainfall during the early growing season in a high B region of Southern Australia by spraying plants with calibrated amounts of water for 16 d. At high B concentrations, rain reduced leaf B by around 50% while simultaneously improving growth of shoots by up to 90%. Rather surprisingly, the rain treatment, which had no significant effect on root B concentrations, caused a two-fold increase in root growth, presumably by improving the supply of photosynthate from the shoot.
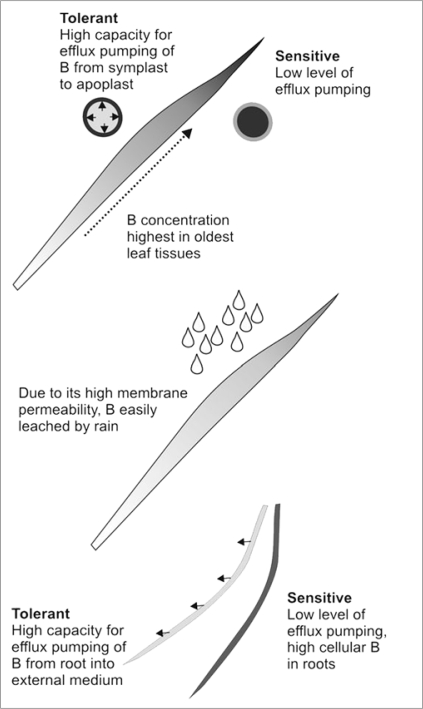
This study has enabled an evaluation of the importance of three main factors in determining the severity of B toxicity; two genetically determined processes, efflux pumping of B in roots and leaves, coupled with the variable leaching of B from leaves by rain (Fig. 1). The results also provide an explanation for the poor correlations observed between toxicity and shoot B concentrations in cereals.7,11
Figure 1.
Summary of processes contributing to reduced B toxicity in wheat and barley. The intensity of shading indicates the level of B in different regions of the plant. Boron (B) enters the leaf via the xylem and continues to accumulate as the leaf grows. When plants are grown in high concentrations of B, the older parts of the leaf become necrotic first while the younger basal tissues continue to expand. In tolerant cultivars, B efflux transporters in leaves pump B from the cytoplasm where it is toxic into the cell walls where it can be tolerated at high concentrations. Sensitive cultivars have a very low capacity for B efflux and therefore retain much higher concentrations inside the cell than in tolerant cultivars. rain can remove large amounts of B from leaves, thereby alleviating toxicity. In roots of tolerant cultivars, the same B efflux transporters that occur in leaves are used to pump B from the cells into the external medium. This reduces the toxicity to roots and limits the amount of B entering the xylem and reaching the leaves.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9798
References
- 1.Reid RJ, Hayes JE, Post A, Stangoulis JCR, Graham RD. A critical analysis of the causes of B toxicity in plants. Plant Cell Environ. 2004;27:1405–1414. [Google Scholar]
- 2.Jefferies SP, Barr AR, Karakousis A, Kretschmer JM, Manning S, Chalmers KJ, et al. Mapping of chromosome regions conferring B tolerance in barley (Hordeum vulgare L.) Theoret Appl Genet. 1999;98:1293–1303. [Google Scholar]
- 3.Hayes JE, Reid RJ. B tolerance in barley is mediated by efflux of B from the roots. Plant Physiol. 2004;136:3376–3382. doi: 10.1104/pp.103.037028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takano J, Miwa K, Yuan L, von Wiren N, Fujiwara T. Endocytosis and degradation of BOR1, a B transporter of Arabidopsis thaliana, regulated by B availability. Proc Natl Acad Sci USA. 2005;102:12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, et al. Arabidopsis B transporter for xylem loading. Nature. 2002;420:337–339. doi: 10.1038/nature01139. [DOI] [PubMed] [Google Scholar]
- 6.Reid R. Identification of B transporter genes likely to be responsible for tolerance to B toxicity in wheat and barley. Plant Cell Physiol. 2007;48:1673–1678. doi: 10.1093/pcp/pcm159. [DOI] [PubMed] [Google Scholar]
- 7.Nable RO, Paull JG, Cartwright B. Problems associated with the use of foliar analysis for diagnosing B toxicity in barley. Plant Soil. 1990;128:225–232. [Google Scholar]
- 8.Emebiri L, Michael P, Moody D. Enhanced tolerance to boron toxicity in two-rowed barley by marker-assisted introgression of favourable alleles derived from Sahara 3771. Plant Soil. 2009;314:77–85. [Google Scholar]
- 9.McDonald GK, Eglinton JK, Barr AR. Assessment of the agronomic value of QTL on chromosomes 2H and 4H linked to tolerance to boron toxicity in barley (Hordeum vulgare L.) Plant Soil. 2009 In Press. [Google Scholar]
- 10.Reid RJ, Fitzpatrick KL. Influence of leaf tolerance mechanisms and rain on boron toxicity in barley and wheat. Plant Physiol. 2009;151:1–8. doi: 10.1104/pp.109.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torun AA, Yazici A, Erdem H, Cakmak I. Genotypic variation in tolerance to B toxicity in 70 durum wheat genotypes. Tur J Agric For. 2006;30:49–58. [Google Scholar]