Abstract
From the earliest work in our laboratory, we hypothesized, and with studies conducted in both clinical research and animal models, we have shown that drugs of abuse, administered or self-administered, on a chronic basis, profoundly alter stress-responsive systems. Alterations of expression of specific genes involved in stress responsivity, with increases or decreases in mRNA levels, receptor and neuropeptide levels, and resultant changes in hormone levels, have been documented to occur after chronic intermittent exposure to heroin, morphine, other opiates, cocaine, other stimulants and alcohol in animal models and in human molecular genetics. The best studied of the stress-responsive systems in humans and mammalian species in general is undoubtedly the HPA axis. In addition, there are stress-responsive systems in other parts in the brain itself, and some of these include components of the HPA axis, such as CRF and CRF receptors, along with POMC gene and gene products. Several other stress-responsive systems are known to influence the HPA axis, such as the vasopressin-vasopressin receptor system. Orexin-hypocretin, acting at its receptors, may effect changes which suggest that it should be properly categorized as a stress-responsive system. However, less is known about the interactions and connectivity of some of these different neuropeptide and receptor systems, and in particular, about the possible connectivity of fast-acting (e.g., glutamate and GABA) and slow-acting (including dopamine, serotonin and norepinephrine) neurotransmitters with each of these stress-responsive components and the resultant impact, especially in the setting of chronic exposure to drugs of abuse. Several of these stress-responsive systems and components, primarily based on our laboratory-based and human molecular genetics research of addictive diseases, will be briefly discussed in this review.
Keywords: Stress responsivity, HPA axis, endorphin, mu opioid receptor, molecular genetics, addiction
Introduction
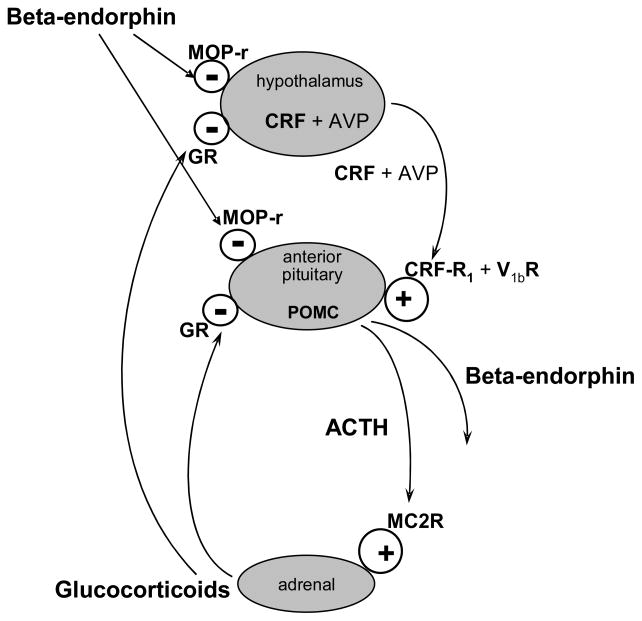
The hypothalamic-pituitary-adrenal (HPA) axis is undoubtedly the best studied stress-responsive system, initially with studies in rodent models and subsequently in humans and non-human primates. Although there are several differences, both in components of this axis and then the regulation of each of the components across species, there are also many similarities (see Figure 1). In this axis, corticotropin-releasing factor (CRF) processed from the CRF gene in the hypothalamus travels to the anterior pituitary where it acts upon CRF-R1 receptors to bring about the production and release of the gene product proopiomelanocortin (POMC). This is further processed in the pituitary to yield several extremely important hormones, including the major stress-responsive hormone in the mammalian species, adrenocorticotropin hormone (ACTH), as well as beta-endorphin, the longest (31 amino acids) and longest acting (over 30 minutes in humans) endogenous opioid, and which is both peripherally active, as well as active in the central nervous system. Other very important neuropeptides are processed from the single gene product of POMC. These include alpha-, beta- and gamma-melanotropin (melanocyte-stimulating hormone or MSH), as well as other neuropeptides. Some of these have been closely linked to appetitive behaviors, and thus, feeding disorders.
Figure 1. Hypothalamic-Pituitary-Adrenal Axis.
Stress causes increased mRNA synthesis and release of hypothalamic CRF and AVP into the portal circulation, which acts on CRF-R1 receptors and V1b receptor in the anterior pituitary, respectively. This induces synthesis of POMC mRNA and peptide in the corticotrope of the anterior pituitary and release into the circulation of beta-endorphin and ACTH, which are derived from processing of POMC. ACTH acts on ACTH receptor (MC2R) in the adrenal cortex and induces release of the stress hormone cortisol (in humans and guinea pigs) or corticosterone (in rats and mice), which are primary mediators of the stress response. Cortisol or corticosterone exerts negative feedback regulation at both the hypothalamus and the pituitary to inhibit the synthesis of POMC and release of ACTH and beta-endorphin via glucocorticoid receptors (GR). In addition to this classical circadian negative feedback regulation by glucocorticoids, the endogenous opioid system, especially the mu-opioid receptors (MOP-r), tonically inhibits this axis.
The focus of this review article is to be on the role of stress responsivity in the impact of drugs of abuse and the implications of those effects on stress responsivity in the development of and perpetuation of specific addictive diseases.
ACTH acts primarily on the adrenal cortex where it brings about the processing and release of all the enzymes in pathways leading to the production of cortisol in humans, non-human primates and guinea pigs, and also corticosterone in rats and mice. In turn, those glucocorticoids, in addition to acting at diffuse sites of the body to assure overall response to stress, including sugar and fat mobilization and metabolism, also act in a negative feedback mode by decreasing production and release of CRF in the hypothalamus and also act directly in the anterior pituitary, bringing about reduction of processing and release of POMC and its neuropeptides. Studies of many other groups have shown that the glucocorticoids act in the hippocampus, where the functional relationships to stress responsivity are less clearly delineated, but seem to be closely associated with reduction in hippocampal size when there is chronic exposure to excessive levels of glucocorticoids and thus to a decrement in learning and memory (see reviews McEwen, 1980; McEwen et al., 1992; Kreek, 1996a; Kreek, 1996b; Kreek, 1997; Kreek and Koob, 1998; Kreek, 2000; Kreek et al., 2002; Kreek et al., 2004; Kreek et al., 2009). In addition to the important negative feedback control of the HPA axis by the glucocorticoids, it has been found that mu-opioid receptor activation by beta-endorphin, or possibly one of the mu-opioid receptor-directed enkephalins, apparently tonically inhibits both production of CRF in the hypothalamus and POMC peptides in the anterior pituitary (Kreek, 1973a; Volavka et al., 1980; Kreek et al., 1983a; Kosten et al., 1986a; Kosten et al., 1986b; Culpepper-Morgan et al., 1992; Culpepper-Morgan and Kreek, 1997; Schluger et al., 1998; Farren et al., 1999; Rosen et al., 1999). It is not known whether this opioid inhibition of CRF and POMC occurs in other parts of the brain. However, negative inhibition of CRF and POMC by glucocorticoids in other regions of the brain has been studied to a modest extent, and in these other regions of gene expression there does not seem to be negative feedback modulation by the glucocorticoid system. All of these neuroendocrine systems discussed in these four paragraphs have been extensively reviewed in textbooks of endocrinology, and do not necessarily represent the work from our laboratory, although we have included comments from our laboratory, when particularly pertinent, such as our contribution of discovering the opioid control of the human HPA stress-responsive axis, which we will not detail further in this review.
