Abstract
The progressive accumulation of β-amyloid (Aβ) in senile plaques and in the cerebral vasculature is the hallmark of Alzheimer’s disease and related disorders. Degradation of Aβ by specific proteolytic enzymes is an important process that regulates its levels in brain. Matrix metalloproteinase 2 (MMP2) was shown to be expressed in reactive astrocytes surrounding amyloid plaques and may contribute to Aβ degradation. Membrane type-1 (MT1)-MMP is the physiological activator for the zymogen pro-MMP2. Here, we show that in addition to MMP2, its activator MT1-MMP is also expressed in reactive astrocytes in regions with amyloid deposits in transgenic mice. Using a Cos-1 cell expression system we demonstrated that MT1-MMP can degrade exogenous Aβ40 and Aβ42. A purified soluble form of MT1-MMP degraded both soluble and fibrillar Aβ peptides in a time dependent manner yielding specific degradation products. Mass spectrometry analysis identified multiple MT1-MMP cleavage sites on soluble Aβ40 and Aβ42. MT1-MMP-mediated Aβ degradation was inhibited with the general MMP inhibitor GM6001 or the specific MT1-MMP inhibitor tissue inhibitor of metalloproteinases-2. Furthermore, in situ experiments showed that purified MT1-MMP degraded parenchymal fibrillar amyloid plaques that form in the brains of Aβ precursor protein transgenic mice. Together, these findings indicate that MT1-MMP possesses Aβ degrading activity in vitro.
A key pathological feature of Alzheimer’s disease (AD)1 is the progressive accumulation of β-amyloid (Aβ) in senile plaques and the cerebral vasculature. Aβ is derived from amyloidogenic processing of the amyloid precursor protein (AβPP), which involves sequential cleavage by β-secretase and γ-secretase (1–2). The steady-state level of Aβ peptides in the brain is controlled by a balance between production and clearance (3). Impaired clearance of Aβ peptides is likely important in the pathogenesis of AD, especially in the more common sporadic form. Several major pathways for Aβ clearance have been identified including receptor-mediated cellular uptake, blood-brain barrier transport (4–5), and direct proteolytic degradation.
Several proteinases/peptidases which can degrade Aβ have been reported including neprilysin (6–7), insulin-degrading enzyme (8), plasmin (9), endothelin-converting enzyme (10), angiotensin-converting enzyme (11), myelin basic protein (12), matrix metalloproteinase (MMP) 2 (13–14), and MMP9 (15). Regarding MMP2, it has been reported to cleave Aβ peptides at several sites (14). MMP2 expression and activity is induced in cultured human cerebrovascular smooth muscle cells in response to pathogenic Aβ (16). Also, in astrocytes the activity of MMP2 is increased in the presence of Aβ (17–20). Reactive astrocytes are found in regions with fibrillar amyloid deposits in brain tissue of human AD subjects and of APPsw (Tg-2576) transgenic mice, and have been shown to participate in the Aβ degradation in the extracellular space (20–23).
MMP2 is released in a latent form (pro-MMP2) that requires activation by membrane-type 1 (MT1)-MMP (24). MT1-MMP was the first MMP to be identified as an integral membrane protein with a single transmembrane domain and a short cytoplasmic C-terminal tail (25). MT1-MMP is inhibited by the endogenous tissue inhibitor of MMPs 2 (TIMP-2) and recruits pro-MMP2 forming a ternary complex. Then, adjacent uninhibited MT1-MMP cleaves the tethered pro-MMP2 (26). MT1-MMP is expressed in a variety of tissues including brain (27). In addition to activating pro-MMP2, MT1-MMP is involved in the breakdown of various extracellular matrix components including collagens, laminins, fibronectin, and proteoglycans (28). This function enables it to participate in numerous normal biological processes, such as reproduction, embryonic development, wound healing, angiogenesis, and apoptosis (29) or in pathological processes, such as rheumatoid arthritis, cardiovascular disease, tumor invasion and metastasis (30).
Expression of MT1-MMP can be induced in human glioma cells and human cerebrovascular smooth muscle cells in response to Aβ (23). It was reported that MT-MMPs induce cleavage and shedding of the AβPP ectodomain and that one of these cleavage sites is within the Aβ peptide region (31). However, any role for MT1-MMP in the degradation of Aβ peptides and the pathology of AD is unknown. In the present study, we show that, like MMP2, MT1-MMP is expressed in reactive astrocytes in regions with fibrillar microvascular amyloid deposits in a human AβPP transgenic mouse model. Subsequently, we show that MT1-MMP expressed in Cos-1 cells is capable of degrading soluble Aβ40 and Aβ42 peptides. A purified soluble truncated form of MT1-MMP also degraded soluble and fibrillar Aβ in-vitro. Mass spectrometry analysis identified multiple MT1-MMP cleavage sites on soluble Aβ40 and Aβ42. Furthermore, in situ experiments show that purified soluble MT1-MMP can degrade parenchymal fibrillar amyloid plaques that form in the brains of AβPP transgenic mice. Together, these data indicate that MT1-MMP possesses Aβ degrading activity.
MATERIALS AND METHODS
Reagents and Chemicals
Synthetic Aβ40 and Aβ42 peptides were synthesized by solid-phase Fmoc (9-fluorenylmethoxycarbonyl) amino acid chemistry, purified by reverse phase high performance liquid chromatography, and structurally characterized as previously described (32). Thioflavin-S (Th-S), Thioflavin-T (Th-T) and TIMP2 were purchased from Sigma-Aldrich (St. Louis, MO). The general MMP inhibitor GM6001 was purchased from Calbiochem (La Jolla, CA).
AβPP Transgenic Mice
Generation of Tg-SwDI transgenic mice on a pure C57BL/6 background was previously described (33). These mice express low levels of human Swedish/Dutch/Iowa mutant AβPP in neurons under control of the mouse Thy1.2 promoter. Tg-SwDI mice accumulate extensive cerebral microvascular fibrillar amyloid. Brain tissues from homozygous 24 months old Tg-SwDI and similarly aged control non-transgenic mice were used in this study. In other experiments brain tissues from 18 months old Tg2576 mice, a model of AD-like parenchymal fibrillar amyloid plaques, were used (44).
Immunofluorescent Labeling
Immunofluorescent stainings were performed on paraffin sections as recently described (33). The following primary antibodies were used for immunostaining: monoclonal antibody 66.1 (1:300), which recognizes residues 1 to 5 of human Aβ (34); rabbit polyclonal antibody to collagen type IV (1:100; Research Diagnostics Inc., Flanders, NJ); mouse monoclonal antibody to glial fibrillary acidic protein (GFAP) for identification of astrocytes (1:300, Chemicon); mouse monoclonal anti-keratan sulfate antibody for the detection of activated microglia (clone: 5D4, 1:200, Seikagaku Corporation, Japan); rabbit polyclonal antibody to MT1-MMP (1:100; Triple Point Biologics Inc., Forest Grove, OR); rabbit polyclonal antibody to MMP2 (1:100; Sigma). Primary antibodies were detected with goat anti-rabbit IgG (Alex 594; 1:2500; Molecular Probes Inc., Eugene, OR) or/and donkey anti-mouse IgG (Alex 488; 1:2500; Molecular Probes, Inc., Eugene, OR). Th-S staining for fibrillar amyloid was performed as described (35).
