Summary of recent advances
Fluorescent dyes based on small organic molecules that function in the near infra red (NIR) region are of great current interest in chemical biology. They allow for imaging with minimal autofluorescence from biological samples, reduced light scattering and high tissue penetration. Herein, examples of ongoing NIR fluorophore design strategies as well as their properties and anticipated applications relevant to the bioimaging are presented.
Introduction
Dyes active in the near infrared (NIR) region have attracted ongoing attention due to their diverse applications in biomedical, materials and related fields. Advantages include minimal interfering absorption and fluorescence from biological samples, inexpensive laser diode excitation, reduced scattering and enhanced tissue penetration depth. However, there are only relatively few classes of NIR dyes that are readily available. These include the phthalocyanines, cyanine dyes and squaraine dyes.
Aqueous insolubility and ease of aggregate formation are problems often encountered with phthalocyanine and squaraine dyes in biological systems. Squaraine dyes are also highly chemically reactive. Cyanine dyes are excellent NIR dyes that have high molar absorptivity, strong fluorescence, and good photostability. However, their intrinsically small Stokes shifts may produce excitation and scattered light interferences.
Great effort has gone into improving the photophysical and photochemical properties of existing NIR dyes. For example, various hydrophilic groups such as sulfonate, pyridinium, glycol and carboxylate, have been appended to increase aqueous solubility. Moreover, the addition of charged functional groups and increased sterics has aided in reducing aggregation. Cyanine dyes have also been functionalized in order to increase their Stokes shifts.
Although the modification of existing dye skeletons with appropriate functional groups has much improved their physicochemical properties, it has also led to new issues. Increasing the molecular weight of the dyes can lead to interference with the functioning of biomolecules or precipitation, apart from synthetic challenges. Large molecular weight dyes cannot be readily used for in vivo amyloid labeling since such studies require blood brain barrier penetration, and can cause increases in the serum pharmacokinetics of drug-dye conjugates.
The development of simple and novel low molecular weight NIR platforms that can be further modified is thus of great interest to the biomedical imaging community. The visualization of tumors and plaques as well as vascular mapping of the heart and brain are aspects of basic biomedical research and disease diagnostics that can continue to benefit substantially from the creation of improved NIR dyes. The development of new and improved fluorophores for trafficking studies, particularly the trafficking of tagged nutrients or drugs, should dramatically increase the number of live cell imaging and in vivo, real-time advanced imaging studies for fundamental research and translational applications.
This review highlights very recent progress (since 2008) towards the synthesis and evaluation of new NIR-active dyes with enhanced optical and physical properties for potential bioimaging applications. In this relatively short period of time research groups from around the world, spanning several disciplines, have created new NIR fluorophores that creatively address a broad scope of significant current challenges. The following sections summarize these ongoing studies.
Novel Cyanine Dyes
The cyanines are a unique class of charged chromophores with an odd number of carbons in a conjugated polymethine framework that has no significant bond alternation. Pyrrolopyrrole cyanine (PPCy) dyes are a new type of NIR fluorophore synthesized via the reaction of diketopyrrolpyrrole with heteroarylacetonitriles. They exhibit narrow and intense absorptions as well as quantum yields ranging from 0.32–0.69 (CHCl3). Imaging applications for these promising materials have been proposed. Aqueous soluble derivatives have not yet been reported [1].