Both CRF and its CRF-R1 receptor (as well as the CRF-R2 receptor, which has as its primary ligands the urocortins, which will not be further detailed in this brief review) are located in other parts of the brain beyond the hypothalamus (see Table 1). Regions which are of particular interest to our group, prefrontal cortex and amygdala, are brain regions in which the genes of the CRF and CRF-R1 are expressed (measurable mRNA levels) (see Table 1). Also, gene expression of POMC has been identified in several brain regions in addition to the anterior pituitary. However, the use of very modern extended-range mass-spectrometric analyses to determine the precise processing of the single gene product, POMC, to specific neuropeptides in different brain regions has not been extensively pursued.
Table 1.
Localization of stress responsive gene mRNAs in specific brain regions, pituitary and adrenal cortex of adult rodents.
| FCx | SN | CPu | VTA | NAc | Amy | Tha | Hip | Hypo | AP | AC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MOP-r | + | +a | + | + | + | + | + | + | + | + | − |
| CRF | + | + | + | + | + | ||||||
| CRF-R1 | + | + | + | + | + | + | +b | ||||
| AVP | + | + | |||||||||
| V1b R | + | − | + | − | + | + | + | + | + | + | |
| POMC | + | + | + | + | + | + | +c | ||||
| MC2R | +d | +d | |||||||||
| Orexin | + | ||||||||||
| OX1 R | +f | +f | +f | +f | +e | +e | |||||
| OX2 R | +f | +f | +f | +e | +e |
MOP-r, mu opioid receptor; CRF, corticotropin-releasing factor; CRF-R1, CRF type 1 receptor; AVP, arginine vasopressin; V1b R, AVP type 1b receptor; POMC; proopiomelanocortin; MC2R, ACTH receptor; OX1 R, orexin type 1 receptor; OX2 R, orexin type 2 receptor.
FCx, frontal cortex; SN, substantia nigra; CPu, caudate-putamen; VTA, ventral tegmental area; NAc, nucleus accumbens; Amy, amygdala; Tha, thalamus; Hip, hippocampus; Hypo, hypothalamus; AP, anterior pituitary; AC, adrenal cortex.
+, positive; −, negative; no notation, no published report found of any attempt to determine expression of these genes (mRNA levels) in brain region or peripheral tissues of adult rodents.
Trivedi et al., 1998. All other data from Zhou et al. 1996a, b and unpublished data.
Another neuropeptide system, the vasopressin system, was identified years ago but only modestly studied over many years; it is also known to directly impact upon the HPA axis in humans. Both early work and more recent work from our Laboratory, and from other scientists in our Center, have shown that this neuropeptide system, the vasopressin-vasopressin receptor system, is profoundly altered by morphine and other opiates in both rodent models and in humans. Much more recently our group has initiated an increased focus on the impact of drugs of abuse, such as morphine, heroin and cocaine, on vasopressin and on the effects of abrupt withdrawal from these compounds on the vasopressin-vasopressin receptor system. Vasopressin is produced primarily in the hypothalamus and acts at the anterior pituitary at a vasopressin receptor (V1b) to bring about the processing of release of POMC. Therefore, vasopressin and its receptor seem to act in parallel with CRF and its CRF-R1 receptor. Very limited studies have been conducted in humans to assess the relative extent of each of these two regulatory components on HPA axis stress-responsive activation. Further, more rigorous basic clinical research studies need to be conducted, utilizing the more modern technology of neuropeptide radioimmunoassays. There is also a peripheral receptor to vasopressin (V2 receptor). Action at this receptor is directly involved in the regulation of elimination and conservation of water at the renal tubule levels. Different brain and anterior pituitary localizations of gene expression of vasopressin and its V1b receptors have been identified (see Table 1).
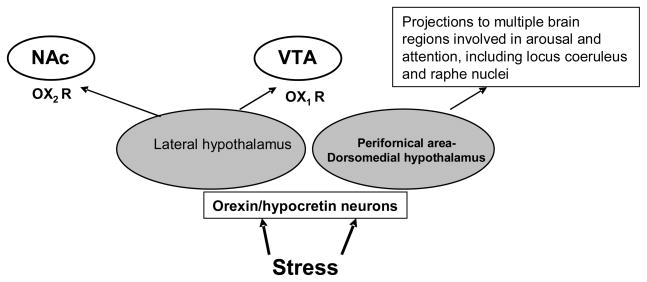
A third stress-responsive system is orexin-hypocretin. Orexin A and B refer to peptides acting on Orexin 1 and 2 receptors (see Figure 2). To date, there is no evidence that orexin-hypocretin acting at its receptors has any direct relationship with or regulation of the HPA axis, as defined above, or the vasopressin system, which in turn also may activate the HPA system. There is, however, extensive evidence that orexin-hypocretin has actions in some of the rewarding areas of the brain, such as the nucleus accumbens and ventral tegmental area (VTA), as well as areas, such as the amygdala, which are involved in fear, emotion, mood, and affect. The various sites in the brain and outside the brain of orexin and orexin receptor gene expressions have been identified (see Table 1).
Figure 2.
Orexin/hypocretin neurons in the lateral hypothalamus (LH) or in the perifornical area or dorsomedial hypothalamus (PFA–DMH) have distinct functions in response to stress. LH orexin neurons project to the nucleus accumbens (NAc) and ventral tegmental area (VTA) and modulate rewarding effects of a drug of abuse through orexin type 1 and type 2 receptors (OX1R and OX2R). The LH orexin neurons are also activated by drug withdrawal stress that may contribute to negative affective states in drug withdrawal. Orexin neurons in PFA–DMH are activated to involve in multiple responses including arousal.
Cocaine, by blocking the presynaptic re-uptake transporters of dopamine, causes the flooding of the extracellular fluid, with dopamine in both the dorsal and ventral striatum (e.g., Maisonneuve et al., 1995), with a resultant increased activation of dopamine D1-like as well as dopamine D2-like receptors in the ventral striatum, including especially the nucleus accumbens and also in the dorsal striatum, including the caudate-putamen. Dopaminergic neurons from the VTA with projections lead to dopamine release in the ventral striatum, and dopamine neurons in the substantia nigra (SN) lead to release of dopamine in the caudate-putamen. However, it should be appreciated at all times that the actual levels of dopamine in extracellular fluid are much higher in the dorsal striatum (caudate-putamen) than in the ventral striatum (nucleus accumbens). Nevertheless, the changes in dopamine tone in the ventral striatum have been related to the rewarding effects of drugs of abuse, as well as to the anticipation of their use, and the locomotor response elicited after their use.
