Abstract
Olfactory floral signals are significant factors in plant-pollinator mutualisms. Recently, unusual fermentation odors have been described in the nectar and flowers of some species. Since yeasts are common inhabitants of many angiosperms nectars, this raises the possibility that nectar yeasts may act as causal agents of fermentation odors in flowers and, therefore, as possible intermediate agents in plant signaling to pollinators. A recent field study has reported that nectar yeasts were quite frequent in floral nectar across three different regions in Europe and America, where they reached high densities (up to 105 cells/mm3). Yeast incidence in floral nectar differed widely across plant host species in all sampling sites. A detailed study currently in progress on one of the species surveyed in that study (Helleborus foetidus, Ranunculaceae) has detected that, in addition to interespecific differences in yeast incidence, there is also a strong component of variance in yeast abundance that takes place at the subindividual level (among flowers of the same plant, among nectaries of the same flower). If yeast metabolism is eventually proved to contribute significantly to floral scent, then multilevel patchiness in the distribution of nectar yeasts (among species, among individuals within species, and among flowers and nectaries of the same individual) might contribute to concomitant multilevel variation in plant signaling and, eventually, also in pollination success, pollen flow and plant fitness.
Key words: nectar, yeast, scent, plant-animal interaction, plant signaling
Pollinators forage on a wide range of flowers that differ in morphology, color, scent and quality and quantity of reward. The majority of these floral features are important visual and olfactory cues that are directly related to plant-pollinators signaling and the pollination process.1–12 Recently, the intriguing possibility has been raised that microbial communities (especially nectarivorous yeasts) inhabiting flowers could explain better than, or in addition to, plant physiology itself, certain floral features that participate in plant-pollinators signaling, like yeasty nectar or floral scent.13,14 However, some of these suggestions are based on circumstantial or indirect evidence indicative of the presence of microbes in flowers. For example, fermentation odors have been described in a number of Angiosperms,14–16 in which different compounds found in nectar were not shared with any other floral parts.13 In addition, yeasty odors (ketones and shortchain alcohols) have only been observed in mature flowers that were already visited by pollinators and thus potentially contaminated with microbes, in contrast, for example, to the sesquiterpenes isolated in immature flowers that are also common in the foliage of many plants.14 Yeasty odors were found in species whose flowers are long-lived, produce large amounts of nectar, and are visited by flies and beetles, which are known to act as yeast vectors to flowers.17–19 In spite of these plausible suggestions, studies indicating a potential role of microbes in the origin of floral scents generally have not looked directly for their presence or abundance in floral nectar, which clearly would provide critical empirical evidence in support of the hypothesis of microbial-mediated signaling in plant-pollinator interactions.
That yeasts are common inhabitants of floral nectars was well known to microbiologists more than a century ago20,21 and has been recently corroborated by Herrera et al.22 This study was conducted at three widely separated areas, which differed greatly in ecological features and biogeographical affinities: two study sites were located in the Southern Iberian Peninsula, about 350 km apart, and one in Yucatán Peninsula, eastern Mexico. Floral nectar samples from 40, 63 and 37 species, belonging to 21, 23 and 21 families, were examined microscopically for yeast cells at these three areas. Yeasts occurred very frequently in floral nectar at all areas, as revealed by the high proportion of nectar samples that contained them (31.8%, 42.3% and 54.4%; samples from all species at each site combined). In addition to being quite frequent in nectar samples, yeast cells often reached extraordinarily high densities in floral nectar at the three areas, which reached roughly 4 × 105 cells/mm3. When plant species, rather than individual nectar samples, were considered as the units for analyses, Herrera et al.22 found wide variation among species in both the frequency of occurrence and the density of yeasts in nectar samples. A significant fraction of such variation was found to be correlated with differences in pollinator composition, a link between pollination ecology and floral nectar microbiology that has remained unexplored until now. Similar results showing high densities and frequency of occurrence of yeasts in nectar, and interespecific differences in these magnitudes related to variation in pollinator composition, have been also reported by de Vega et al.23 for 40 South African plant species, which further supports the generality of the phenomenon. In addition to interespecific differences in the prevalence of nectar yeasts, the data examined by Herrera et al.22 and de Vega et al.23 revealed also considerable intraespecific variability (i.e., among individuals plants of the same species), although this aspect of results was not explicitly considered in their studies.
