Abstract
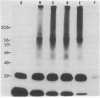
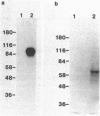
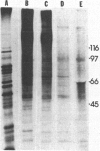
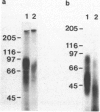
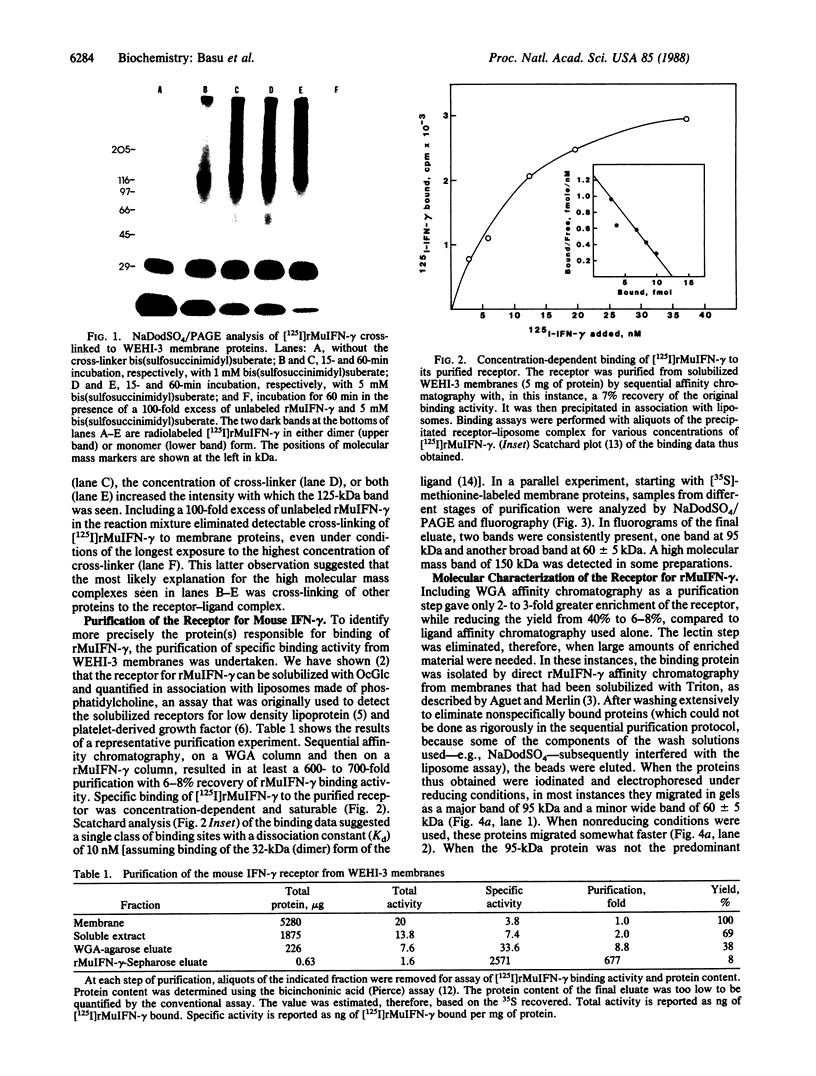
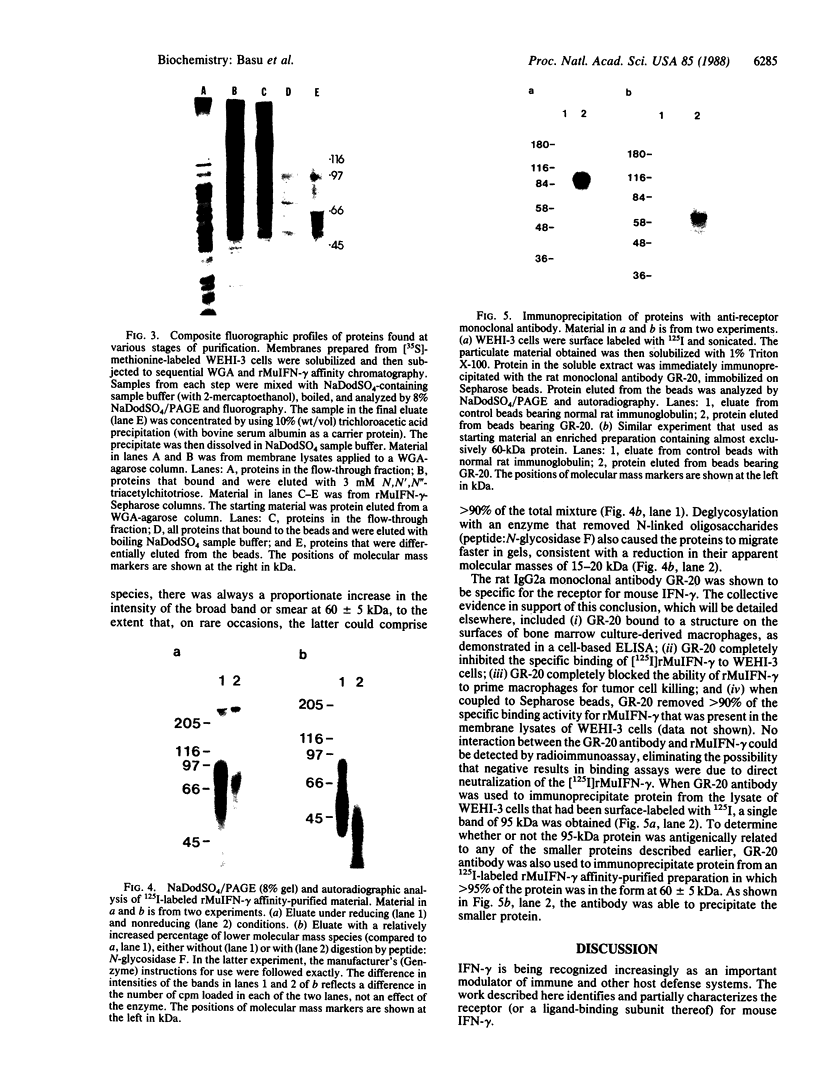
A receptor protein for mouse interferon gamma has been purified from solubilized plasma membranes of the mouse monomyelocytic cell line WEHI-3. Sequential wheat germ agglutinin and ligand affinity chromatography of membranes extracted with octyl beta-D-glucopyranoside resulted in at least a 680-fold purification of the receptor, as measured by precipitating it in association with liposomes composed of phosphatidylcholine. The purified receptor bound 125I-labeled recombinant mouse interferon gamma (rMuIFN-gamma) with a Kd of 10 nM, a value comparable to that obtained with isolated membranes (3.5 nM). PAGE analysis of radiolabeled (with either 35S or 125I) receptor preparations consistently revealed a major band of 95 kDa. This species was degraded with time to smaller fragments, principally one of 60 +/- 5 kDa. Treatment with peptide:N-glycosidase F reduced the apparent molecular masses of the proteins in the 95- and 60-kDa regions by 15-20 kDa each. GR-20, a monoclonal antibody against the receptor, completely inhibited specific binding of 125I-labeled rMuIFN-gamma to WEHI-3 cells, blocked the induction of priming by rMuIFN-gamma of macrophage-mediated tumor cell killing, removed binding activity for 125I-labeled rMuIFN-gamma from solubilized membranes, and immunoprecipitated a single 95-kDa protein from the extract of surface labeled (125I) WEHI-3 cells. Cross-linking of 125I-labeled rMuIFN-gamma to its receptor yielded a complex of 125 +/- 5 kDa, consistent with the binding of the dimeric form of mouse interferon gamma (32 kDa) to a membrane protein of 95 kDa. These data suggest that the receptor for mouse interferon gamma (or a ligand-binding subunit thereof) is a glycoprotein of 95 kDa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Merlin G. Purification of human gamma interferon receptors by sequential affinity chromatography on immobilized monoclonal antireceptor antibodies and human gamma interferon. J Exp Med. 1987 Apr 1;165(4):988–999. doi: 10.1084/jem.165.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon J., Sheehan K. C., Chance C., Thomas M. L., Schreiber R. D. Purification and characterization of the human interferon-gamma receptor from placenta. