Abstract
Background & Aims
Regenerating (Reg) gene IV is predominantly expressed in the gastrointestinal (GI) cells and highly upregulated in many GI malignancies including colorectal cancer (CRC). Human CRC cells expressing higher levels of Reg IV are resistant to conventional therapies including irradiation (IR). However, underlying mechanism is not well defined.
Methods
A murine model of IR-induced intestinal injury, and in vitro and in vivo models of human CRC were used to determine the role of Reg IV in regulation of normal intestinal and colorectal cancer cell susceptibility to IR-induced apoptosis.
Results
Reg IV treatments protected normal intestinal crypt cells from IR-induced apoptosis by increasing the expression of anti-apoptotic genes Bcl-2, Bcl-XL and survivin. However, overexpression of Reg IV in human CRC cells was associated with increased resistance to IR-induced apoptosis. Therefore, we utilized antagonism of Reg IV as a tool to increase CRC cell susceptibility to IR-induced cell death. Two complementary approaches using specific monoclonal antibodies and small interfering RNAs were tested in both in vitro and in vivo models of human CRC. Both approaches resulted in increased apoptosis and decreased cell proliferation leading to a decreased tumor growth and increased animal survival. Furthermore, these approaches increased CRC cell susceptibility to IR-induced apoptosis.
Conclusions
These results implicate Reg IV as an important modulator of gastrointestinal cells susceptibility to IR, hence a potential target for adjunctive treatments for human CRC and other GI malignancies.
INTRODUCTION
The human regenerating (Reg) gene family encodes five small-secreted and structurally unique proteins that share strong structural similarities to the proteins of the calcium-dependent lectin (C-type lectin) super family. Identified members of the human Reg gene family include Reg Iα, Reg Iβ, Reg IIIα, Reg IIIβ and Reg IV.1–5 These genes are constitutively expressed in normal gastrointestinal (GI) mucosa. Individual Reg genes have characteristic expression profiles throughout the proximal to distal axis of the GI tract.6 Reg gene expression is markedly elevated following diverse conditions of mucosal injury, including inflammatory bowel disease or radiation injury.3, 7 Reg genes are also up-regulated in a variety of GI malignancies and have been associated with a more aggressive tumor phenotype.8 Reg IV is especially relevant in CRC as it is prominently expressed in colonic mucosa and is further upregulated during colorectal tumorigenesis. 5, 9, 10 Higher serum levels of Reg IV in CRC patients are also associated with liver metastasis.11 In addition, we observed increased proliferation of human CRC cells when Reg IV protein was added to their culture media.12 In present study, using a murine model of IR-induced intestinal injury, we demonstrated that Reg IV in normal GI tract protected intestinal crypt cells from IR-induced apoptosis, especially at positions 3 to 6 counted from the base, which correspond to the location of the putative stem cells. Reg IV-mediated increases in intestinal crypt cell survival were associated with increased expression of the anti-apoptotic genes Bcl-2, Bcl-XL and survivin. These data implicate Reg IV as a radio-protective agent of normal GI mucosa. However, in previous and present studies we observed that higher levels of Reg IV expression in human CRC cells were associated with reduced susceptibility to IR-induced cell death.6 Therefore, we tested a hypothesis that antagonism of Reg IV signaling would be a useful tool to increase CRC cell susceptibility to IR-induced apoptosis. Two complementary approaches of Reg IV antagonism using specific monoclonal antibodies and small interfering RNAs were tested in both in vitro and in vivo models of human CRC. Both approaches resulted in a significantly reduced tumor growth associated with a decreased cell proliferation and increased apoptosis.
MATERIALS AND METHODS
Cell Lines and Culture
The human CRC cell lines HCT116, SW40 and HT29 (American Type Culture Collection, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (Cambrex, Walkersville MD) containing 10% heat inactivated fetal bovine serum (HyClone, Logan, UT).
Anti-Reg IV Sspecific Polyclonal and Monoclonal Antibodies
Armenian hamster monoclonal antibodies (mAbs; 2H6 and 3E5) and rabbit polyclonal antidody (α-Reg IV 4261) against human Reg IV protein, and recombinant human Reg IV protein (rhR4) were produced and characterized with the help of the Hybridoma Center at the Washington University School of Medicine (http://pathology.wustl.edu/research/hybridoma.php, Fusion No. 4465) by previously described methods.13 A control mAb PIP (hamster anti-bacterial glutathione S-transferase mAb) was kindly provided by Dr. Kathleen Sheehan of the Hybridoma Center.
siRNA Synthesis and Transfection
A panel of siRNAs targeting human Reg IV mRNA was generated using the Silencer® siRNA Construction Kit (Ambion, Austin, Texas). Reg IV (Gene Bank accession no. NM_032044) target mRNA sequences for siRNA oligonucleotides synthesis were as follows:
Reg IV-si1: anti-sense strand, 5′-AAGATGGCTTCCAGAAGCATGcctgtctc-3′; sense strand, 5′-AACATGCTTCTGGAAGCCATCcctgtctc-3′ (nucleotide positions 263–283).
Reg IV-si5: anti-sense strand, 5′ AAGCACTGTGCTGAGATGAGCcctgtctc-3′; sense strand, 5′-AAGCTCATCTCAGCACAGTGCcctgtctc-3′ (nucleotide positions 645–665).
The lowercase nucleotide sequences were complementary to the T7 promoter primer sequence provided with the Kit. The sense and anti-sense siRNA templates were transcribed by T7 RNA polymerase, and the resulting RNA transcripts were hybridized to form double-stranded RNA. The double-stranded RNA sequences were as follows:
Reg IV-si1 (si1): anti-sense siRNA strand, 5′-GAUGGCUUCCAGAAGCAUGUU-3′; sense siRNA strand, 5′-CAUGCUUCUGGAAGCCAUCUU-3′.
Reg IV-si5 (si5): anti-sense siRNA strand, 5′-GCACUGUGCUGAGAUGAGCUU-3′; sense siRNA strand, 5′-GCUCAUCUCAGCACAGUGCUU-3′.
Silencer® Negative Control #1 scrambled siRNA (Neg.1) purchased from Ambion, Austin, Texas and ‘No si’ were used as controls. Reg IV-specific and negative control siRNAs were complexed with the siPORTTM NeoFX Transfection Agent (Ambion, Austin, Texas). HT29 and HCT116 cells were transfected with siRNAs at a final concentration of 100 nM. Protein and mRNA expression was analyzed after 48 hours of transfection.