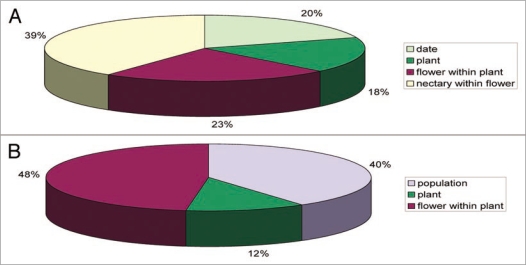
A study currently in progress has documented patterns of intraespecific variability in yeast occurrence in the nectaries of Helleborus foetidus (Ranunculaceae), a winter-flowering, bumble bee-pollinated perennial herb whose long-lived flowers last for roughly two weeks. Frequency of occurrence and cell density of yeasts in nectar were studied at six populations of this species from Sierra de Cazorla (SE Spain). Helleborus foetidus flowers have five separated horn-shaped nectaries hidden at the corolla base, each of which produces up to 5 µl of nectar. This enabled us to study patterns of yeast occurrence also at the within-flower level. At each population, total variance in yeast cell density on a per-nectary basis was partitioned into components due to differences between individual plants, flowers within plants and nectaries within flowers. We found extreme differences concerning the abundance and frequency of yeasts in H. foetidus nectar, the magnitude of intraespecific variation being similar or even greater than variation found in interespecific comparisons in the same study area (Pozo MI, et al. unpublished results). Our data suggest that temporal and spatial factors may explain differences regarding yeast abundance in H. foetidus nectar, and possibly other species as well. The largest component of intraespecific variance in yeast abundance occurred at the subindividual level, and was mainly accounted for by the variance between nectaries in the same flower (Fig. 1). This intraespecific variation in nectarivorous yeast incidence can have some important implications related to plant-pollinators interactions and, more specifically, to plant signaling, as outlined below.
Figure 1.
Hierarchical dissection of variance in yeast abundance in single-nectary nectar samples of Helleborus foetidus. (A) Temporal patterns. Collection dates and plant, flower within plant and nectary within flower as hierarchical levels of variance analyzed. (B) Spatial patterns. Population, plant within populations and flower within plants as hierarchical levels of variance analyzed.
Nectar-inhabiting yeasts modify certain flower characteristics linked to pollinator foraging behavior, such as nectar sugar composition and energetic value, by reducing total sugar concentration and altering the relative proportions of constituent sugars (sucrose, glucose and fructose) and the sucrose:hexose ratio.23–26 Furthermore, as noted above, yeasts could be also implicated in floral volatiles emission.13,14 Consequently, yeast incidence (measured both by frequency and abundance of yeast cells in nectar samples) may have been modifying signaling cues which have been postulated to be intrinsic plant species-specific. Although an empirical connection between yeast presence and fermentation nectar odor is needed, the fact that nectarivorous yeast presence would be as variable as described by our studies could imply the same variability for plant species signaling aspects, along with potential consequences for pollinators, since variance was mainly accounted for by variation below individual plant level. For example, in H. foetidus study variance in yeast abundance occurs mainly at the single nectary, which matches with the smallest scale that is perceived by a foraging insect. The fact that nectar is an important floral reward that plays a decisive role in the establishment of plant-pollinator mutualisms, together with the recently confirmed ubiquity of nectarivorous yeasts which could be acting as parasites of such mutualisms, open up new and exciting avenues to explore their effect on pollination success and pollen flow27–30 and finally on plant fitness.31–35