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4837–4841. doi: 10.1073/pnas.85.13.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Gray P. W., Rinderknecht E., Schreiber R. D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984 Jul 1;160(1):55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. O., Tremble P. M., Frackelton A. R., Jr, Williams L. T. Purification of the platelet-derived growth factor receptor by using an anti-phosphotyrosine antibody. Proc Natl Acad Sci U S A. 1985 May;82(9):2684–2687. doi: 10.1073/pnas.82.9.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hayes M. P., Russell S. W., Trotta P. P., Basu M. Enrichment and initial characterization of the solubilized receptor for mouse gamma interferon. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1096–1105. doi: 10.1016/0006-291x(88)90742-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeBlanc P. A., Katz H. R., Russell S. W. A discrete population of mononuclear phagocytes detected by monoclonal antibody. Infect Immun. 1980 Aug;29(2):520–525. doi: 10.1128/iai.29.2.520-525.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay R. J., Russell S. W. Protein changes associated with stages of activation of mouse macrophages for tumor cell killing. J Immunol. 1986 Aug 15;137(4):1392–1398. [PubMed] [Google Scholar]
- Novick D., Orchansky P., Revel M., Rubinstein M. The human interferon-gamma receptor. Purification, characterization, and preparation of antibodies. J Biol Chem. 1987 Jun 25;262(18):8483–8487. [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Russell J. K., Hayes M. P., Carter J. M., Torres B. A., Dunn B. M., Russell S. W., Johnson H. M. Epitope and functional specificity of monoclonal antibodies to mouse interferon-gamma: the synthetic peptide approach. J Immunol. 1986 May 1;136(9):3324–3328. [PubMed] [Google Scholar]
- Schneider W. J., Goldstein J. L., Brown M. S. Partial purification and characterization of the low density lipoprotein receptor from bovine adrenal cortex. J Biol Chem. 1980 Dec 10;255(23):11442–11447. [PubMed] [Google Scholar]
- Sen-Majumdar A., Murthy U., Das M. A new trophoblast-derived growth factor from human placenta: purification and receptor identification. Biochemistry. 1986 Feb 11;25(3):627–634. doi: 10.1021/bi00351a017. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]