Murine Model of Intestinal Injury
All animal experimentation was conducted in accordance to animal protocol approved by the Washington University Animal studies Committee and performed under Institutional Animal Care. For a murine model of intestinal injury, six-week old female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were γ-irradiated in a Gamacel 40 cesium irradiator at 0.96 cGy/min. Mice were injected intraperitoneally (IP) with vehicle or rhR4 (4 μg/g body weight) 24 h prior of IR (6 or 12 Gy) exposures. Following IR, mice were sacrificed at 6 h and 84 h to assess apoptosis and crypt cell proliferation respectively. The distal jejunum sections exhibited comparatively higher level of IR-injury than any other regions of the GI tract, hence were used for scoring intestinal crypt cell apoptosis and proliferation.
In Vivo Model of Tumor Xenografts
HCT116 cells (6 × 106) were injected subcutaneously into the flanks of 4–6 week-old male anthymic nude mice (Balb/c AnNCr-nu/nu) to develop tumor xenografts. To antagonize Reg IV signaling, treatments of Reg IV-specific mAbs 2H6 and 3E5 (400 μg/day) were started either at day 1 or at day 10 (when all tumors were palpable) of tumor xenografts, and continued for 5 consecutive doses at every alternate day. In separate set of experiments, Reg IV-specific siRNAs were utilized to inhibit Reg IV mRNA expression. Reg IV-specific siRNAs: R4-si1 and R4-si5 (50μl of 5μM siRNA) were administered to the tumors at day 10 of tumor xenografts and continued at every third day for a total of 5 doses. Growth of tumors was assessed at a regular interval by determining the tumor volume (Length × Width × Height × 0.5). Sections from 10% neutral buffer formalin (NBF)-fixed xenografts tumors were immunostained to access the tumor cell apoptosis and proliferation.
Quantification of Apoptosis
NBF-fixed tissues from the distal jejunum of mice sacrificed at 6 h following IR were used to assess the crypt cell apoptosis by hematoxilin and eosin (H&E) and TUNEL staining. Twenty full-length, well-oriented crypts in each cross-section were considered. A total of 12 cross-sections were scored for each mouse to determine the average number of apoptotic cells per crypt. For in vitro model of human CRC, HCT116 and HT29 cells were transfected with Reg IV-specific and negative control siRNAs (100 nM) in 8-well chamber slides at a density of 1.6×104 cell/well. Cells were subjected to 6 Gy IR 24 h after transfection. The cells were then fixed at day 1, 2 or 3 in 10% NBF and immunostained with antibodies specific to activated Caspase-3 or TUNEL to identify apoptotic cells. In tumor xenografts model, apoptotic cells were identified in NBF-fixed tumor sections with H&E and TUNEL staining.
Microcolony Assay
Cell proliferation in intestinal crypts was quantified by immunodetection of 5′-Bromo, 2′-deoxyuridine (BrDU) in distal jejunum sections of mice injected with 120 mg/kg BrDU and 12 mg/kg 5′-Fluoro, 2′-deoxyuridine (FrDU) at 82 h of IR (12 Gy) exposure and sacrificed 2 h later. The viability of each surviving crypt was confirmed by detection of 6 or more BrDU-positive cells. A minimum of 12 complete cross sections were scored for each mouse and average number of surviving crypts per cross section was determined. In tumor xenografts sections, BrDU-positive cells were considered as proliferating cells.
In Vitro Radiation-Survival Colony Assay
HCT116, SW480 and HT29 cells were plated in 6-well culture plates at a very low density (40–50 cells/well) and allowed to adhere to the bottom. Following over night serum-starvation, cells were treated with drugs for Reg IV antagonism (mAbs and siRNAs) for 24 h and then exposed to different doses of IR. Surviving cells were allowed to grow for 15 days until the development of visible colonies. Colonies were stained with Hemoatoxylin 7211 and bluing reagent (Richard-Allan, Kalamazoo, MI) and then counted.
Western Blot Analysis
Equal amount of cell lysate was subjected to SDS-PAGE electrophoresis and transferred to the Immobilon PVDF membrane (Millipore, Bedford, MA). Membrane was then incubated with specific primary antibodies and corresponding bands were detected by the enhanced chemiluminescence system (Amersham, UK).
Real Time RT-PCR
Total RNA isolated from human CRC cells and distal jejunum sections was reverse transcribed and then used for real time RT-PCR analyses. Crossing threshold values for individual genes were normalized to β-Actin gene expression. Primers used in this study are shown in supplementary table 1.
Statistical Analysis
All values were expressed as the mean ± SEM. Data were analyzed using a 2-tailed t test. A ‘P’ value of less than 0.05 was considered to indicate statistical significance.
RESULTS
Reg IV Inhibits IR-Induced Intestinal Cell Apoptosis