Studies from our laboratory have shown that blockage of D1-like and D2-like dopamine receptors by use of selective dopamine receptor antagonists significantly attenuates the cocaine-induced activation of the HPA axis hormones (Zhou et al., 2004). Further, we have shown that in DARPP-32 knockout mice, where there is attenuation of primarily the dopamine D1-like signal transduction mechanisms, again, there is an attenuated HPA axis response to cocaine (Zhou et al., 1999b). These findings suggest that there may be direct connections between the striatum, and particularly the ventral striatum, with the hypothalamic sites of HPA axis stress-responsive initiation.
In our earliest studies of opiate addiction, we addressed the need to develop a medication to treat heroin addiction, research initiated by Dole, Nyswander and Kreek in 1964 at The Rockefeller Institute for Medical Research (Dole et al., 1966). These studies led promptly to prospective studies of the safety, as well as efficacy, and physiological effects of long-acting methadone, as contrasted with the short-acting opiates, such as heroin and morphine. We were struck with the impression that heroin addiction has many of the stigmata of a neuroendocrine disorder, that is, like the result of “a single error in metabolism.” In this case, we found exposure to a single drug brings about profound changes, including endocrine changes, regulated by the brain and controlling many of the critical functions for survival and reproduction (Kreek, 1973a; Kreek, 1978). We therefore built into our initial studies a limited set of then possible assays to be conducted in humans in our clinical research (Kreek, 1973a; Kreek, 1978). What we found was that the HPA axis is profoundly disrupted during cycles of heroin addiction and becomes normalized during long-term methadone maintenance treatment (Kreek 1973a; Kreek, 1978; Kreek et al., 2002). Further studies from our laboratory, as well as research from others, have shown that chronic exposure to cocaine and alcohol also profoundly disrupt the HPA axis (Kreek et al., 2002; Kreek et al., 2004; Kreek et al., 2009).
In our more recent studies, with our first publication in 1998 (Bond et al.,1998), we have shown that a single gene variant of the mu-opioid receptor gene, which we determined to be highly functional (see below), alters stress responsivity and, in fact, is associated not only with changes in stress-responsive systems in healthy human beings, but also is strongly associated in populations with limited admixture with the specific addictive diseases of opiate addiction (Bart et al., 2004; Bart et al., 2005). In this brief review, we will detail some of the pertinent findings in laboratory-based research in animal models and human molecular genetics research primarily from our own laboratory.
Most of the studies in our laboratory, like most laboratories, unfortunately have been conducted almost exclusively in male rats and male mice. However, we have conducted one extremely interesting series of studies in female rats with respect to the impact of gender and female hormones on cocaine-induced behaviors and, conversely, on cocaine effects on one important female sex hormone. Since our work suggests that all of cocaine effects intrinsically involve directly or are interactive with the stress-responsive systems, we will briefly summarize these findings in female rats. We have found that there are significant differences across the estrous cycle in cocaine-induced stereotypic and locomotor behaviors in Fischer female rats (Quiñones-Jenab et al., 1999). Further, we have found that in ovariectomized female rats there are significant effects of ovarian hormone replacement (including estrogen, progesterone, or estrogen plus progesterone-replaced female rats (Quiñones-Jenab et al., 2000a). We have also found that cocaine itself significantly affects progesterone plasma levels in female rats (Quiñones-Jenab et al., 2000b). However, since we have not pursued directly the impact of the female hormones on stress responsivity in the setting of cocaine or other chronic drug of abuse administration, we have not included these studies in any further discussion in this brief review paper.
1. Alterations of CRF and POMC Gene Expression in the HPA Axis and Other Specific Brain Regions
Opioids are important in the control of the secretion of HPA axis stress hormones in rodents as well as in humans. In the rat, acute morphine administration (1 or 2 days) results in increased ACTH and corticosterone secretion, while animals treated on a chronic basis (more than 5 days) with morphine show attenuation of the morphine-induced ACTH and corticoste rone response, with the development of tolerance by 5 days of morphine treatment (Ignar and Kuhn, 1990; Zhou et al., 2006) (Table 2). In humans, in sharp contrast, both acute and chronic morphine administration inhibits the HPA axis. For instance, basal levels of ACTH and cortisol are significantly disrupted in active heroin addicts with suppression of ACTH and cortisol and abnormal diurnal rhythms when heroin or morphine are present in the body, and with activation of the stress-responsive axis in heroin or morphine withdrawal in tolerant and physically-dependent humans and animals (Kreek, 1973a; Cushman and Kreek, 1974; Kreek, 1978). The effects of morphine on HPA-axis activity also depend on the presence or absence of stress. For example, we examined the effect of acute intermittent morphine on pituitary–adrenal function in the rat under the mild stress condition of water restriction (Zhou et al., 1999a). Either acute morphine or water restriction alone increased ACTH secretion as an independent stimulus. When the animals received both morphine and water-restriction stress, as two combined stimuli, however, there was no increase in plasma ACTH levels. Thus, morphine effectively blunts the classical HPA-axis activity caused by this water restriction stressor, probably indicating that opioids play a counter-regulatory role on the stress response by inhibiting the stress response cascade.
Table 2.
Effects of a single morphine injection (10 mg/kg) (A) or chronic intermittent escalating-dose morphine administration (from 3 × 2.5 mg/kg/day at 6-hour intervals on day 1 up to 3 × 40 mg/kg/day on day 10) for 10 days and following its12-hour acute spontaneous withdrawal (B) on mRNA levels (attomole/μg RNA) of MOP-r, POMC, orexin, ppDyn or CRH-R1 in the lateral or medial hypothalamus (Hypo), amygdale (Amy), caudate-putamen (CPu), nucleus accumbens (NAc) core, anterior pituitary (AP), and on plasma levels of ACTH (pg/ml), corticosterone (CORT) (ng/ml), luteinizing hormone (LH) (ng/ml) and testosterone (TEST) (ng/ml).