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/9874
References
- 1.Herrera CM. Selection on floral morphology and environmental determinants of fecundity in a hawkmoth-pollinated violet. Ecol Monogr. 1993;63:251–275. [Google Scholar]
- 2.Herrera CM. The adaptedness of the floral phenotype in a relict endemic, hawkmoth pollinated violet 1. Reproductive correlates of floral variation. Biol J Linn Soc. 1990;40:263–274. [Google Scholar]
- 3.Chittka L, Raine NE. Recognition of flowers by pollinators. Curr Opin Plant Biol. 2006;9:428–435. doi: 10.1016/j.pbi.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Chittka L. Bee color vision is optimal for coding flower color, but flower colors are not optimal for being coded—why? Isr J Plant Sci. 1997;45:115–127. [Google Scholar]
- 5.Schaefer HM, Schaefer V, Levey DJ. How plant-animal interactions signal new insights in communication. Trends Ecol Evol. 2004;19:577–584. [Google Scholar]
- 6.Schiestl FP, Peakall R, Mant JG, Ibarra F, Schulz C, Franke S, et al. The chemistry of sexual deception in an orchid-wasp pollination system. Science. 2004;302:437–438. doi: 10.1126/science.1087835. [DOI] [PubMed] [Google Scholar]
- 7.Ashman T-L, Bradburn M, Cole DH, Blaney BH, Raguso RA. The scent of a male: the role of floral volatiles in pollination of a gender dimorphic plant. Ecology. 2005;86:2099–2105. [Google Scholar]
- 8.Raguso RA. Start making scents: the challenge of integrating chemistry into pollination ecology. Entomol Exp Appl. 2008;128:196–207. [Google Scholar]
- 9.Raguso RA. Pages 83–105 Cognitive Ecology of Pollination: Animal Behaviour and Floral Evolution. Cambridge: Cambridge Univ Press; 2001. Floral scent, olfaction and scent-driven foraging behavior. [Google Scholar]
- 10.Galetto L, Bernardello G. Floral nectaries, nectar production dynamics and chemical composition in six Ipomoea species (Convolvulaceae) in relation to pollinators. Ann Bot. 2004;94:269–280. doi: 10.1093/aob/mch137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaczorowski RL, Gardener MC, Holtsford TP. Nectar traits in Nicotiana section Alatae (Solanaceae) in relation to floral traits, pollinators and mating system. Am J Bot. 2005;92:1270–1283. doi: 10.3732/ajb.92.8.1270. [DOI] [PubMed] [Google Scholar]
- 12.Cnaani J, Thomson JD, Papaj DR. Flower choice and learning in foraging bumblebees: Effects of variation in nectar volume and concentration. Ethology. 2006;112:278–285. [Google Scholar]
- 13.Raguso RA. Why are some floral nectars scented? Ecology. 2004;85:1486–1494. [Google Scholar]
- 14.Goodrich KR, Zjhra ML, Ley CA, Raguso RA. When flowers smell fermented: The chemistry and ontogeny of yeasty floral scent in pawpaw (Asimina triloba: Annonaceae) Int J Plant Sci. 2006;167:33–46. [Google Scholar]
- 15.Omura H, Honda K, Hayashi N. Floral scent of Osmanthus fragrans discourages foraging behavior of cabbage butterfly, Pieris rapae. J Chem Ecol. 2000;26:655. [Google Scholar]
- 16.Goodrich KR, Raguso RA. The olfactory component of floral display in Asimina and Deeringothamnus (Annonaceae) New Phytol. 2009;183:457–469. doi: 10.1111/j.1469-8137.2009.02868.x. [DOI] [PubMed] [Google Scholar]
- 17.Lachance MA, Starmer WT, Rosa CA, Bowles JM, Barker JSF, Janzen DH. Biogeography of the yeasts of ephemeral flowers and their insects. FEMS Yeast Res. 2001;1:1–8. doi: 10.1111/j.1567-1364.2001.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 18.Lachance MA, Rosa CA, Starmer WT, Schlag-Edler B, Barker JSF, Bowles JM. Metschnikowia continentalis var. borealis, Metschnikowia continentalis var. continentalis and Metschnikowia hibisci, new heterothallic haploid yeasts from ephemeral flowers and associated insects. Can J Microbiol. 1998a;44:279–288. [Google Scholar]