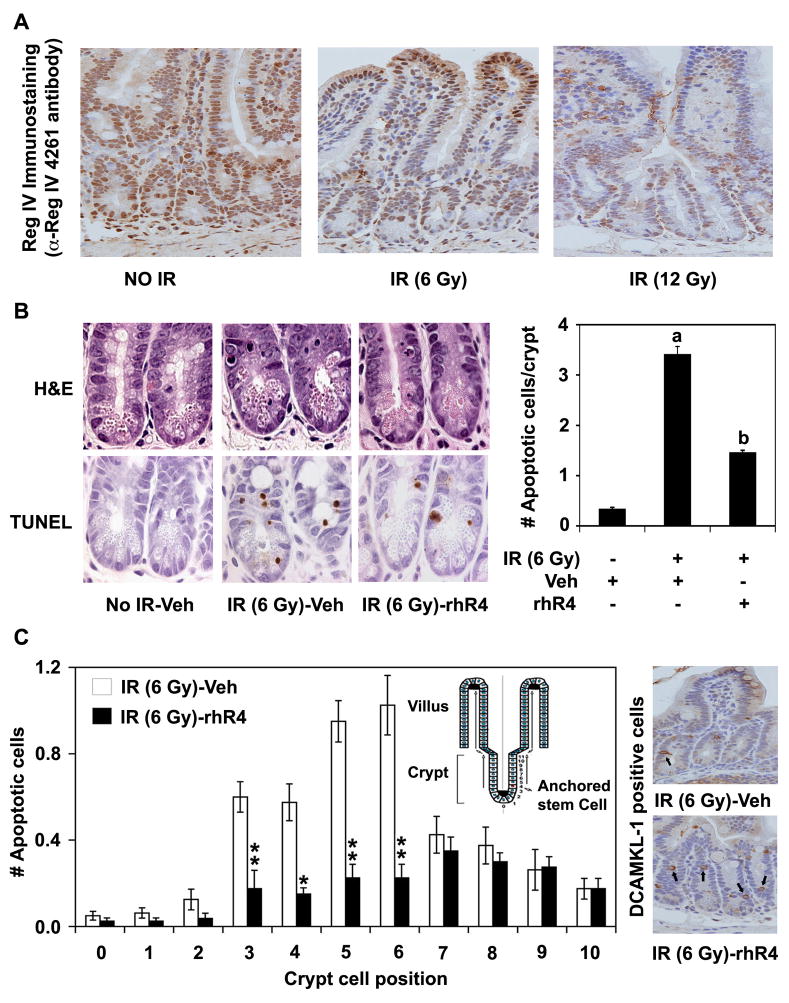
We previously demonstrated that Reg IV regulates apoptosis in human CRC cells.6, 12 However, its role in the normal GI cells was not established. In the present study, we utilized a murine model of IR-induced intestinal injury to determine the role of Reg IV in the normal GI cell apoptosis. Following immunodetection of Reg IV-positive cells in distal jejunum sections using an α-Reg IV 4261 antibody, IR exposures led to a dose-dependent decrease in Reg IV expression (Fig. 1A). Furthermore, IR (6 Gy) exposure to vehicle-treated mice (IR-Veh) led to a significant increase in the number of apoptotic cells per crypt as assessed by H&E and TUNEL staining of the distal jejunum sections. However, a single dose of rhR4 treatment 24 h prior to IR exposure led to a significant decrease in crypt cell apoptosis when compared to IR-vehicle controls (Fig. 1B, left and right panels). Moreover, the rhR4-mediated decrease in the number of apoptotic cells was more confined to crypt cell positions 3 to 6 counted from the base, which corresponded to the locations of the putative stem cell (Fig. 1C, left panel). To confirm these results, we immunostained distal jejunum sections for previously reported stem cell marker doublecortin and CaM kinase-like-1 (DCAMKL-1).14, 15 Reg IV treatments led to a marked increase in the number of DCAMKL-1 positive cells in distal jejunum sections of IR-exposed mice (Fig. 1B, right panel). These data suggest that rhR4 protects GI crypt cells including the crypt stem cell population against IR-mediated apoptosis.
Figure 1.
Reg IV inhibits IR-induced intestinal cell apoptosis. Mice exposed to varying doses of IR and sacrificed 6 h later were used to determine the role of Reg IV in regulation of apoptosis in distal jejunum sections (A) Following immunodetection of Reg IV-positive cells, mice exposed to IR exhibited a dose-dependent decrease in Reg IV expression. (B) Following quantification of apoptosis in distal jejunum sections of mice exposed to IR (6 Gy), the number of apoptotic cells per crypt increased significantly when compared to “No IR-vehicle” controls. However, Reg IV treatment led to a significant reduction in IR-induced apoptosis (left panel). Right panel shows quantitative changes in number of apoptotic cells based on TUNEL staining. (C) The number of cells undergoing apoptosis at crypt cell positions 3 to 6 counted from the base (left panel, inset) corresponding to the location of the putative stem cell was less in Reg IV-treated mice than in vehicle-treated IR-controls (left panel). Following immunodetection of DCAMKL-1 positive cells in distal jejunum sections, Reg IV treatments resulted in a marked increase in its number showing increased survival of stem cells following IR-induced intestinal injury (right panel). Data suggests that Reg IV protects intestinal crypt cells including the crypt stem cell population against IR-mediated apoptosis. Results are representative of 3 different experiments, and data are expressed as mean ± SE. (* P < 0.05, ** P < 0.01, a: P < 0.01; No IR-Veh vs. IR-Veh, b: P < 0.01; IR-Veh vs. IR-rhR4).
Reg IV Increases mRNA Expression of Bcl-2, Bcl-XL & Survivin and Induces Crypt Cell Survival
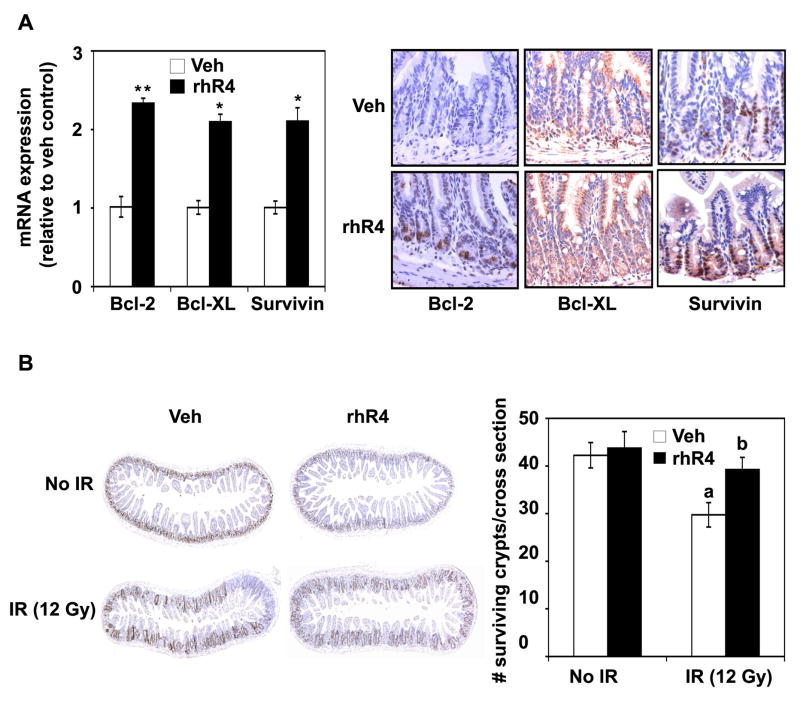
Genes reported in association with altered apoptosis include Bcl-2 and Bcl-XL16–21 and survivin.12, 22 Following real time RT-PCR analyses of mRNA from distal jejunum, we observed that single dose of rhR4 treatment 24 h prior to IR significantly increased the expression of anti-apoptotic genes Bcl-2, Bcl-XL and survivin when compared to vehicle-treated controls (Fig. 2, upper left panel). Results were further supported by the increased expression of Bcl-2, Bcl-XL and survivin when distal jejunum sections from Reg IV-treated mice were immunostained with respective antibodies (Fig. 2, upper right panel). These results suggest that Reg IV treatments induce apoptotic resistance of normal gastrointestinal cells by increasing the expression of anti-apoptotic genes Bcl-2, Bcl-XL and survivin. Following intestinal injury, crypt stem cells proliferate to increase the transit cell population, which form a regenerative crypt, hence play a central role in mucosal regeneration.23 To identify GI crypt cell survival, a microcolony assay was performed, where BrDU-positive cells represented the proliferating crypt cells. A single dose of rhR4 treatment 24 h prior to IR led to a significant increase in numbers of surviving crypts per cross section area when compared to vehicle-treated controls (Fig. 2, lower left and right panels). Results indicate that Reg IV increases crypt cell proliferation that leads to survival of intestinal crypts following IR-induced injury.