Gelatin Substrate Zymography
Cos-1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA). Full-length MT1-MMP and pro-MMP2 in pcDNA3.1 plasmids were the kind gifts of Dr. Jian Cao (Department of Medicine, Stony Brook University, NY, USA). Triplicate near confluent cultures were transfected with plasmids for expression of pro-MMP2, MT1-MMP or both pro-MMP2 and MT1-MMP using FuGENE 6 (Roche, Indianapolis, IN). Transfected cells were incubated in serum-free culture media and 72 h. The conditioned culture media samples were collected and aliquots were electrophoresed on 8% SDS-polyacrylamide gels containing 0.1% gelatin at 100 V for 2 h at 22°C. The gels were removed and incubated in rinse buffer (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 5 mM CaCl2, 2.5% Triton-X 100) for 3 h with several changes, washed 3 × 10 min with ddH2O, then incubated in assay buffer (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 5 mM CaCl2) overnight at 37°C, washed 3 × 10 min with ddH2O, stained with 0.25% Coomassie Brilliant Blue R-250 and then destained. Gelatinolytic MMP activity was observed as clear zones of lysis in the gels.
Aβ Degradation in Cos-1 Cells Expressing Human MT1-MMP
Triplicate near confluent cultures were transfected with purified empty pcDNA3.1 plasmid DNA or full-length MT1-MMP in pcDNA3.1 DNA by FuGENE 6 treatment (Roche, Indianapolis, IN), followed by addition of 2 μg/ml of Aβ40 or Aβ42 in serum-free media for 48 h. The culture media samples were collected and cell lysates were prepared. Aβ in the cell culture media samples was quantitatively analyzed by immunoblotting and sandwich ELISA analysis as described above.
Quantitative Immunoblotting
Samples containing MT1-MMP or Aβ were added directly into SDS-PAGE sample buffer, and stored at −70°C. Aliquots were loaded onto 12% or 10–20% polyacrylamide gels, electrophoresed and transferred onto Hybond-ECL nitrocellulose membranes (Amersham, Arlinton Heights, IL) at 100 V for 1.5 hour at RT. Membranes were blocked in 5% milk/PBS/0.05% Tween20 (PBS-T) for 1 h at RT. Primary antibodies were added (RP1-MMP14 for MT1-MMP; mAb20.1 for Aβ) for 1 h at RT, washed 3 × min with PBS-T. Horseradish peroxidase-conjugated mouse sheep anti-rabbit or anti-mouse IgG (1:5000 Amersham-Pharmacia, Piscataway, NJ), and washed 3 ×5 min with PBS-T. Bands were visualized using the ECL detection method (Amersham-Pharmacia, Piscataway, NJ). Quantitation of MT1-MMP or Aβ bands was performed using a VersaDoc Imaging System (BioRad, Hercules, CA) and the manufacturer’s Quantity Oneton software.
Aβ ELISA Analysis
The levels of soluble Aβ40 and Aβ42 peptides were measured using a quantitative sandwich ELISA as previously described (33).
Purification of Soluble MT1dTM Protein
The cDNA for a soluble, truncated form of MT1-MMP encoding residues Met1-Gly535 that lack the carboxyl-terminal transmembrane and cytosolic domains of full length MT1-MMP (MT1dTM) in pSG5 expression vector was the kind gift of Dr. Jian Cao (Department of Medicine, Stony Brook University, NY, USA). Two hundred ml of serum-free conditioned media from Cos-1 cells overexpressing soluble MT1dTM were passed through gelatin-agarose (Sigma-Aldrich, St. Louis, MO) to remove any gelatinases and then concentrated using an Amicon ultrafiltration unit (NMWL 5000 membrane) (Millipore, Bedford, MA). The enzymatic activity of purified MT1dTM was determined using the specific substrate- Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg (Bachem, California, CA).
In-vitro Soluble Aβ Degradation
Synthetic Aβ40 or Aβ42 were first dissolved in DMSO to a concentration of 1 mg/ml. 40 nM of purified MT1dTM were incubated with 1 μM of synthetic Aβ40 or Aβ42 in zymogen buffer (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 5 mM CaCl2) at 37 °C for specific lengths of time. The Aβ levels were measured in the samples by SDS-PAGE on 10–20% polyacrylamide Tris-Tricine gels and subsequent quantitative immunoblotting (as described above). In some experiments the selective MMP inhibitors GM6001 (10 μM; Calbiochem) or TIMP2 (40 nM; Sigma) were added.
To visualize MT1-MMP generated Aβ cleavage products N-terminal biotin labeled Aβ40 or Aβ42 were incubated with purified MT1dTM for 24 h at 37 C. The samples were diluted in the sample buffer containing 9M urea/5% acetic acid and methyl green. For analysis an acid/urea 22% polyacrylamide gel was prepared and pre run anode to cathode at 250 V for 30 min at 4°C in 5% glacial acetic acid running buffer (36). Following the pre run, the samples were loaded on the gel and electrophoresed at 4 ° from anode to cathode with increasing the voltage every 15 min as follows: 25, 50, 100, 200 volts and then 275 volts for 15 h until the end of the run. Prior to transfer, the acid/urea gel was neutralized in a glass tray by washing 5x with Tris-HCl/glycine transfer buffer on a rocking platform for 15 min. Then the gel was transferred to PVDF membranes be electroblotting for 2.5 hr (80V) at 4°C. After transfer, the membrane was boiled in PBS for 5 min in a glass dish and was cooled down in PBS. The membrane was blocked in 5% milk/PBS/0.05% Tween20 (PBS-T) for 1 h at RT. The membrane was incubated with streptavidin-horseradish peroxidase (1:5000 dilution) for 1 h at RT, and washed 3 × 5 min with PBS-T. Bands were visualized using the ECL detection method as described above.