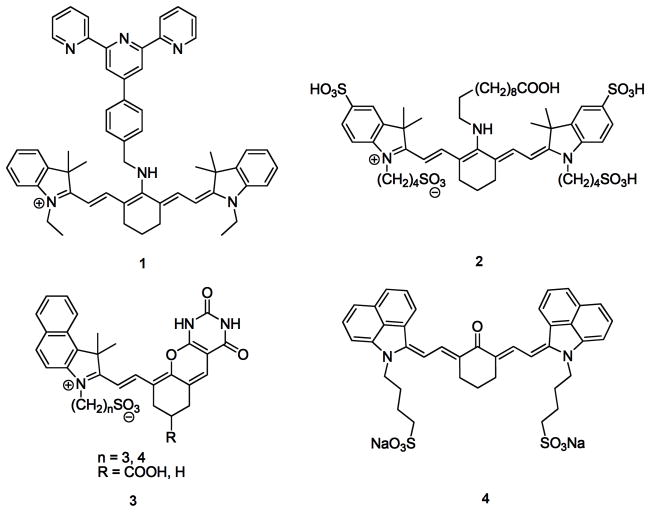
Tang and co-workers appended a terpyridine moiety to a tricarbocyanine dye to afford a water soluble fluorophore (1) that can respond to minor fluctuations in pH (6.70–7.90) via a photoinduced electron transfer (PET) mechanism. Real time imaging and pH detection pH detection was achieved in studies involving live HepG2 and HL-7702 cells [2]. Pham and co-workers modified the cyanine dye NIR820 with four sulfonates to increase water solubility (compound 2). They additionally substituted the chlorine atom at the methine bridge to increase the Stokes shift. Their molecule 4-Sulfonir exhibited a molar extinction coefficient of 1.5 × 105 M−1cm−1, a quantum yield of 0.37 and a Stokes shift of 140 nm. Its potential utility in biological media is under investigation [3]. The Smith group had previously derivatized a carbocyanine in a similar fashion with a zinc (II) dipicolylamine (Zn-DPA) moiety to detect Staphylococcus aureus infection in mice [4,5**]. This embodies a unique example of a non-genetic reporter molecule that specifically targets bacteria in vivo via its inherent affinity for anionic cell surfaces. Similar NIR-mercury ion [6] and NIR cancer [7] probes also have been synthesized.
The Achilefu group has recently reported several studies of interest involving new functional cyanines. A new pyrimidine-fused pH-sensitive NIR active fluorescent probe was discovered via an attempted substitution of the chlorine atom at the cyanine methine moiety with barbituric acid. The product (3) was formed via an unusual debenzoindolation mechanism [8]. In another study, unique cyanine dye-based FRET pairs were developed and conjugated to peptides to monitor caspase activity [9]. The fluorescence lifetimes of a series of cyanines were investigated as a function of solvent and structural features. Decreases in structural rigidity were correlated to losses of excited state energy via non-radiative pathways [10].
Strekowski and co-workers reported the synthesis of a number of benzo[c,d]indolium-derived cyanines (for example, compound 4). Using readily available 1,8-naphtholactam as a substrate they were able to obtain both hydrophobic and water soluble fluorophores. These molecules are expected to be more stable and exhibit enhanced bathochromic shifts as compared to other benzoindolium-derived cyanine congeners [11].
Borohydride-reduced cyanines (“hydrocyanines”) are weakly fluorescent until oxidized. They are thus activated by reactive oxygen species (ROS) including superoxide. Murthy and co-workers successfully measured ROS generation at nanomolar levels and in vivo using hydrocyanines. The hydrocyanines are less prone to autofluorescence as compared to comparable reagents [12].
Iodoacetamide-functionalized cyanines were synthesized by Bruschi et al. They are useful for labeling cysteine residues as alternative dyes for the analysis of plasma proteins via 2-D DIGE (differential display electrophoresis). The authors also report their potential utility in evaluating protein redox status [13].
Commercially available cyanines were linked to radionucleotide-binding moieties and conjugated to tumor-targeting antibodies. This afforded a dual modality positron emission tomography-fluorescence imaging material that exhibited strong binding to HER2-expressing cancer cell lines. This design strategy should be applicable to the generation of libraries of antibody-based multimodal probes [14].
Novel Phthalocyanines and Porphyrin Derivatives
Porphyrins and phthalocyanines and their metal complexes embody some of the most intensively studied NIR-active dyes. A series of conjugated porphyrin dimers with intense absorptions ranging from 650–800 nm and fluorescence emission from 700–800 nm have been used in imaging studies [15]. The authors noted greatly improved photostability as compared to their monomeric counterparts and outstanding potential for photodynamic therapy. These molecules disaggregated and fluoresced upon binding albumin.
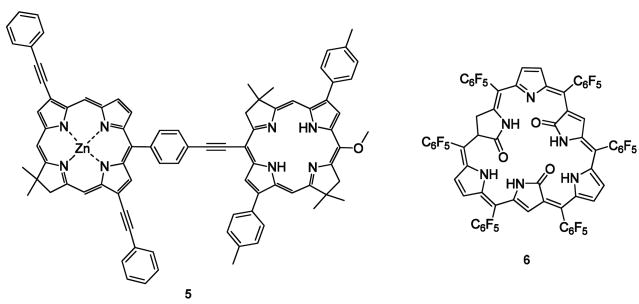
Synthetic chlorin and bacteriochlorin macrocycle dyads (“C-B dyads” such as compound 5) were created that exhibit large Stoke’s shifts (85 – 110 nm). Water soluble analogs are planned. The absorption and emission bandwithds of each constituent of each dyad exhibited very narrow spectral widths (< 20 nm). This feature should enable efficient multicolor imaging applications [16].