| A. Quantitative mRNA levels (attomole/μg RNA) in specific brain regions and plasma hormonal levels (pg/ml for ACTH or ng/ml for other) 30 min after a single morphine injection (10 mg/kg). | |||
|---|---|---|---|
| Saline Control | Single Morphine | ||
| Lateral Hypo | MOP-r | 0.30 ± 0.03 | 0.28 ± 0.04 |
| orexin | 5.1 ± 0.42 | 4.8 ± 0.34 | |
| ppDyn | 5.82 ± 0.31 | 6.00 ± 0.25 | |
| Medial Hypo | POMC | 14 ± 1.7 | 11 ± 1.8 |
| Amy | MOP-r | 0.17 ± 0.02 | 0.20 ± 0.01 |
| POMC | 2.0 ± 0.38 | 1.7 ± 0.28 | |
| CPu | MOP-r | 0.13 ± 0.02 | 0.15 ± 0.01 |
| NAc core | MOP-r | 0.28 ± 0.03 | 0.31 ± 0.02 |
| AP | POMC | 720 ± 42 | 693 ± 45 |
| CRH-R1 | 0.95 ± 0.07 | 0.93 ± 0.08 | |
| Plasma | ACTH | 193 ± 35 | ↑ 380 ± 90* |
| CORT | 234 ± 36 | ↑ 398 ± 36* | |
| LH | 0.25 ± 0.04 | 0.26 ± 0.04 | |
| TEST | 0.87 ± 0.20 | 0.54 ± 0.14 | |
| B. Quantitative mRNA levels (attomole/μg RNA) in specific brain regions and plasma hormonal levels (pg/ml for ACTH or ng/ml for other) 30 min after last morphine injection (40 mg/kg) following chronic intermittent escalating-dose administration (Chronic Morphine group) or 12 hour after last morphine injection as spontaneous withdrawal (Acute Withdrawal group). | ||||
|---|---|---|---|---|
| Saline Control | Chronic Morphine | Acute Withdrawal | ||
| Lateral Hypo | MOP-r | 0.31 ± 0.02 | 0.23 ± 0.01 | ↑0.51 ± 0.07 ** |
| orexin | 5.4 ± 0.56 | 4.0 ± 0.30 | ↑ 8.9 ± 0.22 ** | |
| ppDyn | 6.02 ± 0.37 | 5.65 ± 0.48 | 6.63 ± 0.48 | |
| Medial Hypo | POMC | 14.0 ± 1.0 | 12.7 ± 1.3 | 11.7 ± 1.6 |
| Amy | MOP-r | 0.18 ± 0.01 | 0.17 ± 0.02 | 0.21 ± 0.02 |
| POMC | 2.0 ± 0.19 | 1.8 ± 0.19 | 2.0 ± 0.36 | |
| CPu | MOP-r | 0.16 ± 0.01 | 0.17 ± 0.01 | ↑ 0.21 ± 0.01* |
| NAc core | MOP-r | 0.29 ± 0.03 | 0.21 ± 0.03 | ↑ 0.46 ± 0.07* |
| AP | POMC | 724 ± 35 | 844 ± 96 | ↑ 1073 ± 93** |
| CRH-R1 | 1.17 ± 0.12 | 0.97 ± 0.05 | ↑ 1.51 ± 0.08* | |
| Plasma | ACTH | 203 ± 21 | 188 ± 50 | ↑ 583 ± 40** |
| CORT | 145 ± 26 | 75 ± 13 | 141 ± 25 | |
| LH | 0.19 ± 0.02 | 0.16 ± 0.01 | ↑ 0.26 ± 0.04* | |
| TEST | 0.92 ± 0.13 | ↓ 0.10 ± 0.02** | ↓ 0.31 ± 0.13** | |
Note:↑, significant increase; ↓, significant decrease.
p<0.05,
p<0.01 vs. Saline Control. Data published earlier in graphic form [Zhou et al., 2006].
Methadone (a selective mu-opioid receptor agonist, long-acting in humans) is widely used in the treatment of short-acting opiate (primarily heroin) addiction as well as for the management of chronic pain (Kreek, 1973b). Methadone has a short half-life in rodents, about 60 min in mice and 90 min in rats (Kreek, 1979; Burstein et al., 1980). In several of our animal studies (e.g., Zhou et al., 1996a), therefore, methadone is delivered through osmotic pumps to mimic steady-state methadone maintenance in humans (Kreek, 1973b). In a very early study from our group, we found that chronic (36 days) methadone administration did not alter concentrations of immunoreactive beta-endorphin in the rat amygdala and hypothalamus (Ragavan et al., 1983). The steady-state administration of methadone in rats (10 mg/kg/day) by osmotic pumps achieved a mean plasma level of 123 ng/ml with a range from 100–150 ng/ml (Zhou et al., 1996a), which is comparable to levels achieved and sustained at 24 h after last administration during chronic methadone maintenance treatment at lower doses in humans (60–80 mg/day). On daily oral doses of 60–120 mg/day, we found a range of plasma methadone levels of 74–732 ng/ml in humans (Borg et al., 1995). In this rat model, we did not find any effect of steady-state methadone treatment on the mRNA levels of hypothalamic CRF and POMC, anterior pituitary CRF-R1 and POMC, or circulating corticosterone levels (Zhou et al., 1996a). This demonstrates that steady-state occupancy of mu-opioid receptor with methadone does not have any significant effect on the rat CRF/CRF-R1 or POMC system. Our results support the hypothesis (Kreek, 1973a; Kreek et al., 1981) that there is no disruption of HPA axis activity during steady-state administration of the exogenous opioid methadone (Kreek et al., 1983b).
Recent human studies have demonstrated that severe or unavoidable psychological stressors elevate cocaine and alcohol craving and HPA axis activity, and the HPA responses, in part, predict subsequent drug relapse (Sinha et al., 2006; Brady et al., 2009). In contrast, modest activation of the HPA axis by specific mu-opioid receptor antagonist naltrexone administration reduces alcohol craving and alcohol consumption (O’Malley et al., 2004). Stress-related anxiety and depression are major psychiatric consequences of chronic drug abuse, especially during withdrawal (Koob and Kreek, 2007). For instance, chronic cocaine and its withdrawal dynamically alter HPA activity and expression levels of stress-responsive genes in some specific brain regions of the rat (Table 3) (Zhou et al., 1996b; Zhou et al., 2003a, b). The HPA activation by either acute or chronic “binge” cocaine is apparently mediated through the dopamine receptors (Borowsky and Kuhn, 1991; Spangler et al., 1997) and their intracellular signal pathways (Zhou et al.,1999b). We further found that the cocaine-induced CRF mRNA increase was absent in the rat pretreated with either a selective D1-like or D2-like dopamine receptor antagonist, suggesting that cocaine-induced stimulation of hypothalamic CRF gene expression is secondary to changes in the activity of specific components of dopaminergic systems (Zhou et al., 1996b; Zhou et al., 2004).
Table 3.