Mass Spectrometry
40 nM purified MT1dTM was incubated with 1 μM synthetic Aβ40 or Aβ42 in zymogen buffer (50 mM Tris-HCl, pH 7.5, 200 mM NaCl, 5 mM CaCl2) at 37°C for 2 days. After incubation, the samples were dried in a rotary evaporator (Savant, Farmingdale NY), suspended in 20 μl of 0.1% TFA, ZipTipped using μC18 tips (Millipore, Milford, MA) and then eluted to the target. The addition of 1 μl of matrix consisting of acetonitrile/0.1% trifluoroacetic acid containing ∞-cyano-4-hydroxy cinammic acid (CHCA, 5 mg/ml) was dried on the sample plate. Samples were run on a Voyager-DE STR (Applied Biosystems, Framingham, MA) using a matrix assisted laser desorption ionization - time of flight (MALDI-TOF) mass spectrometer system operated in the reflector mode unless otherwise indicated. The mass scale (m/z 500–5000) was calibrated with a mixture of peptides or internal calibration was performed using a matrix ion at m/z 568.1330 and Aβ42 peptide amino acid 1–13 m/z 1561.6672. For samples acquired in the linear mode, 1 μl was dissolved in 10 μl of a 50% solution of acetonitrile/0.3% trifluoroacetic acid containing sinapinic acid (10 mg/ml) and dried on the sample plate. The mass scale (m/z 1000–25000) was calibrated with myoglobin (400 fM/μl).
In-vitro Fibril Aβ Degradation
To prepare amyloid fibrils, 5 mM Aβ42 in DMSO was diluted in PBS to 100 μM, vortexed for 30 sec, and incubated at 37 °C for 5 days (37). Triplicate samples of 10 μM of aged fibrillar Aβ was then incubated with 100 nM of purified MT1dTM in absence or presence of 100 μM GM6001 at 37 °C for 5 days. After digestion, the remaining fibrillar Aβ was quantitated using a Th-T fluorescence binding assay. Briefly, 5 μl of 100 μM Th-T was added to 100 μl of sample, mixed and incubated at RT in the dark for 10 min. Th-T fluorescence, an indicator of fibril Aβ binding, was measured at λex of 446 nm and λem of 490 nm.
Transmission Electron Microscopy
Sample aliquots were deposited onto carbon-coated copper mesh grids. Sample grids were allowed to stand for 60 sec, and excess solution was wicked away. Sample grids were then negatively stained with 2% (w/v) uranyl acetate and allowed to dry. The samples were viewed with an FEI Tecnai 12 BioTwin transmission electron microscope at 80 kV, and digital images were taken with an Advanced Microscopy Techniques camera.
In-Situ Fibrillar Amyloid Plaque Degradation
For this analysis the well-characterized Tg2576 (APPsw) mouse model of AD that develops abundant fibrillar amyloid pathology (38) was used. Brains were removed from anesthetized 18 months old Tg2576 mice after perfusion with cold saline and snap-frozen on dry ice. Five-μm cryostat sections will be collected on slides. Every other section was flipped 180° so that identical faces of adjacent sections were exposed (37). Paired adjacent sections (one incubated with zymogen buffer, the other with 100 nM purified MT1dTM in absence or presence of 100 μM GM6001) in triplicate were incubated at 37°C for 5 days, stained with thioflavin-S (ThS), and then imaged with fluorescence microspcopy. The parenchymal plaque amyloid area of ThS fluorescence was determined using image analysis software (Image J). Fractional area was compared between paired sections.
Statistical Analysis
Data were analyzed by Student’s t-test at the 0.05 significance level.
RESULTS
MT1-MMP and MMP2 are expressed in brain regions with prominent cerebral microvascular fibrillar Aβ deposits in Tg-SwDI mice
Previously, MMP2 was found increased in reactive astrocytes adjacent to parenchymal amyloid plaques in aged AβPP transgenic mouse brain (39). MMP2 expressed by reactive astrocytes is implicated in extracelluar Aβ catabolism (39). We have generated the Tg-SwDI mouse model, which develops early-onset and progressive accumulation of regional cerebral microvascular fibrillar amyloid deposition (33). Th-S staining of brain sections of aged Tg-SwDI mice revealed extensive fibrillar Aβ accumulation in the microvessels of the thalamus, but not in the cortex (Fig. 1A,B). MMP2 is also expressed in cells in the thalamus where fibrillar microvascular Aβ accumulates, but not the cortex (Fig. 1C,D). MMP2 is expressed as an inactive zymogen (pro-MMP2) requiring proteolytic activation by MT1-MMP. Labeling for MT1-MMP also showed strong expression by cells in the thalamus where fibrillar microvascular Aβ accumulates, but not the cortex (Fig. 1E,F).
Fig. 1. MT1-MMP and MMP2 are expressed in regions of fibrillar Aβ accumulation in Tg-SwDI mouse brain.
Brain sections from twenty four month old Tg-SwDI mice were labeled for fibrillar Aβ using Th-S (green) showing that the cortex (A) lacks appreciable fibrillar amyloid whereas the thalamic region (B) contains extensive microvascular amyloid accumulations. Immunolabeling for MMP2 or MT1-MMP (red) in adjacent brains sections shows weak expression in the cortex (C and E, respectively) but strong expression in the thalamic region (D and F, respectively) containing abundant microvascular amyloid. Scale bars = 50 μm.
MT1-MMP and MMP2 are selectively expressed in reactive astrocytes in brain regions with microvascular fibrillar amyloid deposits in aged Tg-SwDI mice
To identify the cell type that expresses MMP2 and MT1-MMP near microvascular amyloid deposits the Tg-SwDI mouse brain sections were double immunolabeled for GFAP to detect reactive astrocytes and either MMP2 or MT1-MMP (Fig. 2). Immunolabeling for MMP2 and its activator MT1-MMP strongly co-localized with GFAP-positive cells. Immunolabeling for activated microglia in these tissue sections failed to show co-localization with MMP2 or MT1-MMP (data not shown). These data show that like MMP2, its activator MT1-MMP is selectively expressed in reactive astrocytes near cerebral microvascular fibrillar amyloid deposits in aged Tg-SwDI mouse brain.
Fig. 2. MT1-MMP and MMP2 are expressed in reactive astrocytes near fibrillar microvascular amyloid deposits in Tg-SwDI mouse brain.
Brain sections from twenty four month old Tg-SwDI mice were double immunolabeled for GFAP to identify astrocytes (green) and MMP2 or MT1-MMP (red). The thalamic region, which contains extensive microvascular fibrillar amyloid, is shown. Numerous reactive astrocytes were observed (A and E) as well as strong immunolabeling for MMP2 (B) and MT1-MMP (F). Merging of the images showed strong co-localization of GFAP and MMP2 (C) or MT1-MMP (G). Scale bars = 50 μm. Higher magnifications of the merged images are shown in (D) and (H), respectively. Scale bars = 10 μm.