- 19.Lachance MA, Rosa CA, Starmer WT, Schlag-Edler B, Barker JSF, Bowles JM. Wickerhamiella australiensis, Wickerhamiella cacticola, Wickerhamiella occidentalis, Candida drosophilae and Candida lipophila, five new related yeast species from flowers and associated insects. Int J Syst Bacteriol. 1998b;48:1431–1443. doi: 10.1099/00207713-48-4-1431. [DOI] [PubMed] [Google Scholar]
- 20.Guillermond A. In: The Yeasts. Tanner FW, translator. New York: John Wiley & Sons, Inc; 1920. [Google Scholar]
- 21.Nadson GA, Krasilnikov NA. La levure du nectar des fleurs: Anthomyces reukaufii Gruess. Bull Soc Mycol France. 1927;43:232–244. (Fre). [Google Scholar]
- 22.Herrera CM, de Vega C, Canto A, Pozo MI. Yeasts in floral nectar: a quantitative survey. Ann Bot. 2009;103:1415–1423. doi: 10.1093/aob/mcp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vega C, Herrera CM, Johnson SD. Yeasts in floral nectar of some South African plants: quantification and associations with pollinator type and sugar concentration. S Afr J Bot. 2009 [Google Scholar]
- 24.Canto A, Herrera CM, Medrano M, Pérez R, García IM. Pollinator foraging modifies nectar sugar composition in Helleborus foetidus (Ranunculaceae): An experimental test. Am J Bot. 2008;95:315–320. doi: 10.3732/ajb.95.3.315. [DOI] [PubMed] [Google Scholar]
- 25.Canto A, Pérez R, Medrano M, Castellanos MC, Herrera CM. Intra-plant variation in nectar sugar composition in two Aquilegia species (Ranunculaceae): Contrasting patterns under field and glasshouse conditions. Ann Bot. 2007;99:653–660. doi: 10.1093/aob/mcl291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrera CM, García IM, Pérez R. Invisible floral larcenies: Microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology. 2008;89:2369–2376. doi: 10.1890/08-0241.1. [DOI] [PubMed] [Google Scholar]
- 27.Kevan PG, Eisikowitch D, Fowle S, Thomas K. Yeast-contaminated nectar and its effect on bee foraging. J Apic Res. 1988;27:26–29. [Google Scholar]
- 28.Eisikowitch D, Kevan PG, Lachance MA. The nectar-inhabiting yeasts and their effect on pollen germination in common milkweed, Asclepias syriaca L. Isr J Bot. 1990a;39:217–225. [Google Scholar]
- 29.Eisikowitch D, Lachance MA, Kevan PG, Willis A, Collins-Thompson DL. The effect of the natural assemblage of microorganisms and selected strains of the yeast Metschnikowia reukaufii in controlling the germination of pollen of the common milkweed, Asclepias syriaca. Can J Bot. 1990b;68:1163–1165. [Google Scholar]
- 30.Johnson SD, Hargreaves AL, Brown M. Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology. 2006;87:2709–2716. doi: 10.1890/0012-9658(2006)87[2709:dbnfaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Adler LS. The ecological significance of toxic nectar. Oikos. 2000;91:409–420. [Google Scholar]
- 32.Adler LS, Irwin RE. Ecological costs and benefits of defenses in nectar. Ecology. 2005;86:2968–2978. [Google Scholar]
- 33.Wiens F, Zitzmann A, Lachance MA, Yegles M, Pragst F, Wurst FM, et al. Chronic intake of fermented floral nectar by wild treeshrews. PNAS. 2008;105:10426–10431. doi: 10.1073/pnas.0801628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pyke GH, Day LP, Wale KA. Pollination ecology of Christmas bells (Blandfordia nobilis Sm.): effects of adding artificial nectar on pollen removal and seed set. Aust J Ecol. 1988;13:279–284. [Google Scholar]
- 35.Jersakova J, Johnson SD, Kindlmann P, Pupin AC. Effect of nectar supplementation on male and female components of pollination success in the deceptive orchid Dactylorhiza sambucina. Acta Oecol-Int J Eco. 2008;33:300–306. [Google Scholar]