Figure 2.
Reg IV increases the expression of anti-apoptotic genes and induces crypt cell survival. (A) Following real time RT-PCR and immunostaining analyses, Reg IV treatments significantly increased the expression of anti-apoptotic genes Bcl-2, Bcl-XL and survivin when compared to vehicle-treated controls (left and right panels). (B) Following microcolony assays of tissues from distal jejunum, mice exposed to IR (12 Gy) and sacrificed 84 h later exhibited a significant decrease in number of surviving crypts, when compared to vehicle-treated “No IR” controls. However, Reg IV treatments resulted in a significant increase in numbers of surviving crypts when compared to vehicle-treated IR controls. Right panel indicates a quantitative analysis of above experiments. Results are based on 3 different experiments, and data are expressed as mean ± SE (* P < 0.5, ** P < 0.01, a: P < 0.01; No IR-Veh vs IR-Veh, b: P < 0.01; IR-Veh vs IR-rhR4)
Reg IV Expression in Human CRC is Associated With Cell Susceptibility to IR-Induced Apoptosis
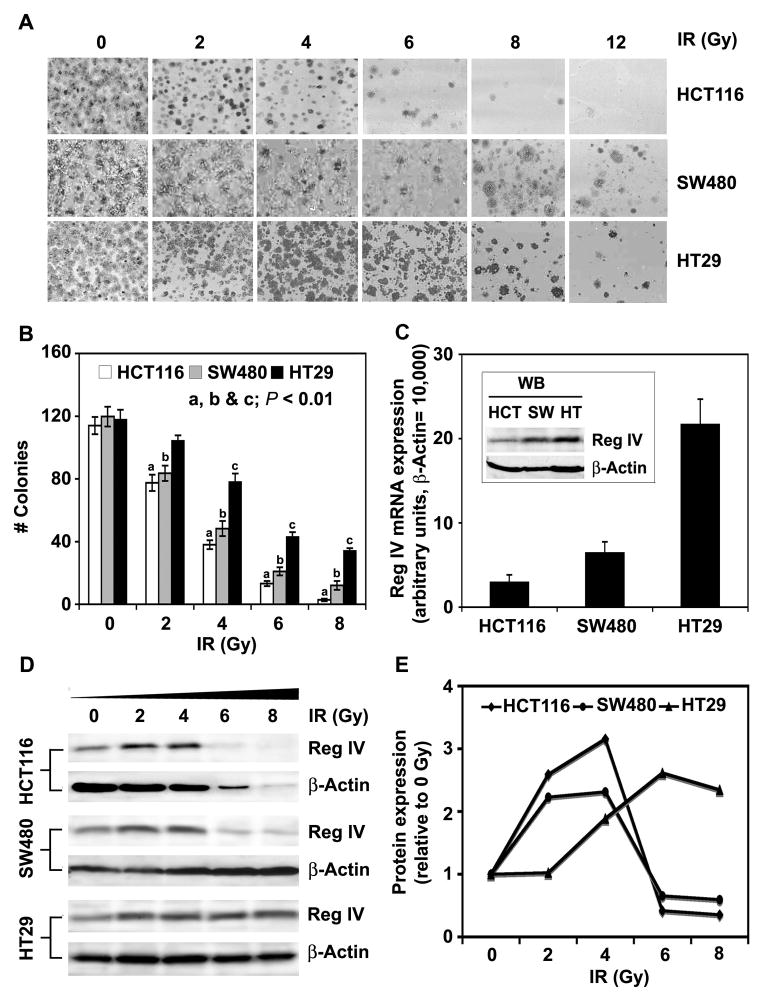
Higher levels of Reg IV expression were reported in human CRC cells selected for in vitro resistance to the cancer chemotherapeutic agent 5-FU.10 We previously demonstrated that addition of rhR4 to the cultures of human CRC cells led to increased cell survival following IR exposure.6 Here we show that Reg IV expression in different human CRC cells was associated with cell susceptibility to IR-induced apoptosis. Following in vitro radiation-survival colony assays, human CRC cells (HCT116, SW480 & HT29) exposed to increasing doses of IR (0, 2, 4, 6, 8 & 12Gy) exhibited a dose-dependent decrease in surviving colonies (Fig. 3A & B). A higher number of surviving colony counts in SW480 and HT29 cells in comparison to HCT116 cells was associated with comparatively higher expression of Reg IV (Fig. 3C & inset). Furthermore, results demonstrated that Reg IV protein expression in human CRC cells increased following exposure of lower IR (2 or 4 Gy) doses (Fig. 3D & E) and was associated with fractional cell survival. However, higher IR doses leading to a decreased expression of Reg IV were associated with decreased cell survival shown in panel A of figure 3. These results suggest that an alteration in the Reg IV expression occurs as a cellular response to IR leading to altered cell survival in human CRC.
Figure 3.