Exogenous Aβ40 and Aβ42 degradation in Cos-1 cells expressing human MT1-MMP
We next determined if MT1-MMP, like MMP2, could play a role in Aβ degradation using a cell culture expression system. Cos-1 cells were chosen since they do not normally express either MT1-MMP or MMP2. Therefore, Cos-1 cells were transfected to express pro-MMP2 alone, MT1-MMP alone or both pro-MMP2 and MT1-MMP. Post transfection, the cells were incubated with serum-free media for an additional 48 h. The cell lysates were collected and analyzed by immunoblotting using an anti-MT1-MMP antibody demonstrating protein expression in the cells transfected with the MT1-MMP plasmid (Fig. 3A). The culture media samples were collected and subjected to gelatin zymography to assay for MMP2 activities (Fig. 3B). The zymography assayed showed that pro-MMP2 was only expressed in the Cos-1 cells transfected with the pro-MMP2 plasmid. Whereas pro-MMP2 alone migrated at ≈72 kDa the co-transfection with pro-MMP2 and MT1-MMP exhibited activated MMP2 which migrated as a doublet at ≈66 kDa. These experiments demonstrated that MT1-MMP expressed in Cos-1 cells was enzymatically active.
Fig. 3. Activation of pro-MMP2 by MT1-MMP expressed in Cos-1 cells.
Triplicate cultures of Cos-1 cells were transfected with empty plasmid vector (pcDNA3.1), Pro-MMP2 vector alone, MT1-MMP vector alone, or both Pro-MMP2 vector and MT1-MMP vector. Twenty four hours post transfection, the cells were incubated in serum-free medium for an additional 48 h. (A) The cell lysates were collected and analyzed by immunoblotting by using anti-MT1-MMP. (B) The culture media samples were collected and analyzed by gelatin zymography. Co-expression of Pro-MMP2 and MT1-MMP led to conversion of pro-MMP2 to MMP2 demonstrating the MT1-MMP was proteolytically active.
To determine if MT1-MMP expressed in Cos-1 cells could degrade Aβ, the cells were transfected with either empty plasmid vector (pcDNA3.1) or the MT1-MMP plasmid vector. Post transfection; the cells were incubated with 2 μg/ml of freshly prepared soluble Aβ40 or Aβ42 in serum-free media for an additional 48 h. The cell lysates were collected and analyzed by immunoblotting using the anti-MT1-MMP antibody confirming MT1-MMP protein expression in the transfected cells (Fig. 4A). Although small amounts of Aβ peptides were found associated with the cells present in the cell lysates there was no difference in the amounts between control and MT1-MMP expressing Cos-1 cells (data not shown). The media samples were collected and analyzed for Aβ40 and Aβ42 peptide levels by immunoblotting using monoclonal anti-Aβ (Fig. 4B,D, respectively) and by quantitative ELISA measurements (Fig. 4C,E, respectively). These results indicate that both Aβ40 and Aβ42 were strongly reduced by about 50% and 70%, respectively, in MT1-MMP transfected Cos-1 cells.
Fig. 4. Aβ40 and Aβ42 are degraded by MT1-MMP expressed in Cos-1 cells.
Triplicate cultures of Cos-1 cells were transfected with empty plasmid vector (pcDNA3.1) or MT1-MMP vector. Twenty four hours post transfection, the cells were incubated with 2 μg/ml of freshly solublized Aβ40 or Aβ42 in serum-free media for an additional 48 h. (A) The cell lysates were collected and analyzed by immunoblotting using anti-MT1-MMP. The culture media samples were collected and analyzed for Aβ40 and Aβ42 peptides levels by immunoblotting using anti-Aβ (B and D, respectively) and by ELISA (C and E, respectively). The data shown are the mean ± S.D. (n=3). *, p < 0.05; **, p < 0.01.
In-vitro Aβ40 and Aβ42 degradation by purified soluble MT1-MMP
MT1-MMP is normally expressed as a membrane bound enzyme. However, a soluble transmembrane domain-lacking form of MT1-MMP (MT1dTM) can be used to study the proteolytic function of the enzyme in solution. Therefore, we used purified MT1dTM protein to investigate if Aβ peptides could be degraded in-vitro. Aβ40 or Aβ42 (1 μM) was incubated at 37°C in the presence or absence of purified MT1dTM (40 nM) up to 24 h. At designated time points, samples were collected and analyzed for Aβ levels by quantitative immunoblotting using the anti-Aβ mAb. As shown in Fig. 5, Aβ40 and Aβ42 were degraded by purified MT1dTM in-vitro in a time-dependent manner with ≈40% reduction in the levels of both peptides in 24 h.
Fig. 5. Aβ40 and Aβ42 degradation by soluble MT1dTM.
Aβ40 (A and B) or Aβ42 (C and D) was incubated at 37°C in the presence or absence of purified 40 nM of MT1dTM. At each time point, samples were collected and analyzed for Aβ level by quantitative immunoblotting using anti-Aβ mAb. The data shown are the mean ± SD of three separate determinations.
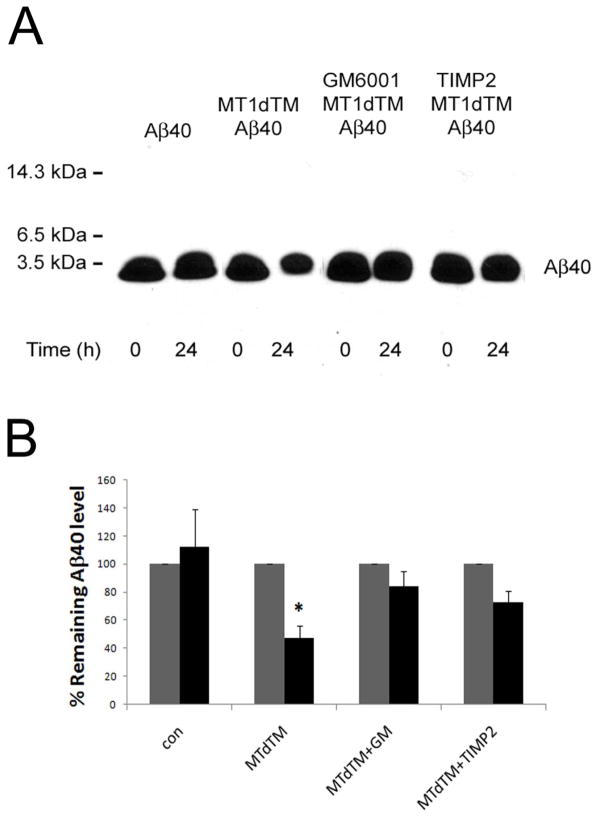
To confirm that the enzymatic activity of purified MT1dTM was required for the Aβ degradation in-vitro we used the general MMP inhibitor GM6001 and specific MT1-MMP inhibitor TIMP2, Aβ40 was incubated with purified MT1dTM at 37°C in the presence or absence of GM6001 or TIMP2 for 24 h. The immunoblotting data presented in Fig. 6 shows that MT1dTM mediated Aβ40 degradation was blocked by GM6001 and TIMP2 indicating that the proteolytic activity of MT1dTM was responsible for the observed Aβ degradation.