Hammer and co-workers have solved many long-standing classical challenges involving the difficult synthesis and purification of discreet phthalocyanine isomers by employing innovative solid-phase synthesis methods [17]. Their asymmetrically-substituted phthalocyanines and their oligonucleotide conjugates have found immediate utility in PCR applications [18] and as molecular beacons [19].
Vicente has synthesized and evaluated cationic water soluble Zn and Si pyridyloxy phthalocyanines [20]. Amphiphilic Si phthalocyanines localized preferentially within the lysosomes and exhibited high phototoxicity towards human Hep2 cells with an IC50 of 2.2 μM at a 1 J cm−2 light dose.
Rurak and colleagues have designed an expanded porphyrin based on a rubyrin core functionalized with sulfur atoms and polycylic aromatic units fused to the pyrrole rings. This hexaphyrin demonstrated preferable detection of Hg2+ over other metal cations [21]. The extended conjugation resulted in exceptional bathochromic shifts and good molar absorptivity at common laser lines from 322–780 nm.
Furota and Osuka synthesized triangular shaped triply-N-confused expanded porphyrins. One of these (6) displays a broad band at 475 nm in dichloromethane, Q-bands at 835, 947 and 1087 nm, and changes the color upon protonation from blue to green. A study of how related shape changes influence fundamental properties is ongoing [22*].
A benzotexaphyrin with an extensively delocalized π-electron system was synthesized and demonstrated a distinctive red-shifted Q-like absorption band in the near-IR region (λmax 730 nm and λex 825 nm). It possesses low triplet excited-state energy, high triplet quantum yield and efficient singlet oxygen generation. The synthesis of derivatives suitable for bioimaging studies is planned [23].
Expanded pentafluorphenyl porphyrins were prepared by the condensation of meso-pentafluorofipyrromethane and pentafluorobenzaldehyde. Two of the compounds obtained exhibited emission bands at 939 and 953 nm [24]. The acid-catalyzed condensation of sterically hindered 3,5-bis(trifluoromethyl)benzaldehyde and pyrrole afforded fluorinated hexa- and heptaphyrins with near-IR optical activity [25]. A near IR fluorescent chemodosimeter for Ag+ cation based on an expanded fluorine-containing porphyrin was synthesized [26*]. The authors reported intense fluorescence with λex = 514 nm and λem = 1050 nm in MeOH.
Squaraine derivatives
The squaraines are dyes with an electron deficient central four-membered ring and a resonance stabilized zwitterionic structure. The central ring is typically appended with donor moieties to afford a donor-acceptor-donor motif. Suzuki and co-workers [27] introduced multiple water-solubilizing sulfonate moeities into a squaraine framework. A bovine serum albumin (BSA) conjugate containing a squaraine tetrasulfonate exhibited absorption maxima at 787 nm, excitation maxima at 760 nm, emission maxima at 812 nm and a quantum yield of 0.08. The authors envision these dyes to be useful in protein detection, as covalent labeling probes, and as contrast agents for in vivo imaging.
The Smith group has reported recent progress [28, 29**] on their pioneering studies of the squaraine rotaxanes [30–32] in which a macrocycle encapsulates the highly electrophilic squaraine thereby shielding it from nucleophilic attack as well as from self-aggregation. In the latest embodiments, highly soluble rotaxane stopper groups were shown to possess excellent stability and solubility in aqueous media [28] and new capping chemistry was developed, allowing for tunable fluorescence properties [29**].
Nucleophilic sulfur generally reacts readily with unprotected squaraines. This property had been previously used to develop chemsodosimeters for biological thiols. Ajayaghosh and co-workers report that they have significantly improved upon previous squaraines used in the detection of cysteine and homocysteine. They developed a ratiometric probe which loses its extended conjugation and NIR fluorescence upon reaction with a thiol while developing a very strongly emissive band in the visible region [33].