Endogenous level of Reg IV in human CRC is associated with cell susceptibility to IR-induced cell death. In vitro radiation-survival colony assays were performed to determine the effect of varying IR doses on cellular death of HCT116, SW480 and HT29 human CRC cells. (A & B) Human CRC cells exhibited IR dose-dependent decreases in surviving colony counts. HCT116 cells were comparatively more susceptible to IR-induced cell death, as the number of surviving colonies in HCT116 cells was lower than that of SW480 and HT29 cells. (C) Higher number of surviving colony counts in SW480 and HT29 cells in comparison to HCT116 was associated with increased expression of Reg IV. (D & E) As a cellular response, an increase in Reg IV protein expression was noted following exposure of lower IR doses (2 & 4 Gy). However, higher IR doses led to marked decreases in Reg IV expression and were associated with the higher levels of IR-induced cell death. Left panel shows specific protein bands from western blot analysis and right panel shows change in Reg IV protein expression following densitometric scanning of corresponding bands. Results are representative of three different experiments and expressed as mean SEM. (a, b & c: P < 0.01 in HCT116, HT29 and SW480 cells respectively)
Function Blocking of Reg IV Protein Following Specific mAbs Treatments Increases Human CRC Cell Susceptibility to IR-Induced Apoptosis
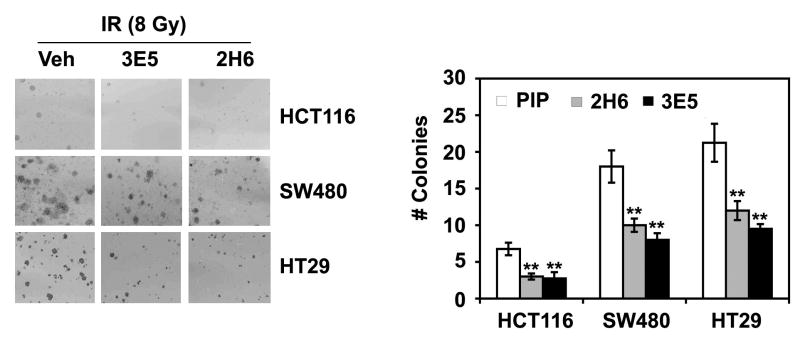
Function blocking of Reg IV protein using specific mAbs (2H6 and 3E5) leads to significant decreases in proliferation of human CRC cells (Supplementary Figure 1). We further performed in vitro radiation-survival colony assay to determine CRC cell susceptibility to IR-induced apoptosis. Function blocking of Reg IV protein using specific mAbs led to significant decrease in surviving cells following IR. In comparison to PIP controls, addition of 2H6 and 3E5 mAbs to the cultures of HCT116, SW480 and HT29 CRC cells led to significant decreases in the number of surviving colonies following IR (8 Gy) exposure (Fig. 4 left & right panels). IR-induced cell death in these cells exhibited a direct correlation with endogenous levels of Reg IV expression. Following IR, HCT116 cells with comparatively lower level of Reg IV expression exhibited higher level of cell death, while SW480 and HT29 cells with comparatively higher levels of Reg IV expression exhibited lower levels of cell death.
Figure 4.
Antagonism of Reg IV signaling with specific mAbs treatments increases CRC cell susceptibility to IR-induced death. (A & B) In vitro radiation-survival colony assays were performed to determine human CRC cell susceptibility to IR-induced death. As assessed by the number of surviving colonies, pretreatment of HCT116, SW480 and HT29 cells with Reg IV-specific mAbs (2H6 & 3E5; 400 μg/ml) for 24 h led to significant decrease in surviving colony counts following IR (8 Gy). (** P < 0.01)
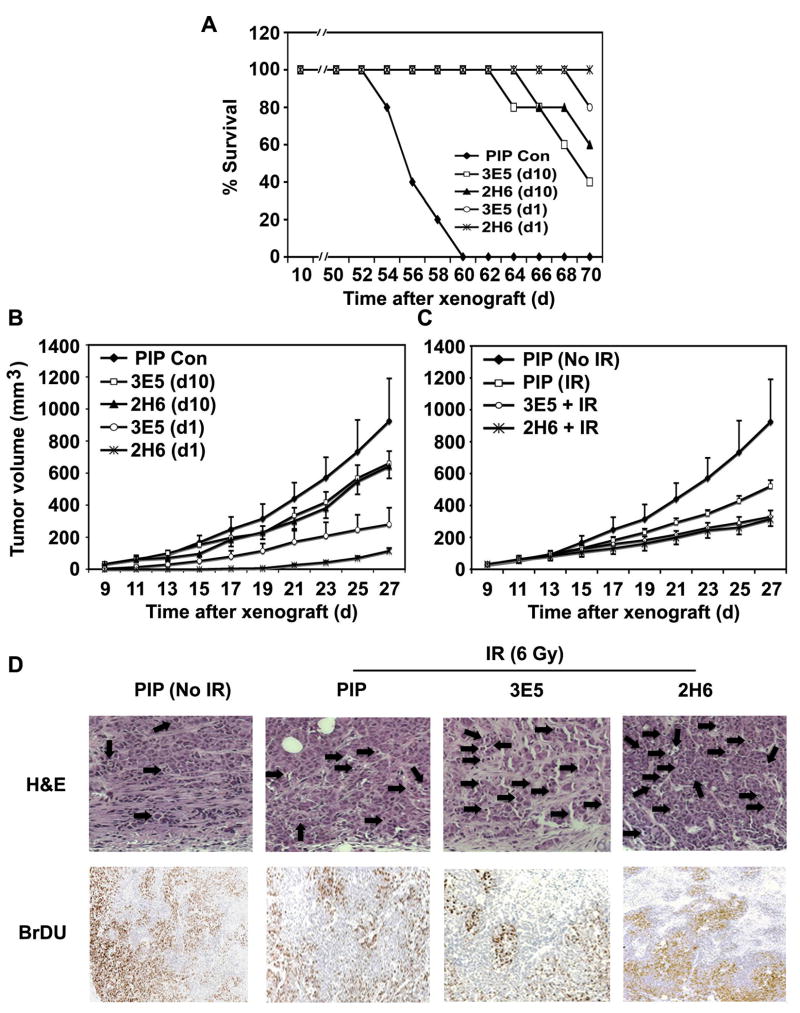
Treatment of Reg IV-Specific mAbs Increases Survival Rate and Reduces Tumor Growth in a Murine Tumor Xenografts Model
To show the effect of Reg IV antagonism on tumor growth and animal survival, we established murine tumor xenografts using HCT116 cells, and different regimens of Reg IV-specific mAbs treatments were tested. Mice treated with 5 consecutive doses of Reg IV-specific mAb (3E5 and 2H6; 400 mg/day) administered at every alternate day starting either from day 1 (d1) or day 10 (d10) of tumor xenografts exhibited a significantly increased survival rate (Fig. 5A) and reduced tumor growth (Fig. 5B) in comparison to mice treated with the equivalent doses PIP control antibody. Furthermore, in comparison to the animals of d10 treatment group, a higher survival rate (Fig. 5A) and lower tumor growth (Fig. 5B) was noted in animals of d1 treatment group, which exhibited a greater effectiveness of Reg IV antagonism if started at early time points of tumor development. In separate experiments, mice with tumor xenografts were also treated with Reg IV-specific mAbs at day 10 and exposed to 6 Gy IR 24 h later. Treatments of mAbs were continued for 5 alternate days, and tumor growth was assessed. Similar to Reg IV-specific mAbs treatments, IR exposure significantly reduced tumor xenografts volume. Combined therapy using Reg IV-specific mAbs and IR significantly increased the overall therapeutic effect (Fig. 5C). Sections of tumor xenografts from treatment groups shown in Fig. 5C were stained with H&E to identify apoptotic cells. Mice exposed to IR exhibited an increased number of apoptotic cells in tumor xenograft sections. Treatments with Reg IV-specific mAbs further increased the number of apoptotic cells showing an additive effect on cellular apoptosis following combined therapy (Fig. 5D upper panel). Reg IV-specific mAbs-mediated increase in tumor cell apoptosis was also associated with a significant reduction in expression of previously reported anti-apoptotic genes Bcl-2, Bcl-XL and Survivin (data not shown). In addition, Reg IV- specific mAbs treatments also led to a significant reduction in tumor cell proliferation as assessed by BrDU incorporation in dividing cell nuclei (Fig. 5D lower panel). These results indicate that Reg IV has potent anti-apoptotic and pro-proliferative effects. Results further suggest that antagonism of Reg IV signaling increases tumor cell susceptibility to IR-induced apoptosis, leading to a greater therapeutic effect of radiotherapy on tumor growth.