Fig. 6. Aβ40 degradation by soluble MT1dTM is inhibited by GM6001 and TIMP2.
Aβ40 was incubated with purified soluble MTdTM at 37°C in the presence or absence of the general MMP inhibitior GM6001 (10 μM) or the specific MT1-MMP inhibitor TIMP2 (40 nM) for 24 h. Following incubation, the samples were collected and analyzed for Aβ levels by quantitative immunoblotting using anti-Aβ (A). The data shown are the mean ± SD of three separate determinations (B). *, p < 0.05, paired t test.
The data above demonstrates that MT1-MMP exhibits proteolytic activity towards Aβ40 or Aβ42 in-vitro or in Cos-1 cells expressing MT1-MMP. However, these analyses only show loss of intact Aβ peptides based on immunoblotting or ELISA analysis. To identify MT1-MMP mediated Aβ cleavage products we performed acid/urea gel analysis, a technique that can resolve low molecular mass peptides. For this analysis soluble amino-terminal, biotinylated Aβ40 or Aβ42 peptides were incubated with purified MT1dTM for 48 h. Following incubation, the samples were electrophoresed on a 22% polyacrylamide acid/urea gels, transferred to membranes, and analyzed for biotin-labeled intact Aβ and amino-terminal fragments using a streptavidin-horseradish peroxidase conjugate. As shown in Fig. 7, the levels of intact biotin-labeled Aβ40 and Aβ42 were markedly reduced by digestion with MT1dTM and several biotin-labeled amino-terminal fragments of each Aβ peptide were observed. These data further confirm that MT1dTM degrades soluble Aβ in-vitro.
Fig. 7. Analysis of MT1dTM mediated Aβ cleavage fragments on acid/urea gels.
Soluble amino terminal, biotinylated Aβ40 or Aβ42 were incubated with purified soluble MT1dTM for 48 h. Following incubation, samples were separated on 22% polyacrylamide acid/urea gels, transferred to membranes, and analyzed for Aβ products by using a streptavidin-horseradish peroxidase conjugate to detect biotinylated peptides and fragments. Lane 1, biotinylated-Aβ40; lane 2, biotinylated-Aβ40 + MT1dTM; lane 3, biotinylated-Aβ42; and lane 4, biotinylated-Aβ42 + MT1dTM. The brackets denote amino terminal cleavage products common to Aβ40 and Aβ42.
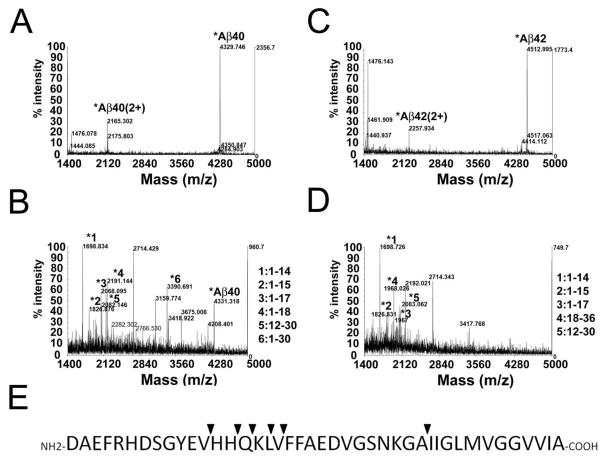
To identify specific cleavage products, synthetic Aβ40 or Aβ42 was digested with purified MT1dTM and analyzed by MALDI-TOF mass spectrometry (Fig. 8). The major fragments generated from proteolytic cleavage of Aβ40 were similar to those generated from Aβ42. Several cleavage sites were identified mainly around V12 through L17, generating major fragments of 1–14 to 1–17, which were consistent with the amino terminal major cleavage products shown in the acid/urea gels (Fig. 7).
Fig. 8. MALDI-TOF MS analysis of Aβ fragments released from by purified MT1dTM.
Synthetic Aβ40 alone (A), Aβ42 alone (C), Aβ40 and purified MT1dTM (B) or Aβ42 and purified MTdTM (D) were incubated with 37°C for 2 days. After incubation the samples were analyzed by MALDI-TOF mass spectrometry. Comparing with Aβ40 or Aβ42 alone, several specific peaks were identified as Aβ fragments (reflector mode). (E) Summary of the MT1dTM cleavage sites on Aβ (▼).
In-vitro fibrillar Aβ degradation by purified soluble MT1-MMP
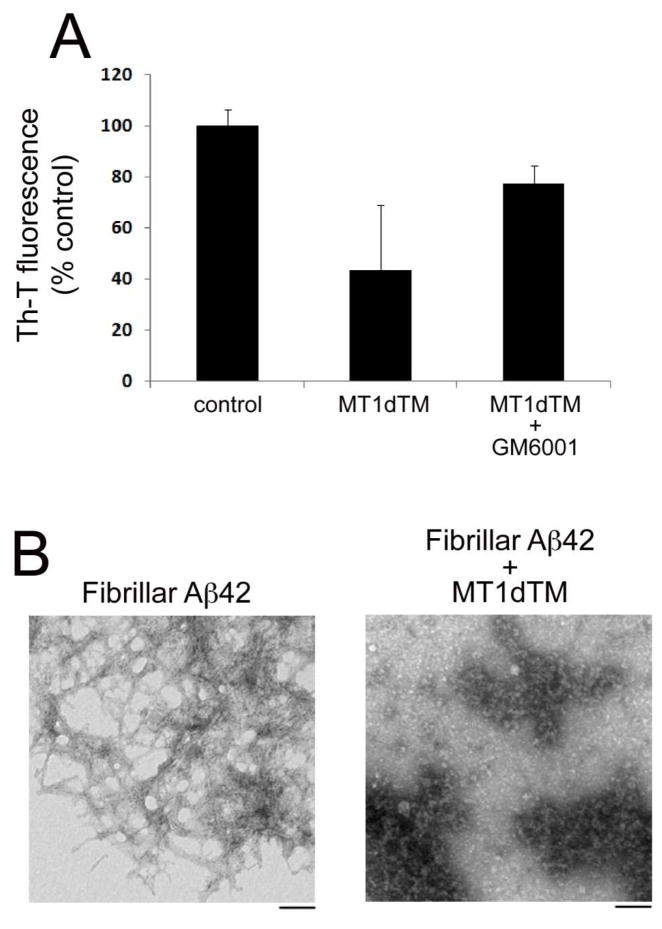
Aβ peptides largely accumulate in the AD brain in the form of fibrillar amyloid deposits. To determine whether MT1-MMP could degrade fibrillar Aβ, we prepared aged fibrillar Aβ42, subsequently incubated it with purified MT1dTM at 37° for 5 days and measured the remaining fibrillar Aβ using a Th-T fluorescence binding assay. Fig. 9A shows a >50% reduction in the Th-T fluorescence signal in the fibrillar Aβ sample treated with MT1dTM. Importantly, the MMP inhibitor GM6001 largely blocked MT1dTM mediated fibrillar Aβ degradation indicating the loss of fibrillar Aβ was dependent on the enzymatic activity of MT1dTM. To further confirm this finding at the ultrastructural level fibrillar Aβ was incubated in the absence or presence of purified MT1dTM for 5 days and then TEM analysis was performed to visualize the extent fibrillar Aβ structure (Fig. 9B). Fibrillar Aβ incubated with MT1dTM showed a marked reduction in the number and length of amyloid fibrils. Together, these data indicate that MT1dTM is also capable of degrading the assembled fibrillar form of Aβ.