Effects of chronic “binge” pattern cocaine administration (3 × 15 mg/kg/day i.p. at hourly intervals) up to 14 days (A) and following its 10-day withdrawal (B) on plasma ACTH (pg/ml) and corticosterone (CORT) (ng/ml) levels and on POMC, CRF, and CRF-R1 mRNA levels (pg/μg total RNA) in the hypothalamus (Hypo), anterior pituitary (AP), frontal cortex (FCx) and amygdala (Amy) of the rat.
| A. Plasma hormonal levels (pg/ml for ACTH and ng/ml for CORT) and quantitative mRNA levels (attomole/μg RNA) in specific brain regions 30 min after last “binge” cocaine injection (15 mg/kg) | ||||
|---|---|---|---|---|
| Saline Control | 1-day Cocaine | 3-day Cocaine | 14-day Cocaine | |
| ACTH | 17 ± 4 | 60 ± 10 | ↑145 ± 27* | 50 ± 20# |
| CORT | 10 ± 2 | ↑55 ± 7* | ↑90 ± 16* | ↑47 ± 11# |
| CRF in Hypo | 0.39 ± 0.03 | ↑1.10 ± 0.20* | 0.34 ± 0.03 | ↓0.26±0.02* |
| POMC in Hypo | 32.3 ± 3.5 | ↓26.0 ± 2.0 | 29.0 ± 1.2 | 33.0 ± 1.5 |
| CRF-R1 in AP | 0.79 ± 0.03 | 0.74 ± 0.05 | 0.85 ± 0.04 | ↑0.96±0.05* |
| POMC in AP | 260 ± 15 | 270 ± 12 | 310 ± 22 | ↑350 ± 25* |
| CRF in FCx | 0.21 ± 0.01 | 0.22 ± 0.02 | ↑0.29±0.02* | 0.24 ± 0.02 |
| CRF in Amy | 0.057±0.003 | ↑0.119±0.026* | 0.063±0.003 | 0.063±0.002 |
| B. Plasma hormonal levels (pg/ml for ACTH and ng/ml for CORT) and quantitative mRNA levels (attomole/μg RNA) in specific brain regions after 1 or 2 days (Acute Withdrawal group) or after 10 days (Chronic Withdrawal group) from chronic “binge” cocaine administration for 14 days. | ||||
|---|---|---|---|---|
| Acute Withdrawal Saline Control | Acute Withdrawal | Chronic Withdrawal Saline Control | Chronic Withdrawal | |
| ACTH | 50 ± 6 | ↑138 ± 39*a | 33 ± 13 | 62 ± 19 |
| CORT | 4.9 ± 0.7 | ↑14.0 ± 3.9*a | 7.0 ± 1.7 | 6.2 ± 1.3 |
| CRF in Hypo | 0.33 ± 0.06 | 0.34 ± 0.04b | 0.29 ± 0.02 | 0.27 ± 0.02 |
| POMC in Hypo | 33 ± 4.0 | 42 ± 5.0a | 31 ± 2.0 | 30 ± 3.0 |
| CRF-R1 in AP | 0.85 ± 0.08 | 1.13 ± 0.25a | 0.59 ± 0.07 | 0.70 ± 0.10 |
| POMC in AP | 213 ± 18 | 307 ± 62a | 182 ± 15 | 183 ± 7 |
| CRF in FCx | 0.22±0.01 | 0.25±0.02b | 0.22 ± 0.01 | 0.20 ± 0.05 |
| CRF in Amy | 0.055±0.003 | ↑0.069±0.004*b | 0.051±0.008 | 0.043±0.002 |
Note: ↑, significant increase; ↓, significant decrease;
p<0.05 vs. Saline Control,
p<0.05 vs 3-day cocaine. Data published earlier in graphic form [Zhou et al., 1996a, 2003a, b].
1 day withdrawal;
2 day withdrawal
2. Involvement of Arginine Vasopressin and V1b Receptor in Drug Withdrawal and Heroin Seeking Precipitated by Stress
A growing body of evidence suggests that vasopressinergic neuronal activity in the amygdala and hypothalamus represents an important element in the neurobiology of stress-related behaviors in rodent models (Griebel et al., 2002; Wigger et al., 2004). Our laboratory has recently begun to explore the role of arginine vasopressin (AVP) in drug addiction, by examining AVP gene expression in the rat amygdala and hypothalamus after chronic intermittent escalating-dose heroin or during early and late spontaneous withdrawal. We found that amygdalar AVP mRNA levels were increased during early heroin withdrawal only. Of note, this heroin withdrawal-induced AVP mRNA increase was replicated in a separate study after early spontaneous morphine withdrawal (Zhou et al., 2008b).
In collaboration with Leri of the University of Guelph in Canada, we found that foot shock stress increased AVP mRNA level in the amygdala of rats withdrawn from heroin self-administration, but not in heroin-naive rats, suggesting that AVP and its receptors may be involved in stress-induced reinstatement of heroin-seeking behavior (an animal model for studying drug relapse in humans). There are three subtypes of AVP receptors: V1a, V1b, and V2. While V2 is located in the kidney and mediates the antidiuretic action, V1a and V1b are expressed in extended amygdala (see Table 1). We went on to investigate whether the blockade of central AVP receptors (V1a or V1b receptor) would alter heroin-seeking during tests of reinstatement induced by foot shock stress and by heroin primes, and HPA-axis hormonal responses to foot shock. The selective V1b receptor antagonist SSR149415 (but not V1a antagonist) dose-dependently attenuated foot shock-induced reinstatement, blocked heroin-induced reinstatement, and blunted the HPA-axis activation by foot shock. In another study, we also found an increase in AVP mRNA levels in the amygdala after acute withdrawal from chronic cocaine exposure (Zhou et al., 2005). In conclusion, these data in rats together suggest that the stress-responsive AVP/V1b receptor system (including the amygdala) may be critical components of the neural circuitry underlying the aversive emotional consequences of drug withdrawal, and the effect of negative emotional states on drug-seeking behavior (Zhou et al., 2008b). It may be worthwhile to explore the effectiveness of systemic V1b receptor antagonists for the management of withdrawal and for the prevention of drug relapse to opiates, alcohol or other drugs of abuse.
3. Alterations of Orexin/Hypocretin and Preprodynorphin Gene Expression in the Lateral Hypothalamus in Animal Models of Opiate or Cocaine Dependence after Chronic Intermittent Escalating-Dose Administration
The orexins/hypocretins are neuropeptides mainly expressed in cells of the lateral hypothalamus, perifornical area and dorsomedial hypothalamus, with extensive CNS projections (de Lecea et al., 1998). It has been established that hypothalamic orexins/hypocretins (orexin A acting at both orexin type 1 and 2 receptors [OX1R and OX2R], while orexin B acts only on OX2R) are involved in the regulation of sleep–wakefulness, arousal, feeding and stress. A growing body of evidence suggests that orexins may have an important role in the modulation of drug reward and drug-seeking behaviors (Fig. 2). Studies of acute morphine administration have shown an attenuated increase in extracellular dopamine levels in the nucleus accumbens by orexin receptor blockade in the VTA (Narita et al., 2006). Orexin A in the VTA has been found to be critical for the development of cocaine-induced synaptic plasticity and behavioral sensitization (Borgland et al., 2006). Further, the interaction between orexins and their receptors has been found to underlie motivated behaviors (Boutrel et al., 2005; Harris et al., 2005). It has been reported that i.c.v. infusion of orexin A (hypocretin-1) decreases brain reward function, as circuitry (Boutrel et al., 2005).