Figure 5.
Reg IV-specific mAbs treatments increase survival rate and reduce tumor growth in murine model of tumor xenografts. (A) Mice treated with multiple doses Reg IV-specific mAb (3E5 and 2H6; 400 μg/day) starting from day 1 of tumor xenografts exhibited an increased survival rate in comparison to mice treated with equivalent doses of mAbs starting from day 10 (when tumors were palpable) and PIP controls. (B) Treatments of Reg IV-specific mAbs started from day 1 of tumor xenografts led to a significant reduction in tumor growth when compared to the animals treated with equivalent doses of Reg IV-specific mAbs started from day 10 and PIP controls. These results exhibited an enhanced effectiveness of mAbs treatments, when injected at earlier time points of tumor growth. (C) In a separate experiment, mice were treated with Reg IV-specific mAbs at day 10 of tumor xenografts and exposed to 6 Gy IR 24 h later. Treatments of mAbs were continued for 5 doses at every alternate day. Significant reduction in tumor growth was observed showing additive effect of IR and mAbs treatments. (D) Staining of tumor tissues with H&E exhibited an increased number of apoptotic cells following IR. Treatments of Reg IV-specific mAbs further increased the number of apoptotic cells showing an additive effect on cellular apoptosis. In addition, Reg IV-specific mAbs treatments also led to a significant reduction in tumor cell proliferation as assessed by BrDU incorporation. These results indicated that Reg IV is a potent mediator of cellular apoptosis and proliferation hence antagonism of Reg IV signaling increases tumor cell susceptibility to IR-induced cell death.
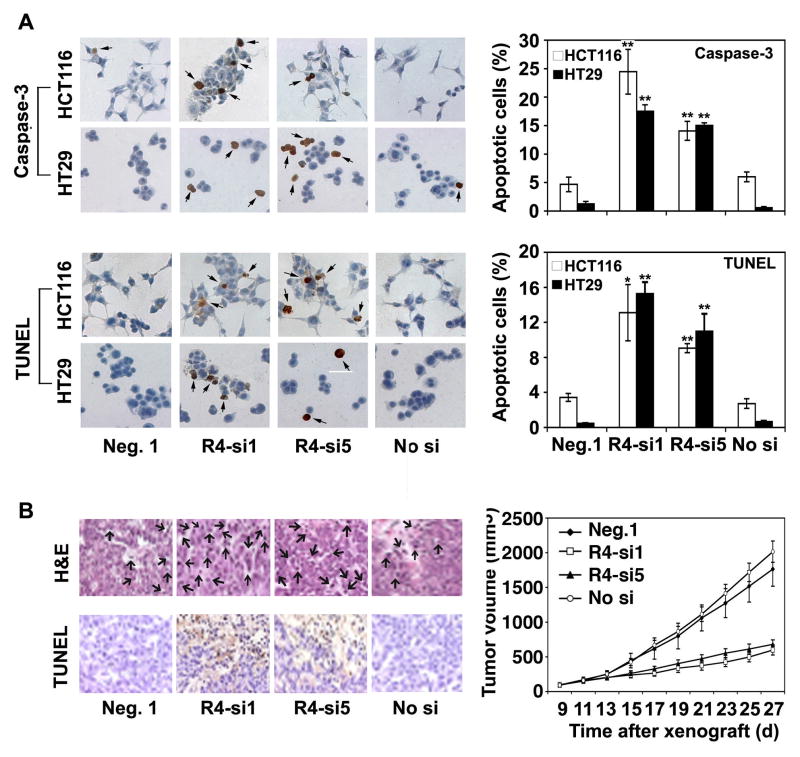
Treatment of siRNAs Targeting Reg IV mRNA Sequence Leads to Increased Apoptosis and Reduced Cell Proliferation
As a second approach of Reg IV antagonism, we used a panel of siRNAs (si1 and si5) targeting Reg IV mRNA to decrease human CRC cell proliferation (supplementary Fig. 1). Following immunostaining for activated caspase-3 and TUNEL, transfection of HCT116 and HT29 cells with Reg IV-specific siRNAs (R4-si1 & R4-si5; 100 nM) led to a significant increase in number of apoptotic cells in comparison to Neg. 1 (a scrambled siRNA) or ‘No si’ controls (Fig. 6A left and right panels). Furthermore, intratumoral injection of these siRNAs (R4-si1 & R4-si5; 50μl of 5μM siRNA/day for 5 consecutive doses at every third day) in an in vivo model of human CRC led to a significant increase in apoptotic cell number in solid viable (Fig. 6B left panel) and the adjacent liquefied portions (data not shown) of tumor xenografts. Increased apoptotic cell death following Reg IV-specific siRNAs treatment resulted in significantly reduced growth of tumor xenografts (Fig. 6B right panel). In both in vitro and in vivo models of human CRC, administration of Reg IV-specific siRNAs was associated with decreased expression of anti-apoptotic genes, including Bcl-2, Bcl-XL and survivin (data not shown). These results supported our hypothesis that Reg IV antagonism is a useful tool to reduce colorectal tumor growth and increase tumor cell susceptibility to IR-induced apoptotic cell death.
Figure 6.