Fig. 9. Fibrillar Aβ degradation by soluble MT1dTM.
(A) Fibrillar Aβ42 was incubated alone or with MT1dTM in the presence or absence of the MMP inhibitor GM6001 for 37°C for 5 days. The remaining fibrillar Aβ was quantitated using a Th-T binding fluorescence assay. The data shown are the mean ± SD of three separate determinations. (B) Fibrillar Aβ42 was incubated alone or with purified MT1dTM at 37°C for 5 days. The samples were collected an analyzed by TEM. Scale bars = 100 nm.
MT1dTM degrades parenchymal fibrillar Aβ plaques in situ
The above data showed that purified MT1dTM was capable of degrading soluble and fibrillar synthetic Aβ peptides in vitro. We next determined if purified MT1dTM could degrade actual amyloid deposits that form in the brains of APP transgenic mice. To do this, adjacent brain slices of aged Tg2576 mice, which contain abundant amyloid plaques, were incubated at 37°C for 5 days with buffer alone or purified MT1dTM in the presence or absence of the MMP inhibitor GM6001. After incubation the sections were stained with Th-S and the area of fluorescence between matching fibrillar plaque deposits from adjacent sections was measured. The area of Th-S fluorescence of adjacent brain sections did not show a difference when incubated with buffer alone, while the area of parenchymal amyloid plaque deposits was significantly decreased (p < 0.001) in the brain sections incubated with purified MT1dTM (Fig. 10). Importantly, amyloid plaque degradation by purified MT1dTM was effectively blocked with the MMP inhibitor GM6001. These results suggest that purified MT1dTM is capable of degrading fibrillar amyloid plaques in brain tissue.
Fig. 10. In Situ brain fibrillar amyloid plaque degradation by soluble MT1dTM.
Adjacent 5 μm fresh frozen brain sections from 18 months old Tg2576 mice were incubated alone (A,B,C,E) or with purified MTIdTM (D) or GM6001-treated MT1dTM (F) at 37°C for 5 days. The sections were then fixed, and stained with Th-S. Insets show parallel representative plaques enlarged. Scale bars = 50 μm. (G) The parallel cortical fibrillar amyloid plaque areas were quantified in the treated and untreated sections and expressed as percent remaining Th-S area. The data presented are the mean ± S.D. of n = 25 plaques (buffer alone); n = 37 plaques (incubated with MT1dTM); n = 27 plaques (incubated with GM6001-treated MT1dTM). *, p < 0.001, paired t test.
DISCUSSION
In the present study we show that MT1-MMP, the physiological activator of pro-MMP2, can degrade soluble and fibrillar forms of Aβ in vitro. The MMP superfamily consists of secreted and membrane types of metalloproteinases largely involved in degradation and remodeling of the extracellular matrix. It was previously shown that MMP2 and MMP9 are produced by reactive astrocytes surrounding amyloid plaques in aged human AβPP transgenic mice (17–20, 39). Similarly, we found that MMP2 and its activator MT1-MMP are expressed in reactive astrocytes in brain regions with microvascular amyloid deposits in aged Tg-SwDI mice (Figs. 1 and 2). Previously, we reported that pathogenic Aβ stimulates the expression and activation of MT1-MMP and MMP2 in the cultured human cerebrovascular smooth muscle cells (16, 40). Consistent with these earlier in vitro findings, in aged Tg-SwDI mice we also found MMP2 and MT1-MMP expression in the smooth muscle cell medial layer of meningeal vessels that occasionally contained fibrillar Aβ deposits (data not shown).
MT1-MMP was shown to degrade Aβ peptides in both its natural transmembrane form expressed in Cos-1 cells and as a purified soluble form lacking the carboxyl-terminal transmembrane region (MT1dTM). However, more robust degradation of Aβ peptides was observed when MT1-MMP was expressed in Cos-1 cells compared to using the soluble MT1dTM form in vitro. This disparity may reflect different levels of MT1-MMP present in each type of experiment or, more likely, is a consequence of the soluble MT1dTM exhibiting less enzymatic activity than its natural transmembrane counterpart (41). In any case, soluble MT1dTM provided a useful tool to demonstrate degradation of soluble and fibrillar Aβ in in vitro and in situ experiments.
Most of the well-known Aβ-degrading enzymes such as endothlein converting enzyme, insulin-degrading enzyme, and neprilysin largely show degradative activity towards soluble forms of Aβ, but not fibrillar Aβ. However, plasmin and MMP9 are two Aβ-degrading enzymes shown to be capable of degrading fibrillar Aβ in-vitro (37, 42). In the initial experiments of the present study we mainly focused on the degradation of the monomer form of soluble Aβ in the in-vitro assay or in Cos-1 cells. However, in Fig. 4 above the prominent Aβ monomer a faint Aβ dimer band was observed which was also degraded in Cos-1 cells expressing MT1-MMP. Although we did not investigate the specific degradation of other soluble forms of Aβ such as trimers, tetramers, or higher order oligomers our experiments showed that fibrillar Aβ was degraded by soluble MT1dTM. Based on this latter finding we predict that other soluble oligomeric forms of Aβ are likely degraded by MT1-MMP although this will need to be confirmed.
Several structural models of amyloid fibrils have been proposed (43). The common feature is the β-pleated sheet structure perpendicular to the fibril axis with a hairpin loop at the C-terminus. The conversion of soluble Aβ to fibrillar amyloid is accompanied by an increased resistance to proteolytic degradation (44). In this regard it is noteworthy that purified soluble MT1dTM can similarly degrade fibrillar Aβ in-vitro and fibrillar amyloid deposits in brain tissue sections of human AβPP transgenic mice. MMP9, like MT1-MMP, cleaves between residues A30-I31 (37). This site is exposed on the surface of Aβ fibrils allowing access for cleavage by MMP9 and MT1-MMP (37). In contrast, Aβ fibrils were observed to be more resistant to degradation by MMP2. It was proposed that the major MMP2 cleavage site of Leu34-Met35 within the hydrophobic domain of Aβ would be inaccessible within an amyloid structure (44). Collectively, these findings suggest that the various Aβ-degrading enzymes likely work at different sites in the brain for Aβ catabolism. For example, secreted Aβ-degrading enzymes such as IDE, MMP2, and MMP9 may effectively target soluble forms of Aβ in interstitial fluid whereas membrane-bound Aβ-degrading enzymes such as neprilysin and MT1-MMP are better suited for deposited fibrillar Aβ or Aβ associated with cell surfaces.