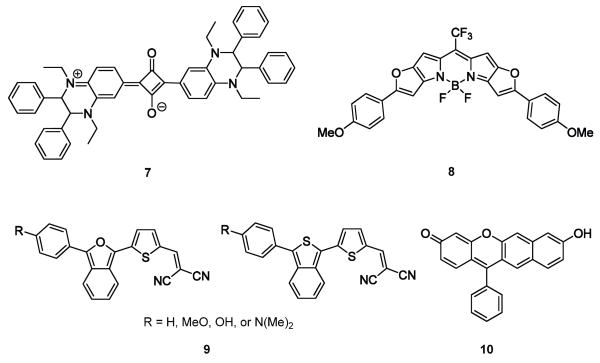
Patil et al. have created tetrahydroquinoxaline-based squaraines (e.g., 7). The rationale for incorporating a quinoxaline was to use its strong electron donor properties to afford enhanced bathochromic shifts. The squaraine quinoxalines were additionally shown to function as effective chemical sensors for copper ions [34].
BODIPY analogs
The BODIPY (borondipyrromethane) dyes typically have relatively shorter wavelength emission maxima and smaller extinction coefficients as compared to the cyanines and other NIR active fluorophores. Suzuki found that by fusing π-excessive aromatic rings to the BODIPY core (e.g., compound 8) significant increases in quantum yield, higher extinction coefficients, improvements in photostability and NIR optical activity could be achieved [35,36].
The O’Shea group has synthesized tetraarylazadipyrromethenes which contain aryl groups bonded to the chromophore resulting in emission in the range of 650–789 nm [37]. Judicious positioning of receptors on the aryl rings has afforded photoinduced electron transfer (PET) or internal charge transfer (ICT) chemosensors [38, 39]. The tetraarylazadipyrromethenes exhibit an interesting intramolecular oxygen-fluorine displacement reaction [40] which has recently been used as a key step in a facile covalent immobilization strategy [41].
Benzo[c]heterocycles
Swager and co-workers [42**] have recently reported eight unique push-pull-type near-infrared dyes (9) that contain isobenzofuran or isothianaphthene moieties. The isobenzofuran fluorophores exhibit red-shifted absorptions relative to the isothianaphthenes. This was attributed to isobenzofuran’s relatively greater pro-quinoidal character. These dyes may be of great promise for imaging applications, particularly for the visualization of β-amyloid plaque [43,44]. To date, their photophysical properties have been studied in CHCl3 and MeOH. Strong emission in the NIR and large (>200 nm) Stokes shifts have been achieved.
Xanthenes
The xanthene dyes include some of the most common fluorophores such as fluorescein and rhodamine; however, they are typically not active in the NIR region. A series of seminaphthofluorone xanthene dye regioisomers, synthesized by the authors’ group, exhibits dual excitation and emission from fluorescent neutral and anion forms. Systematic alterations to the angle of benzannulation and the placement of ionizable moieties afforded deep-red to NIR emission from the dyes’ anionic states. Unusually large Stokes shifts for xanthene dyes (up to ~200 nm in aqueous buffer, compound 10) was observed. allowing for NIR emission upon excitation in the blue/green wavelength region. These fluorophores embody minimalist structures and are thus potentially highly useful templates for further functionalization and optimization [45].
Summary
Outstanding, rapid progress of great scope has been made in designing new NIR-active probes. These studies promote collaborative efforts from scientists specializing in divergent fields. Despite the major ongoing efforts, significant opportunities remain. For example, despite the widely accepted utility of NIR small molecule probes, apparently the only clinically-approved material to date is indocyanine green (ICG). ICG has a quantum yield of only 0.01 in aqueous solution, and there have been reports of poor stability, rapid clearance form the liver and cytotoxicity [46]. In the near future improved NIR fluorophores will continue to be discovered that address issues such as improved molar absorptivity, spectral bandwidths, quantum yield, Stokes shift, lifetime, photostability, solubility, molecular targeting, systematic clearance, low toxicity, synthesis, purification and ease of functionalization and conjugation.
Figure.
Table 1.