Treatments of siRNAs targeting Reg IV mRNA sequence increase apoptosis and reduce CRC growth. (A) Following Caspase-3 and TUNEL immunostainings, HCT116 and HT29 cells transfected with Reg IV-specific siRNAs (R4-si1 and R4-si5; 100 nM) exhibited significantly increased number of apoptotic cells in comparison to Neg. 1 (a scrambled siRNA) or “No si” controls (left and right panels). (B) Following H&E and TUNEL staining, intra-tumoral injections of R4-si1 & R4-si5 (50μl of 5μM siRNA/day for 5 consecutive doses at every third day) resulted in a significantly increased number of apoptotic cells in solid viable (left panel) and adjacent liquefied portions (not shown) of tumor xenografts leading to a significantly reduced tumor growth (right panel). These results suggested that Reg IV antagonism is a useful tool to reduce colorectal tumor growth and increase tumor cell susceptibility to apoptosis. (* P < 0.05, ** P < 0.01)
DISCUSSION
The normal gastrointestinal (GI) epithelial architecture is frequently disrupted following injury. The homeostasis in a normal GI epithelial cells population is maintained by their continuous replacement with replicating undifferentiated epithelial cells (transit cells) located in the crypts, followed by their subsequent differentiation and migration away from the zone of replication.24, 25 Crypt stem cells play a central role in maintaining GI architecture both in normal and injury states.23, 26 Stem cells proliferate into a transit cell population, which expand rapidly to form a regenerative crypt.26, 27 Disrupted architecture of GI epithelial cells following injury leads to a dysregulated expression of anti-apoptotic genes. In the present study, we investigated the possible mechanisms for apoptosis and survival of intestinal epithelial crypt cells using a well-defined murine model of IR-induced intestinal injury. The observations clearly identified Reg IV as a factor regulating the intestinal crypt cell apoptosis. Reg IV treatments to the mice led to a significant reduction in number of IR-induced apoptotic cells, especially at crypt cell positions 3 to 6 counted from the base, corresponding to the location of the putative stem cell. Following intestinal injury, Reg IV provided a degree of protection to the intestinal crypt stem cell against IR-induced apoptotic cell death. The surviving stem cell proliferated to increase their numbers and give rise to a more rapidly proliferating transit cell population to form a regenerative crypt. A single surviving clonogenic crypt stem cell is sufficient to give rise to a regenerating crypt. In the present study, Reg IV-mediated protection to intestinal crypt stem cells played a very important role in restoring normal epithelial architecture and function by differentiation of epithelium and its subsequent migration to respective intestinal locations. Reg IV-mediated regulation of intestinal crypt cell apoptosis was also associated with increased expression of previously reported anti-apoptotic genes Bcl-2, Bcl-XL and survivin. These results clearly suggest that Reg IV protects intestinal crypt cell from IR-induced apoptosis and increases their survival especially in the putative stem cell zone. The surviving stem cells proliferate to increase their number and give rise to the more rapidly proliferating transit cell population, which expands rapidly to form a regenerative crypt. The proliferating transit cells are then differentiated, migrated to their respective positions of the intestinal crypt-villus axis, and reestablish a normal intestinal epithelial architecture.
Most human adenocarcinomas are relatively resistant to chemotherapy (CT) and IR-induced cell death.28–30 Efforts to overcome this resistance by increasing concentration of cytotoxic drugs or dosage of irradiation have failed to significantly improve the therapeutic response. Novel cancer treatment strategies targeting the genes that regulate the cell susceptibility to CT or IR-induced cellular apoptosis may prove to be a helpful adjuvant in the treatment of many malignancies. Reg IV was reported to be overexpressed in human CRC cells HT29 selected for increased in vitro resistance to chemotherapeutic drug 5-FU.10 Our previous and current studies clearly identified an association of Reg IV expression in human CRC cells with their susceptibility to IR-induced cell death. These studies indicated that Reg IV could be a potential target of new therapeutics designed to increase tumor cell susceptibility to conventional CT or IR. The present study further finds that antagonism of Reg IV signaling is a useful tool to increase cell susceptibility to IR-induced apoptosis in human CRC. Addition of mAbs specific to Reg IV protein and transfection of cells with siRNAs targeting the Reg IV mRNA sequence were selected as two complementary approaches for blocking Reg IV signaling. In both approaches led to similar decreases in cell proliferation and increases in cell susceptibility to IR-induced death in HCT116, SW480 and HT29 human CRC cells. Antagonism of Reg IV signaling resulted in an increased number of apoptotic cells, which was found in association with decreased expression of anti-apoptotic genes, including Bcl-2, Bcl-XL and survivin. Since we have previously reported that Reg IV regulates the expression of these anti-apoptotic factors through EGFR-Akt-Ap1 signaling pathway, this could be proposed as a possible mechanism of Reg IV mAbs-mediated increase in human CRC cell apoptosis. Data from the tumor xenografts studies also exhibited reduced tumor growth and increased apoptosis following antagonism of Reg IV signaling. Anti-tumor effects were more pronounced when Reg IV mAbs were administered at early phases of tumorigenesis.
In summary, Reg IV is an important regulator of cell proliferation, apoptosis and cell susceptibility to IR. Increased expression of Reg IV at injury protects GI mucosa, including the crypt stem cell population. However, overexpression of Reg IV in many human GI malignancies including CRC leads to a tumor phenotype resistant to radio/chemotherapy-induced apoptosis. Antagonism of Reg IV signaling decreases cell proliferation and increases tumor cell susceptibility to IR-induced apoptosis. These results identify Reg IV as a previously unappreciated regulator of cell proliferation and apoptosis that might contribute to increased resistance to apoptotic death during therapy. Hence, antagonism of Reg signaling could be a new potential therapeutic intervention for human CRC and other GI malignancies.
Supplementary Material
Acknowledgments
Supported by NIH grants DK060106 & P30 DK52574 to Brian K. Dieckgraefe and DK62265 & CA109269 to Shrikant Anant.