MT1-MMP appears to be highly expressed in brain regions exhibiting amyloid pathology and neuroinflammation (Figs. 1 and 2). On the other hand, in normal brain or in the absence of amyloid pathology little, if any, expression of MT1-MMP is observed. This suggests that under normal conditions MT1-MMP likely has little involvement in regulating basal brain Aβ levels compared with other Aβ-degrading enzymes that are constitutively expressed. However, when amyloid deposition and neuroinflammation occur, as in AD, reactive astrocytes and vascular smooth muscle cells markedly increase their expression of MT1-MMP which may then play a significant role degrading soluble and deposited Aβ peptides. This increased expression in response to amyloid deposition implies that MT1-MMP may be an opportunistic Aβ-degrading enzyme. Future experimentation will be needed to determine if MT1-MMP does indeed contribute to Aβ degradation in vivo under pathological conditions when it is likely expressed.
Various members of the MMP superfamily may play some role regulating the levels of Aβ in the CNS. For example, MMP2, MMP9, and MT1-MMP possess Aβ-degrading activity (15, 45). MMP2, MMP3, MMP9 and MT1-MMP exhibit increased expression in response to Aβ (16, 23, 46). However, the protein levels and activity of MMP2, MMP3, and MMP9 showed no difference in the frontal cortex of AD patients compared with control patients (47). This may reflect a very limited, focal expression in specific cells that was not discerned in this study. It was reported that MT1-MMP, MT3-MMP and MT5-MMP have α secretase-like shedding activity on AβPP which would preclude Aβ formation (31). More specifically, recombinant MT3-MMP showed multiple cleavage sites on AβPP within the Aβ domain. Since the shedding pattern for MT1-MMP and MT3-MMP are very similar, MT1-MMP may also cleave AβPP within the same sites. Here, our mass spectrometry data showed an MT1-MMP cleavage site at the H14-Q15, which is the same as an MT3-MMP shedding site on AβPP (30). However, Aβ peptide was not degraded by recombinant MT3-MMP or by cells expressing MT3-MMP. Therefore, regarding MT-MMPs the Aβ degradation activity appears specific to MT1-MMP.
In conclusion, we have demonstrated that MT1-MMP is selectively expressed in reactive astrocytes near fibrillar amyloid deposits in human AβPP transgenic mouse brain. MT1-MMP was found to degrade soluble Aβ40 and Aβ42 as well as fibrillar amyloid. Together, our data suggest MT1-MMP could function as an opportunistic Aβ degrading enzyme when expressed by reactive astrocytes adjacent to fibrillar amyloid deposits. Future in vivo studies are needed to determine its role in relation to other identified Aβ degrading enzymes in regulating Aβ levels in brain.
Footnotes
Abbreviations used are: AD, Alzheimer’s disease; Aβ, amyloid β-protein; MMP, matrix metalloproteinase; MT1-MMP, membrane type 1 matrix metalloproteinase; Th-T, thioflavin T; Th-S, thioflavin S; DMSO, dimethyl sulfoxide; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; PBS, phosphate-buffered saline; TIMP2, tissue inhibitor of metalloproteinase-2, MT1dTM: soluble transmembrane domain-lacking form of MT1-MMP; GFAP, glial fibrillary acidic protein.
This work was supported by National Institutes of Health grants HL72553 and NS55118. We thank Dr. Mahiuddin Ahmed and Dr. Steven Smith for performing the TEM analysis of fibrillar Aβ. ELISA antibody reagents were generously provided by Eli Lilly Laboratories.
References
- 1.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evin G, Weidemann A. Biogenesis and metabolism of Alzheimer’s disease Abeta amyloid peptides. Peptides. 2002;23:1285–1297. doi: 10.1016/s0196-9781(02)00063-3. [DOI] [PubMed] [Google Scholar]
- 3.Saido TC. Alzheimer’s disease as proteolytic disorders: anabolism and catabolism of beta-amyloid. Neurobiol Aging. 1998;19:S69–75. doi: 10.1016/s0197-4580(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 4.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1–40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. The Journal of clinical investigation. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlokovic BV. Clearing amyloid through the blood-brain barrier. J Neurochem. 2004;89:807–811. doi: 10.1111/j.1471-4159.2004.02385.x. [DOI] [PubMed] [Google Scholar]
- 6.Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nature medicine. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- 7.Iwata N, Tsubuki S, Takaki Y, Shirotani K, Lu B, Gerard NP, Gerard C, Hama E, Lee HJ, Saido TC. Metabolic regulation of brain Abeta by neprilysin. Science. 2001;292:1550–1552. doi: 10.1126/science.1059946. [DOI] [PubMed] [Google Scholar]
- 8.Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. The Journal of biological chemistry. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 9.Ledesma MD, Da Silva JS, Crassaerts K, Delacourte A, De Strooper B, Dotti CG. Brain plasmin enhances APP alpha-cleavage and Abeta degradation and is reduced in Alzheimer’s disease brains. EMBO reports. 2000;1:530–535. doi: 10.1093/embo-reports/kvd107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckman EA, Reed DK, Eckman CB. Degradation of the Alzheimer’s amyloid beta peptide by endothelin-converting enzyme. The Journal of biological chemistry. 2001;276:24540–24548. doi: 10.1074/jbc.M007579200. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. The Journal of biological chemistry. 2001;276:47863–47868. doi: 10.1074/jbc.M104068200. [DOI] [PubMed] [Google Scholar]
- 12.Liao MC, Ahmed M, Smith SO, Van Nostrand WE. Degradation of amyloid beta protein by purified myelin basic protein. The Journal of biological chemistry. 2009;284:28917–28925. doi: 10.1074/jbc.M109.050856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamada T, Miyazaki K, Koshikawa N, Takahashi M, Akatsu H, Yamamoto T. Selective localization of gelatinase A, an enzyme degrading beta-amyloid protein, in white matter microglia and in Schwann cells. Acta neuropathologica. 1995;89:199–203. doi: 10.1007/BF00309334. [DOI] [PubMed] [Google Scholar]
- 14.Roher AE, Kasunic TC, Woods AS, Cotter RJ, Ball MJ, Fridman R. Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochem Biophys Res Commun. 1994;205:1755–1761. doi: 10.1006/bbrc.1994.2872. [DOI] [PubMed] [Google Scholar]
- 15.Backstrom JR, Lim GP, Cullen MJ, Tokes ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40) J Neurosci. 1996;16:7910–7919. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung SS, Zhang W, Van Nostrand WE. Pathogenic A beta induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J Neurochem. 2003;85:1208–1215. doi: 10.1046/j.1471-4159.2003.01745.x. [DOI] [PubMed] [Google Scholar]