Summary of features in representative compounds.
| Compound | Spectral Properties | Application | Reference |
|---|---|---|---|
| 1 | Excitation wavelength: 648 nm; emission: 750 nm. | Rapid monitoring of minor pH fluctuations and live cell imaging (HepG2 and HL-7702) | 2 |
| 2 | Stokes shift of 140 nm | Multichannel imaging | 3 |
| 3 | Absorption maxima: 690 nm (neutral pH), 605 nm (acid pH); excitation maxima: 605 nm; emission maxima: 690 nm. | pH monitoring | 8 |
| 4 | Absorption maxima in MeOH, 645 nm (ε = 50800 M−1cm−1); Absorption maxima in acidified MeOH (pH < 2), 914 nm (ε= 142000 M−1cm−1) | pH monitoring | 11 |
| 5 | Absorption maxima for the chlorin component of the dyad: 650 nm (ε= 60000 M−1cm−1); emission maxima: 760 nm. Large Stokes shift (110 nm). Solvent: toluene. | Potential application in NIR imaging upon further modifications to create water-soluble bioconjugatable chlorin-bacteriochlorin (B–C) dyads. | 16 |
| 6 | Absorption maximas: 475, 591. 835, 947 and 1087 nm (free base), 638, 809, 888 and 1016 nm (protonated form); excitation maxima: 600 nm; emission maxima: 1094 nm (free base), 1044 nm (protonated). Solvent: CH2Cl2. | No specific application is mentioned. | 22 |
| 7 | Absorption maxima: 717 nm (ε= 104400 M−1cm−1); emission maxima: 774 nm. Solvent: CHCl3. | Affinity for copper | 34 |
| 8 | Absorption maxima: 723 nm (ε= 253000 M−1cm−1); emission maxima: 738 nm. Solvent: CHCl3. | High-resolution multicolor bioanalysis and bioimaging. | 35, 36 |
| 9 | In the isothianaphthene family, for the compound with R=N(Me)2 the absorption maxima is 579 nm and the emission maxima is 785 nm. Stokes shift = 206 nm | Under testing as NIR contrast agents for biomedical applications. | 42 |
| 10 | Absorption maxima: 536 nm; emission maxima: 733 nm. Stokes shift: 197 nm. Solvent: Aqueous phosphate buffer with 1% DMSO. | Live cell imaging | 45 |
Acknowledgments
The authors thank the National Institutes of Health for continued support of the efforts in creating functional molecular probes via grant RO1 EB2044.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fischer GM, Isomaki-Krondahl M, Gottker-Schnetmann I, Daltrozzo E, Zumbusch A. Pyrrolopyrrole cyanine dyes: a new class of near-infrared dyes and fluorophores. Chem Eur J. 2009;15:4857–4864. doi: 10.1002/chem.200801996. [DOI] [PubMed] [Google Scholar]
- 2.Tang B, Yu F, Li P, Tong LL, Duan X, Xie T, Wang X. A near-infrared neutral pH fluorescent probe for monitoring minor pH changes: imaging in living HepG2 and HL-7702 cells. J Am Chem Soc. 2009;131:3016–3023. doi: 10.1021/ja809149g. [DOI] [PubMed] [Google Scholar]
- 3.Pham W, Cassell L, Gillman A, Koktysh D, Gore JC. A near-infrared dye for multichannel imaging. Chem Commun. 2008;16:1895–1897. doi: 10.1039/b719028j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leevy WM, Gammon ST, Jiang H, Johnson JR, Maxwell DJ, Jackson EN, Marquez M, Piwnica-Worms D, Smith BD. Optical imaging of bacterial infection in living mice using a fluorescent near-infrared molecular probe. J Am Chem Soc. 2006;128:16476–16477. doi: 10.1021/ja0665592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Leevy WM, Gammon ST, Johnson JR, Lampkins AJ, Jiang H, Marquez M, Piwnica-Worms D, Suckow MA, Smith BD. Noninvasive optical imaging of staphylococcus aureus bacterial infection in living mice using a bis-dipicolylamine-zinc(II) affinity group conjugated to a near-infrared fluorophore. Bioconjugate Chem. 2008;19:686–692. doi: 10.1021/bc700376v. The authors created a robust imaging probe combining a carbocyanine with zinc(II) dipicolylamine (Zn-DPA) for the detection of localized S. aureus infection. The probe is easily visualized at a leg infection site within 3 hours of administration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang B, Cui LJ, Xu KH, Tong LL, Yang GW, An LG. A sensitive and selective near-infrared fluorescent probe for mercuric ions and its biological imaging applications. ChemBioChem. 2008;9:1159–1164. doi: 10.1002/cbic.200800001. [DOI] [PubMed] [Google Scholar]