Our laboratory has recently investigated the modulation of orexin/hypocretin gene expression in animal models of drug addiction. Most of the lateral hypothalamic orexin neurons express the preprodynorphin gene (Chou et al., 2001). Therefore, we examined levels of the preprodynorphin and orexin mRNAs in the lateral hypothalamus. During the aversive state of acute withdrawal from chronic intermittent escalating-dose morphine, we found increased orexin mRNA levels in rat lateral hypothalamus, supporting our hypothesis that enhanced lateral hypothalamic orexin neuronal activity, resulting from its increased gene expression, contributes to negative affective states in opiate withdrawal (Table 2B). Under this withdrawal-related stress condition, there was no change in the lateral hypothalamic preprodynorphin mRNA levels (Zhou et al., 2006).
To extend our research to the effects of cocaine, we further determined whether orexin reflected by an elevated intracranial self-stimulation threshold in the lateral hypothalamus, further supporting the hypothesis that orexin negatively regulates the activity of brain reward and preprodynorphin mRNA levels in rat lateral hypothalamus or medial hypothalamus (including perifornical and dorsomedial areas) are altered following (1) cocaine rewarding process using cocaine conditioned place preference (CPP); and (2) chronic cocaine exposure using both “binge”-pattern administration in steady-dose (45 mg/kg/day) and escalating-dose (45 to 90 mg/kg/day) regimens (Zhou et al., 2008a). We found that orexin mRNA levels in the the lateral hypothalamus were decreased after cocaine place conditioning. A decreased lateral hypothalamic orexin mRNA level was also observed after chronic escalating-dose cocaine (but not CPP pattern regimen without conditioning, or steady-dose regimen). Furthermore, acute withdrawal from chronic escalating-dose cocaine administration resulted in increases in both the lateral hypothalamic orexin and preprodynorphin mRNA levels, which were unaltered by naloxone or after chronic withdrawal. Together, our results suggest that (1) alteration of the lateral hypothalamic orexin gene expression is region-specific after cocaine place conditioning and dose-dependent after chronic exposure; and (2) increased orexin and preprodynorphin gene expressions in the lateral hypothalamus may contribute to negative affective states in cocaine withdrawal. Therefore, the orexin system may be a target for therapeutic interventions.
In addition, the preprodynorphin/kappa-opioid receptor system in the nucleus accumbens and caudate-putamen has been implicated in regulating the behavioral effects of cocaine by playing a homeostatis role that opposes dopaminergic alterations (Spangler et al., 1993; Koob and Kreek, 2007). In our recent study using tissue plasminogen activator (tPA) deficient mice, we found increased basal preprodynorphin mRNA and Homer 1a protein levels in the nucleus accumbens, and the tPA-deficient mice showed the lack of behavioral sensitization to repeated “binge” cocaine and cocaine rewarding effect (as measured by CPP) (Maiya et al., 2009). It has been demonstrated that acute pretreatment with Dyn A(1-17) or kappa-opioid receptor agonists is effective in decreasing mesoaccumbal DA neurotransmission and cocaine reward (Spanagel et al., 1992; Zhang et al., 2004; Shippenberg et al., 2007). On the basis of the above evidence, we hypothesize that increased basal levels of endogenous preprodynorphin gene expression lead to altered (lowered) dopaminergic signaling in the nucleus accumbens and blunted cocaine-related rewarding behavior and sensitization (Maiya et al., 2009).
4. Involvement of Mu-Opioid Receptor in Animal Models of Cocaine Seeking
Methadone maintenance at appropriate doses may be effective in reducing cocaine abuse in heroin-dependent individuals. There is a growing body of evidence, in fact, that at appropriate doses, methadone maintenance can decrease cocaine use in heroin–cocaine co-users. Borg et al. (2002) found that 69% of patients stopped regular cocaine abuse when maintained on a mean methadone dose of 67 mg/day. Similarly, in a clinical trial by Strain et al. (1994), it was found that rates of cocaine use were lower among patients maintained on a mean of 50 mg/day (53%) compared to those maintained on a mean of 20 mg/day (62%). Stine et al. (1991) developed a contingency treatment program in which the patient’s methadone dose increased by 5 mg in response to each cocaine-positive urine screen, up to a maximum dose of 120 mg/day. Under these conditions, when the methadone dose achieved a mean of 115 mg/day, cocaine-positive urine samples decreased by 89%. Schottenfeld et al. (2005) reported that methadone maintenance (65–85 mg/day), without formal contingency management, promoted retention in treatment, significantly reduced both opioid and cocaine use, and enhanced length of abstinence from both drugs. A recent study by Peles et al. (2006) reported that 69% of patients stopped cocaine use after 1 year of methadone maintenance treatment when mean stabilized doses of methadone reached 176 mg/day.
Higher doses of methadone may be required in patients who display severe cocaine abuse because cocaine exposure increases mu-opioid receptors in specific brain regions. In our early and recent animal studies, we have found that there is significant increase in mu-opioid receptor density after chronic (14 days) binge-pattern cocaine administration, which is found in both the mesocorticolimbic and nigrostriatal dopaminergic systems, including the nucleus accumbens, amygdala and anterior cingulate, and caudate-putamen (Unterwald et al., 1994). After acute cocaine exposure (1 or 5 days), an increase in mu-opioid receptor mRNA levels has been found in the same, specific brain regions (Azaryan et al., 1996; Yuferov et al., 1999). Using positron emission tomography (PET) technology, up-regulation of mu-opioid receptor binding has been observed in cocaine-dependent individuals, and it has been associated with cocaine craving (Zubieta et al., 1996; Gorelick et al., 2005; Gorelick et al., 2008). We have shown that there is a relative endorphin deficiency in cocaine addicts and also in chronically cocaine-abusing, methadone-maintained former heroin addicts, just as we showed years ago that there is a persistent endorphin deficiency in medication-free, illicit-heroin-free, former heroin addicts (Kreek et al., 1984; Borg et al., 1995; Kreek, 1996a; Schluger et al., 2001).
In collaboration with Leri, we investigated the effect of high-dose steady-state methadone on cocaine CPP and cocaine intravenous self-administration in the rodent models for cocaine-taking and -seeking behaviors. We found that (1) rats implanted with osmotic pumps delivering steady-state methadone prior to cocaine conditioning did not express cocaine CPP (Leri et al., 2006); (2) rats with steady-state methadone after cocaine conditioning displayed neither spontaneous nor cocaine-precipitated CPP (Leri et al., 2009); and (3) but steady-state methadone did not alter the intravenous self-administration (continuous schedule of reinforcement) of various doses of cocaine (Leri et al., 2006). Further, mu-opioid receptor mRNA levels in the nucleus accumbens core were significantly elevated in rats after exposure to cocaine conditioning. However, the up-regulation of mu-opioid receptor mRNA levels was reduced by steady-state methadone in a dose-dependent manner (Leri et al., 2006; Leri et al., 2009). In conclusion, our results suggest that high-dose steady-state methadone does not alter the direct reinforcing effect of cocaine but blocks spontaneous and cocaine-precipitated cocaine seeking, possibly by preventing mu-opioid receptor alterations in the nucleus accumbens core induced by cocaine conditioning (Leri et al., 2006; 2009). Our results in animal studies parallel and support the clinical findings in former heroin- and cocaine-co-dependent individuals maintained on high-dose methadone who consume less cocaine.