Footnotes
No conflicts of interests exist
Repository URL and data accession numbers: Hybridoma cell lines 2H6.3.1 and 3E5.4.1 (http://pathology.wustl.edu/research/hybridoma.php; Fusion No. 4465), Reg IV mRNA (Gene Bank accession no. NM_032044)
Author contributions: K.S.B.: conception and design, collection and assembly of data, data analysis and integration, manuscript writing, and final approval of the manuscript; Q.L.: collection and assembly of data, and final approval of the manuscript; S.K.S.: collection and assembly of data, and final approval of the manuscript; K.K.: collection and assembly of data, and final approval of the manuscript; S.M.S.: collection and assembly of data, and final approval of the manuscript; M.S.: collection and assembly of data, and final approval of the manuscript; J.H.G: collection and assembly of data, and final approval of the manuscript; K.A.: collection and assembly of data, and final approval of the manuscript; R.M.: collection and assembly of data, and final approval of the manuscript; C.W.H.: data analysis and integration and final approval of the manuscript; S.A.: data analysis and integration, financial support, and final approval of the manuscript; B.K.D.: conception and design, financial support, data analysis and integration, and final approval of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chakraborty C, Katsumata N, Myal Y, Schroedter IC, Brazeau P, Murphy LJ, Shiu RP, Friesen HG. Age-related changes in peptide-23/pancreatitis-associated protein and pancreatic stone protein/reg gene expression in the rat and regulation by growth hormone-releasing hormone. Endocrinology. 1995;136:1843–9. doi: 10.1210/endo.136.5.7720628. [DOI] [PubMed] [Google Scholar]
- 2.Laurine E, Manival X, Montgelard C, Bideau C, Berge-Lefranc JL, Erard M, Verdier JM. PAP IB, a new member of the Reg gene family: cloning, expression, structural properties, and evolution by gene duplication. Biochim Biophys Acta. 2005;1727:177–87. doi: 10.1016/j.bbaexp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Dieckgraefe BK, Crimmins DL, Landt V, Houchen C, Anant S, Porche-Sorbet R, Ladenson JH. Expression of the regenerating gene family in inflammatory bowel disease mucosa: Reg Ialpha upregulation, processing, and antiapoptotic activity. J Investig Med. 2002;50:421–34. doi: 10.1136/jim-50-06-02. [DOI] [PubMed] [Google Scholar]
- 4.Lasserre C, Simon MT, Ishikawa H, Diriong S, Nguyen VC, Christa L, Vernier P, Brechot C. Structural organization and chromosomal localization of a human gene (HIP/PAP) encoding a C-type lectin overexpressed in primary liver cancer. Eur J Biochem. 1994;224:29–38. doi: 10.1111/j.1432-1033.1994.tb19991.x. [DOI] [PubMed] [Google Scholar]
- 5.Hartupee JC, Zhang H, Bonaldo MF, Soares MB, Dieckgraefe BK. Isolation and characterization of a cDNA encoding a novel member of the human regenerating protein family: Reg IV. Biochim Biophys Acta. 2001;1518:287–93. doi: 10.1016/s0167-4781(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 6.Bishnupuri KS, Luo Q, Korzenik JR, Henderson JO, Houchen CW, Anant S, Dieckgraefe BK. Dysregulation of Reg gene expression occurs early in gastrointestinal tumorigenesis and regulates anti-apoptotic genes. Cancer Biol Ther. 2006;5:1714–20. doi: 10.4161/cbt.5.12.3469. [DOI] [PubMed] [Google Scholar]
- 7.Dieckgraefe BK, Stenson WF, Korzenik JR, Swanson PE, Harrington CA. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics. 2000;4:1–11. doi: 10.1152/physiolgenomics.2000.4.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Zenilman ME, Kim S, Levine BA, Lee C, Steinberg JJ. Ectopic expression of reg protein: A marker of colorectal mucosa at risk for neoplasia. J Gastrointest Surg. 1997;1:194–201. doi: 10.1016/s1091-255x(97)80109-6. discussion 201–2. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Lai M, Gu X, Luo M, Shao L. Reg IV, a differentially expressed gene in colorectal adenoma. Chin Med J (Engl) 2003;116:918–22. [PubMed] [Google Scholar]
- 10.Violette S, Festor E, Pandrea-Vasile I, Mitchell V, Adida C, Dussaulx E, Lacorte JM, Chambaz J, Lacasa M, Lesuffleur T. Reg IV, a new member of the regenerating gene family, is overexpressed in colorectal carcinomas. Int J Cancer. 2003;103:185–93. doi: 10.1002/ijc.10788. [DOI] [PubMed] [Google Scholar]
- 11.Oue N, Kuniyasu H, Noguchi T, Sentani K, Ito M, Tanaka S, Setoyama T, Sakakura C, Natsugoe S, Yasui W. Serum concentration of Reg IV in patients with colorectal cancer: overexpression and high serum levels of Reg IV are associated with liver metastasis. Oncology. 2007;72:371–80. doi: 10.1159/000113147. [DOI] [PubMed] [Google Scholar]
- 12.Bishnupuri KS, Luo Q, Murmu N, Houchen CW, Anant S, Dieckgraefe BK. Reg IV activates the epidermal growth factor receptor/Akt/AP-1 signaling pathway in colon adenocarcinomas. Gastroenterology. 2006;130:137–49. doi: 10.1053/j.gastro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Li A, Crimmins DL, Luo Q, Hartupee J, Landt Y, Ladenson JH, Wilson D, Anant S, Dieckgraefe BK. Expression of a novel regenerating gene product, Reg IV, by high density fermentation in Pichia pastoris: production, purification, and characterization. Protein Expr Purif. 2003;31:197–206. doi: 10.1016/s1046-5928(03)00164-5. [DOI] [PubMed] [Google Scholar]
- 14.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–7. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 15.Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S, Houchen CW. Selective Blockade of DCAMKL-1 Results in Tumor Growth Arrest by a Let-7a MicroRNA-Dependent Mechanism. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed JC, Miyashita T, Takayama S, Wang HG, Sato T, Krajewski S, Aime-Sempe C, Bodrug S, Kitada S, Hanada M. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J Cell Biochem. 1996;60:23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Ogura E, Senzaki H, Yamamoto D, Yoshida R, Takada H, Hioki K, Tsubura A. Prognostic significance of Bcl-2, Bcl-xL/S, Bax and Bak expressions in colorectal carcinomas. Oncol Rep. 1999;6:365–9. doi: 10.3892/or.6.2.365. [DOI] [PubMed] [Google Scholar]
- 19.Maurer CA, Friess H, Buhler SS, Wahl BR, Graber H, Zimmermann A, Buchler MW. Apoptosis inhibiting factor Bcl-xL might be the crucial member of the Bcl-2 gene family in colorectal cancer. Dig Dis Sci. 1998;43:2641–8. doi: 10.1023/a:1026695025990. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene. 2000;19:6627–31. doi: 10.1038/sj.onc.1204087. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe J, Kushihata F, Honda K, Sugita A, Tateishi N, Mominoki K, Matsuda S, Kobayashi N. Prognostic significance of Bcl-xL in human hepatocellular carcinoma. Surgery. 2004;135:604–12. doi: 10.1016/j.surg.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Sarela AI, Macadam RC, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000;46:645–50. doi: 10.1136/gut.46.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–20. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 24.Gordon JI, Hermiston ML. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol. 1994;6:795–803. doi: 10.1016/0955-0674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 25.Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78:219–43. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 27.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–9. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 28.Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–39. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- 29.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–26. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 30.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–9. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.