- 7.Tanisaka H, Kizaka-Kondoh S, Makino A, Tanaka S, Hiraoka M, Kimura S. Near-infrared fluorescent labeled peptosome for application to cancer imaging. Bioconjugate Chem. 2008;19:109–117. doi: 10.1021/bc7001665. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Berezin MY, Guo K, Kao J, Achilefu S. Near-infrared fluorescent ph-sensitive probes via unexpected barbituric acid mediated synthesis. Org Lett. 2009;11:29–32. doi: 10.1021/ol802363x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Fan J, Cheney PP, Berezin MY, Edwards WB, Akers WJ, Shen D, Liang K, Culver JP, Achilefu S. Activatable molecular systems using homologous near-infrared fluorescent probes for monitoring enzyme activities in vitro, in cellulo, and in vivo. Mol Pharm. 2009;6:416–427. doi: 10.1021/mp800264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, Berezin MY, Henary M, Strekowski L, Achilefu S. Fluorescence lifetime properties of near-infrared cyanine dyes in relation to their structures. J Photochem Photobiol, A. 2008;200:438–444. doi: 10.1016/j.jphotochem.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henary M, Mojzych M, Say M, Strekowski L. Functionalization of benzo[c,d]indole system for the synthesis of visible and near-infrared dyes. J Heterocycl Chem. 2009;46:84–87. [Google Scholar]
- 12.Kundu K, Knight SF, Willett N, Lee S, Taylor WR, Murthy N. Hydrocyanines: a class of fluorescent sensors that can image reactive oxygen species in cell culture, tissue, and in vivo. Angew Chem, Int Ed. 2009;48:299–303. doi: 10.1002/anie.200804851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruschi M, Grilli S, Candiano G, Fabbroni S, Della Ciana L, Petretto A, Santucci L, Urbani A, Gusmano R, Scolari F, et al. New iodo-acetamido cyanines for labeling cysteine thiol residues. A strategy for evaluating plasma proteins and their oxido-redox status. Proteomics. 2009;9:460–469. [Google Scholar]
- 14.Xu H, Eck PK, Baidoo KE, Choyke PL, Brechbiel MW. Toward preparation of antibody-based imaging probe libraries for dual-modality positron emission tomography and fluorescence imaging. Bioorg Med Chem. 2009;17:5176–5181. doi: 10.1016/j.bmc.2009.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuimova MK, Collins HA, Balaz M, Dahlstedt E, Levitt JA, Sergent N, Suhling K, Drobizhev M, Makarov NS, Rebane A, Anderson HL, Phillips D. Photophysical properties and intracellular imaging of water-soluble porphyrin dimers for two-proton excited photodynamic therapy. Org Biomol Chem. 2009;7:889–896. doi: 10.1039/b814791d. [DOI] [PubMed] [Google Scholar]
- 16.Kee HL, Nothdurft R, Muthiah C, Diers JR, Fan D, Ptaszek M, Bocian DF, Lindsey JS, Culver JP, Holten D. Examination of chlorin bacteriochlorin energy-transfer dyads as prototypes for near-infrared molecular imaging probes. Photochem Photobiol. 2008;84:1061–1072. doi: 10.1111/j.1751-1097.2008.00409.x. [DOI] [PubMed] [Google Scholar]
- 17.Erdem SS, Nesterova IV, Soper SA, Hammer RP. Solid-phase synthesis of asymmetrically substituted “AB(3)-type” phthalocyanines. J Org Chem. 2008;73:5003–5007. doi: 10.1021/jo800536v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nesterova IV, Verdree VT, Pakhomov S, Stricker KL, Allen MW, Hammer RP, Soper SA. Metallo-phthalocyanine near-IR fluorophores: oligonucleotide conjugates and their application in PCR assays. Bioconjugate Chem. 2007;18:2159–2168. doi: 10.1021/bc700233w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesterova IV, Erdem SS, Pakhomov S, Hammer RP, Soper SA. Phthalocyanine dimerization-based molecular beacons using near-IR fluorescence. J Am Chem Soc. 2009;131:2432–2433. doi: 10.1021/ja8088247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li HR, Jensen TJ, Fronczek FR, Vicente MGH. Synthesis and properties of a series of cationic water-soluble phthalocyanines. J Med Chem. 2008;51:502–511. doi: 10.1021/jm070781f. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Descalzo AB, Weik F, Emmerling F, Shen Z, You X-Z, Rurack K. A core-modified rubyrin with meso-aryl substituents and phenanthrene-fised pyrrole rings: a highly conjugated near-infrared dye and Hg2+ probe. Angew Chem Int Ed. 2008;47:193–197. doi: 10.1002/anie.200702854. [DOI] [PubMed] [Google Scholar]
- 22*.Xie YS, Yamaguchi K, Toganoh M, Uno H, Suzuki M, Mori S, Saito S, Osuka A, Furuta H. Triply N-confused hexaphyrins: near-infrared luminescent dyes with a triangular shape. Angew Chem Int Ed. 2009;48:5496–5499. doi: 10.1002/anie.200900596. A new class of triply N-confused hexaphyrins exhibiting triangular shapes and NIR emission. These molecules are unique templates for understanding and controlling structure-property relationships. [DOI] [PubMed] [Google Scholar]
- 23.Lu T, Shao P, Mathew I, Sand A, Sun W. Synthesis and photophysics of benzotexaphyrin: a near-infrared emitter and photosensitizer. J Am Chem Soc. 2008;130:15782–15783. doi: 10.1021/ja807021n. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Shin J-Y, Osuka A. Facile synthesis of large meso-pentafluorophenyl-substituted expanded porphyrins. Eur J Org Chem. 2008;8:1341–1349. [Google Scholar]
- 25.Kang S, Hayashi H, Umeyama T, Matano Y, Tkachenko NV, Lemmetyinen H, Imahori H. meso-3,5-Bis(trifluoromethyl)phenyl-substituted expanded porphyrins: synthesis, characterization, and optical, electrochemical, and photophysical properties. Chem Asian J. 2008;3:2065–2074. doi: 10.1002/asia.200800229. [DOI] [PubMed] [Google Scholar]
- 26*.Zhu X, Fu S, Wong WK, Wong WY. A near-infrared fluorescent chemodosimeter for silver(I) ion based on an expanded porphyrin. Tetrahedron Lett. 2008;49:1843–1846. doi: 10.1002/anie.200600248. Significant UV-Vis and fluorescence changes in the NIR region upon addition of Ag+ in methanol. High sensitivity and selectivity with an association constant in the range of 1010 M−1. [DOI] [PubMed] [Google Scholar]
- 27.Umezawa K, Citterio D, Suzuki K. Water-soluble NIR fluorescent probes based on squaraine and their application for protein labeling. Anal Sci. 2008;24:213–217. doi: 10.2116/analsci.24.213. [DOI] [PubMed] [Google Scholar]
- 28.Fu N, Gassensmith JJ, Smith BD. Effect of stopper size on squaraine rotaxane stability. Supramol Chem. 2009;21:118–124. doi: 10.1080/10610270802468454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Gassensmith JJ, Barr L, Baumes JM, Paek A, Nguyen A, Smith BD. Synthesis and photophysical investigation of squaraine rotaxanes by “clicked capping”. Org Lett. 2008;10:3343–3346. doi: 10.1021/ol801189a. An advance of the authors’ studies towards creating squaraines that don’t suffer from chemical reactivity and aggregation whereby heightened control over synthesis and physical properties is achieved. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arunkumar E, Forbes CC, Noll BC, Smith BD. Squaraine-derived rotaxanes: sterically protected fluorescent near-IR dyes. J Am Chem Soc. 2005;127:3288–3289. doi: 10.1021/ja042404n. [DOI] [PubMed] [Google Scholar]