- 17.Gottschall PE, Yu X, Bing B. Increased production of gelatinase B (matrix metalloproteinase-9) and interleukin-6 by activated rat microglia in culture. Journal of neuroscience research. 1995;42:335–342. doi: 10.1002/jnr.490420307. [DOI] [PubMed] [Google Scholar]
- 18.Muir EM, Adcock KH, Morgenstern DA, Clayton R, von Stillfried N, Rhodes K, Ellis C, Fawcett JW, Rogers JH. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Brain research. 2002;100:103–117. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 19.Deb S, Gottschall PE. Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. J Neurochem. 1996;66:1641–1647. doi: 10.1046/j.1471-4159.1996.66041641.x. [DOI] [PubMed] [Google Scholar]
- 20.Deb S, Wenjun Zhang J, Gottschall PE. Beta-amyloid induces the production of active, matrix-degrading proteases in cultured rat astrocytes. Brain Res. 2003;970:205–213. doi: 10.1016/s0006-8993(03)02344-8. [DOI] [PubMed] [Google Scholar]
- 21.Vehmas AK, Kawas CH, Stewart WF, Troncoso JC. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol Aging. 2003;24:321–331. doi: 10.1016/s0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 22.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nature medicine. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 23.Deb S, Zhang JW, Gottschall PE. Activated isoforms of MMP-2 are induced in U87 human glioma cells in response to beta-amyloid peptide. Journal of neuroscience research. 1999;55:44–53. doi: 10.1002/(SICI)1097-4547(19990101)55:1<44::AID-JNR6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. The Journal of biological chemistry. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 25.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 26.Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Experimental cell research. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 27.Yamada T, Yoshiyama Y, Sato H, Seiki M, Shinagawa A, Takahashi M. White matter microglia produce membrane-type matrix metalloprotease, an activator of gelatinase A, in human brain tissues. Acta neuropathologica. 1995;90:421–424. doi: 10.1007/BF00294800. [DOI] [PubMed] [Google Scholar]
- 28.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annual review of cell and developmental biology. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Current opinion in cell biology. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 30.Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Brain Res Rev. 2001;36:249–257. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad M, Takino T, Miyamori H, Yoshizaki T, Furukawa M, Sato H. Cleavage of amyloid-beta precursor protein (APP) by membrane-type matrix metalloproteinases. Journal of biochemistry. 2006;139:517–526. doi: 10.1093/jb/mvj054. [DOI] [PubMed] [Google Scholar]
- 32.Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, Yates J, Cotman C, Glabe C. Assembly and aggregation properties of synthetic Alzheimer’s A4/beta amyloid peptide analogs. Journal of Biological Chemistry. 1992;267:546–554. [PubMed] [Google Scholar]
- 33.Davis J, Xu F, Deane R, Romanov G, Previti ML, Zeigler K, Zlokovic BV, Van Nostrand WE. Early-onset and robust cerebral microvascular accumulation of amyloid beta-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid beta-protein precursor. The Journal of biological chemistry. 2004;279:20296–20306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 34.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nature medicine. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 35.Dickson DW, Wertkin A, Mattiace LA, Fier E, Kress Y, Davies P, Yen SH. Ubiquitin immunoelectron microscopy of dystrophic neurites in cerebellar senile plaques of Alzheimer’s disease. Acta neuropathologica. 1990;79:486–493. doi: 10.1007/BF00296107. [DOI] [PubMed] [Google Scholar]
- 36.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. [see comment] Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, Lee JM. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. Journal of Biological Chemistry. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- 38.Ross SA, Cunningham RT, Johnston CF, Rowlands BJ. Neuron-specific enolase as an aid to outcome prediction in head injury. British Journal of Neurosurgery. 1996;10:471–476. doi: 10.1080/02688699647104. [DOI] [PubMed] [Google Scholar]
- 39.Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee JM. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis J, Cribbs DH, Cotman CW, Van Nostrand WE. Pathogenic amyloid beta-protein induces apoptosis in cultured human cerebrovascular smooth muscle cells. Amyloid. 1999;6:157–164. doi: 10.3109/13506129909007321. [DOI] [PubMed] [Google Scholar]
- 41.Cao J, Sato H, Takino T, Seiki M. The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro-gelatinase A activation. Journal of Biological Chemistry. 1995;270:801–805. doi: 10.1074/jbc.270.2.801. [DOI] [PubMed] [Google Scholar]
- 42.Tucker HM, Kihiko M, Caldwell JN, Wright S, Kawarabayashi T, Price D, Walker D, Scheff S, McGillis JP, Rydel RE, Estus S. The plasmin system is induced by and degrades amyloid-beta aggregates. Journal of Neuroscience. 2000;20:3937–3946. doi: 10.1523/JNEUROSCI.20-11-03937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wetzel R. Ideas of order for amyloid fibril structure. [see comment] Structure. 2002;10:1031–1036. doi: 10.1016/s0969-2126(02)00809-2. [DOI] [PubMed] [Google Scholar]
- 44.Crouch PJ, Tew DJ, Du T, Nguyen DN, Caragounis A, Filiz G, Blake RE, Trounce IA, Soon CP, Laughton K, Perez KA, Li QX, Cherny RA, Masters CL, Barnham KJ, White AR. Restored degradation of the Alzheimer’s amyloid-beta peptide by targeting amyloid formation. Journal of Neurochemistry. 2009;108:1198–1207. doi: 10.1111/j.1471-4159.2009.05870.x. [DOI] [PubMed] [Google Scholar]
- 45.Roher AE, Kasunic TC, Woods AS, Cotter RJ, Ball MJ, Fridman R. Proteolysis of A beta peptide from Alzheimer disease brain by gelatinase A. Biochemical & Biophysical Research Communications. 1994;205:1755–1761. doi: 10.1006/bbrc.1994.2872. [DOI] [PubMed] [Google Scholar]
- 46.Lee JM, Yin KJ, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Annals of Neurology. 2003;54:379–382. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- 47.Baig S, Kehoe PG, Love S. MMP-2, -3 and -9 levels and activity are not related to Abeta load in the frontal cortex in Alzheimer’s disease. Neuropathology & Applied Neurobiology. 2008;34:205–215. doi: 10.1111/j.1365-2990.2007.00897.x. [DOI] [PubMed] [Google Scholar]