However, we have not found any cocaine-induced changes of POMC gene expression or one of its products, beta-endorphin, which is the longest of the endogenous opioid peptides, that normally acts at mu-opioid receptors. Therefore with increased mu-opioid receptors but with no increase in beta-endorphin, a relative “endorphin deficiency” develops.
We investigated potential roles that dopamine D1-like or D2-like receptors could play in regulation of POMC gene expression in the hypothalamus (major beta-endorphin neurons in the CNS) in response to acute “binge” cocaine. We found that the dopamine D2-like receptor blockade by a selective antagonist sulpiride increased POMC mRNA levels in the hypothalamus, indicating that the dopamine D2-like receptor exerts a tonic inhibitory effect on hypothalamic POMC gene expression. The POMC mRNA increases induced by the dopamine D2-like receptor blockade were attenuated by acute “binge” cocaine. In contrast to the dopamine D2-like receptor, the dopamine D1-like receptor blockade by a selective antagonist SCH23390, acute “binge” cocaine or their combination had no effect on hypothalamic POMC mRNA levels. These results suggest a specific role for the dopamine D2-like receptor in acute cocaine’s effects on hypothalamic POMC gene expression (Zhou et al., 2004).
5. Alterations of Mu-Opioid Receptor and POMC Gene Expression in Animal Models of Opiate Dependence after Chronic Intermittent Escalating-Dose Administration
Many different studies have examined the effect of chronic opioid agonist treatment and also opiate withdrawal precipitated by opioid antagonists on mu-opioid receptor mRNA levels in different brain regions. The results obtained are conflicting; a decrease, an increase, or no changes in mu-opioid receptor mRNA levels have been reported, and these apparently contradictory results may depend on differences in the dose, route and mode of the opioid agonist administered, exposure time, and the brain regions examined. In many previous studies, morphine or other short-acting mu-opioid agonists were chronically administered by pellets. This differs from heroin addicts who use an intermittent pattern of self-administration, in which they experience both rewarding effects and chronic stress induced by repeated heroin injection and withdrawal (Kreek and Koob, 1998). Therefore, an administration paradigm of chronic intermittent escalating-dose morphine and heroin has been developed in our laboratory in order to mimic the multiple and escalating doses that human heroin abusers seek daily to achieve rewarding effects and suffer the symptoms of withdrawal during between-dose intervals. As reviewed by Kreek and Koob (Kreek and Koob, 1998; Koob and Kreek, 2007), endogenous opioidergic systems, especially POMC-derived beta-endorphin, exert inhibitory effects on the HPA axis in both humans and rodents. Furthermore, the lateral portion of the hypothalamus is an important brain region for reward and other motivated behaviors. However, the question of whether morphine withdrawal influences mu-opioid receptor gene expression in the lateral hypothalamus has not yet been studied.
We conducted this particular study to determine the effects of acute single-dose morphine administration, 10-day chronic intermittent escalating-dose morphine administration, or its 12-h spontaneous withdrawal on mu-opioid receptor mRNA levels (Zhou et al., 2006). We specifically selected several brain regions considered to play an important role in the reinforcing or motivational effects of drugs of abuse, such as the nucleus accumbens core, amygdala, lateral hypothalamus, and caudate-putamen (Kreek and Koob, 1998). We found that either acute or chronic intermittent escalating-dose morphine did not modify mu-opioid receptor mRNA levels in the brain regions listed above. However, in contrast, acute spontaneous morphine withdrawal led to an increase in mu-opioid receptor mRNA levels in the nucleus accumbens core, lateral hypothalamus, and caudate-putamen, but not in the amygdala (Table 2B). Our data clearly showed that morphine withdrawal increases mu-opioid receptor mRNA levels in a region-specific manner. Our finding of increased mu-opioid receptor gene expression by morphine withdrawal suggests that endogenous opioid agonists and exogenous opiates also have an inhibitory effect on mu-opioid receptor gene expression (Zhou et al., 2006).
In early studies using morphine pellets, several research groups have shown that POMC mRNA in the hypothalamus tended to decrease after chronic morphine pellets in either normal rats (Mocchetti et al., 1989; Bronstein et al., 1990), castrated male rats (Wardlaw et al., 1996) or ovariectomized female guinea pigs (Fang et al., 1998). We recently examined intermittent heroin-induced changes of the POMC gene expression during early withdrawal from chronic intermittent escalating-dose heroin administration in normal male rats. After 12-hour withdrawal, there was a slight, but significant, decrease in the POMC mRNA levels in the hypothalamus (Zhou et al., in preparation). Given the fact that the POMC neurons in the arcuate nucleus project to the lateral hypothalamus and that beta-endorphin binds to mu-opioid receptor to exert an inhibitory effect on lateral hypothalamic neurons (Georgescu et al., 2003), we found the increase in mu-opioid receptor mRNA levels in the lateral hypothalamus after 12-hour opiate withdrawal (Zhou et al., 2006), suggesting a decrease in opioidergic activity caused by decreased POMC gene expression from the arcuate nucleus. Since POMC neurons also project to the CRF and AVP cells in the paraventricular nucleus, and opioids exert a tonic inhibitory effect on the HPA activity, one could expect enhanced HPA activity during 12-hour withdrawal if lower opioidergic activity is found. Indeed, we observed an elevation of plasma ACTH and corticosterone levels at this withdrawal time point (Zhou et al., 2006). This relative endogenous opioid-deficiency hypothesis is supported by evidence in humans that heroin addicts have decreased mu-opioid receptor-relevant activity (Kreek et al., 1984; Schluger et al., 2001). Also, our hypothesis is further supported by recent studies showing that opioid agonist methadone reduces early withdrawal-associated increases in heroin self-administration in heroin-dependent monkeys (Negus, 2006; Negus and Rice, 2009).