- 31.Arunkumar E, Fu N, Smith BD. Squaraine-derived rotaxanes: Highly stable, fluorescent near-IR dyes. Chem-Eur J. 2006;12:4684–4690. doi: 10.1002/chem.200501541. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JR, Fu N, Arunkumar E, Leevy WM, Gammon ST, Piwnica-Worms D, Smith BD. Squaraine rotaxanes: superior substitutes for Cy-5 in molecular probes for near-infrared fluorescence cell imaging. Angew Chem Int Ed. 2007;46:5528–5531. doi: 10.1002/anie.200701491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sreejith S, Divya KP, Ajayaghosh A. A near-infrared squaraine dye as a latent ratiometric fluorophore for the detection of aminothiol content in blood plasma. Angew Chem Int Ed. 2008;47:7883–7887. doi: 10.1002/anie.200803194. [DOI] [PubMed] [Google Scholar]
- 34.Chandrasekaran Y, Dutta GK, Kanth RB, Patil S. Tetrahydroquinoxaline based squaraines: synthesis and photophysical properties. Dyes Pigments. 2009;83:162–167. [Google Scholar]
- 35.Umezawa K, Matsui A, Nakamura Y, Citterio D, Suzuki K. Bright, color-tunable fluorescent dyes in the Vis/NIR region: establishment of new “tailor-made” multicolor fluorophores based on borondipyrromethene. Chem-Eur J. 2009;15:1096–1106. doi: 10.1002/chem.200801906. [DOI] [PubMed] [Google Scholar]
- 36.Umezawa K, Nakamura Y, Makino H, Citterio D, Suzuki K. Bright, color-tunable fluorescent dyes in the visible-near-infrared region. J Am Chem Soc. 2008;130:1550–1551. doi: 10.1021/ja077756j. [DOI] [PubMed] [Google Scholar]
- 37.Killoran J, Allen L, Gallagher JF, Gallagher WM, O’Shea DF. Synthesis of BF2 chelates of tetraarylazadipyrromethenes and evidence for their photodynamic therapeutic behaviour. Chem Commun. 2002;17:1862–1863. doi: 10.1039/b204317c. [DOI] [PubMed] [Google Scholar]
- 38.Loudet A, Bandichhor R, Wu LX, Burgess K. Functionalized BF2 chelated azadipyrromethene dyes. Tetrahedron. 2008;64:3642–3654. doi: 10.1016/j.tet.2008.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Killoran J, McDonnell SO, Gallagher JF, O’Shea DF. A substituted BF2-chelated tetraarylazadipyrromethene as an intrinsic dual chemosensor in the 650–850 nm spectral range. New J Chem. 2008;32:483–489. [Google Scholar]
- 40.Loudet A, Bandichhor R, Burgess K, Palma A, McDonnell SO, Hall MJ, O’Shea DF. B,O-chelated azadipyrromethenes as near-IR probes. Org Lett. 2008;10:4771–4774. doi: 10.1021/ol8018506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palma A, Tasior M, Frimannsson DO, Vu TT, Meallet-Renault R, O’Shea DF. New on-bead near-infrared fluorophores and fluorescent sensor constructs. Org Lett. 2009;11:3638–3641. doi: 10.1021/ol901413u. [DOI] [PubMed] [Google Scholar]
- 42**.Meek ST, Nesterov EE, Swager TM. Near-infrared fluorophores containing benzo[c]heterocycle subunits. Org Lett. 2008;10:2991–2993. doi: 10.1021/ol800988w. Highly creative series of dyes that will allow the authors to continue their groundbreaking studies on NIR compounds that cross the blood brain barrier and image β-amyloid plaque. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nesterov EE, Skoch J, Hyman BT, Klunk WE, Bacskai BJ, Swager TM. In vivo optical imaging of amyloid aggregates in brain: design of fluorescent markers. Angew Chem Int Ed. 2005;44:5452–5456. doi: 10.1002/anie.200500845. [DOI] [PubMed] [Google Scholar]
- 44.Xiang ZM, Nesterov EE, Skoch J, Lin T, Hyman BT, Swager TM, Bacskai BJ, Reeves SA. Detection of myelination using a novel histological probe. J Histochem Cytochem. 2005;53:1511–1516. doi: 10.1369/jhc.5A6704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang YJ, Lowry M, Xu XY, Escobedo JO, Sibrian-Vazcluez M, Wong L, Schowalter CM, Jensen TJ, Fronczek FR, Warner IM, et al. Seminaphthofluorones are a family of water-soluble, low molecular weight, NIR-emitting fluorophores. Proc Natl Acad Sci U S A. 2008;105:8829–8834. doi: 10.1073/pnas.0710341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pauli J, Vag T, Haag R, Spieles M, Wenzel M, Kaiser WA, Resch-Genger U, Hilger I. An in vitro characterization study of new near infrared dyes for molecular imaging. Eur J Med Chem. 2009;44:3496–3503. doi: 10.1016/j.ejmech.2009.01.019. [DOI] [PubMed] [Google Scholar]