6. Mu-Opioid Receptor Variants
Following the first cloning of any opioid receptor, and the delta-opioid receptor from a cell line, by two groups independently in 1993, and subsequently the cloning of all three types of opioid receptors, mu, delta and kappa, first from rodent models and then with cloning of the human genes, we made the decision to begin work in human molecular genetics. This, on one hand, was to complement our laboratory work, and on the other, our basic clinical research (which is not reviewed in this very brief paper.) In our human molecular genetics work over the intervening years, we have used two very different approaches. First, we have used a hypothesis-driven approach, in which specific genes or pathways including various genes which code for the proteins which are targets of drugs of abuse, or other genes that code for proteins that are part of the signal transduction pathways, or are in related neurotransmitter neuropeptide systems, which we have documented to be impacted upon by drugs of abuse, have been studied. Some of the work leading towards the decision of which genes are to be studied in this way have come from our laboratory-based research, and specifically, from our research on stress responsivity, as reviewed above. Other selections have come directly from our basic clinical research. Since our laboratory has always engaged in bidirectional translational research, many of our hypothesis-driven selections for genes to be rigorously studied have come from both laboratory and clinical science.
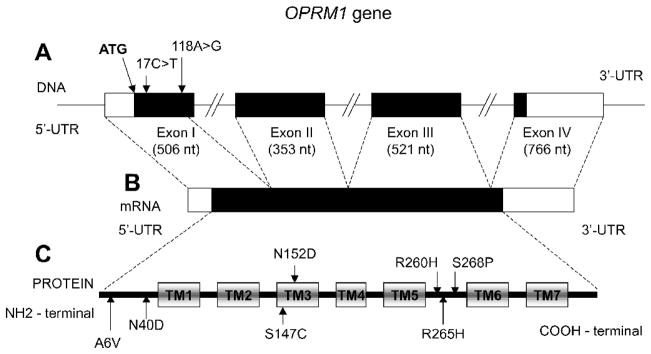
In our first human molecular genetic efforts, we joined in full collaboration with the laboratory of Lei Yu, and decided to first identify variants of the mu-opioid receptor gene. The mu-opioid receptor gene encodes the peptide which is well established to be the direct target for a major drug of abuse, heroin (once biotransformed to 6-acetyl- morphine), as well as the major treatment agent for heroin addiction, methadone, which is a synthetic opioid that is long-acting in humans, as well as many types of pain medications, including the naturally-derived morphine and its congeners, and synthetic fentanyls and other related mu-opioid receptor-directed compounds. In our first work from 1995 to 1998, we used the older techniques of longer gel-sequencing. We therefore focused on sequencing the first three exons of the mu-opioid receptor gene, which codes for a large part of the mu-opioid receptor peptide. These three exons include the N-terminus and the seven transmembrane regions, but not the intracellular carboxy-terminus tail (see Figure 3). In our first work, we identified several mu-opioid gene variants (Bond et al., 1998).
Figure 3. The structure of the OPRM1 gene and location of the polymorphisms.
A- Exon-intron structure of the OPRM1 gene reported in Wendel and Hoehe (1998). The position of the ATG initiation codon is indicated. The numbering of the polymorphism position is based upon a system that gives value +1 to the first nucleotide of this codon; bases belonging to the coding region have positive values; bases belonging to the upstream 5’ to the initiation codon have negative values. The protein coding region is indicated in black.
B- The structure of OPRM1 mRNA. The protein coding region is indicated in black.
C- The seven transmembrane domain structure of the OPRM1 protein. Transmembrane domains (TM1-TM7) are indicated in rectangles.
However, we had made the decision to study functionality of an identified gene variant with a high allelic frequency, prior to any publication. Our original study was conducted in three different ethnic/cultural groups, Caucasian, African American and Hispanic; we found highly significant differences in the allelic frequencies of the two most common gene variants, A118G and C17T, across these three groups. One paper had reported identification of the A118G mu- opioid receptor gene variant (Bergen et al., 1997) after we had also discovered it, but before our first report, and while we were conducting the functionality studies of A118G. The group that identified the A118G was particularly focused on alcoholism in specific populations, and not on gene functionality; they found no association of this variant with alcoholism in three different populations.
We conducted studies in several appropriate molecular and cell biological constructs and systems. We selected the A118G variant, because it codes for the changes in asparagine to aspartic acid in amino acid position 40, and because it is the site of putative glycosylation, a phenomenon which we later proved to be correct (Kroslak et al., 2007). We found that when appropriate constructs were made, the A118G variant receptor bound beta-endorphin three times more tightly than did the prototype receptor (Bond et al., 1998). There was no difference in binding of any other endogenous (e.g., met-enkephalin and endomorphin) or exogenous (e.g., morphine and methadone) opioids or opiates. Further, we also found, using a cell biological-molecular construct, that G-protein-coupled potassium inwardly-rectifying channels (GIRKs) were activated to a far greater extent when beta-endorphin bound to the variant receptor than to the prototype. Again, this was only found for long, natural beta-endorphin and not for other endogenous or exogenous opioids or opiates (Bond et al., 1998). Beta-endorphin is by far the longest (31 amino acids) and longest-acting (more than 30 min half-life in human blood) of the endogenous opioids, and probably demands the greatest fidelity of receptors for binding and activation with signal transduction. In our initial publication (Bond et al., 1998) and in a later publication (LaForge et al., 2000), we predicted that this A118G variant would be functional in humans and specifically in HPA axis function, since our laboratory has shown that the mu-opioid receptor system is involved in regulating the HPA axis in humans by tonic inhibition. We predicted that stress responsivity would be altered with one or more copies of this A118G variant receptor (Bond et al., 1998). Further, we predicted that any other mammalian physiological system in which the mu-opioid receptor plays a physiological modulatory role would be potentially altered by presence of this variant (Bond et al., 1998). Our earliest studies had suggested that the A118G variant has much lower cell membrane receptor presentation than does the prototype, although this was not rigorously studied for our initial report (Bond et al., 1998). Therefore we studied this in subsequent work, and showed that, indeed, in three different cell types, there is much lower cell-surface presentation of the A118G variant receptor than the prototype (Kroslak et al., 2007). The net potential or physiological effect in humans may be offset by the greater beta-endorphin binding and more effective signal transduction. Therefore, only in the setting of need for additional endogenous beta-endorphin, as the major mu-opioid receptor-directed endogenous peptide, would one see any abnormalities in function.
In an early study, Wand and colleagues showed that one copy of the G variant profoundly altered the HPA response (cortisol) to a specific opiate antagonist, naloxone, given in high repeated doses to healthy volunteers (Wand et al., 2002). Wand followed up with a second more extensive study in a large number of healthy volunteers, and again made the same finding (Chong et al., 2006). Also, the group of Kranzler (Hernandez-Avila et al., 2003) made similar findings. Our laboratory has shown that healthy persons with one or two copies of this A118G variant have higher baseline levels of cortisol than persons with the prototype mu-opioid receptor (A118A), under stress-minimized circumstances. Further, we found females with A118G variant contribute to this basal hormonal difference to a greater extend than males (Bart et al., 2006). More recently, in work in progress, we have found in healthy volunteers that other aspects of the HPA axis, under tonic inhibition of the mu-opioid receptor system, are significantly modified in individuals with one or two copies of the A118G variant (Ducat et al., in preparation). Also, other groups have found that the A118G variant may be associated with different responses to pain of specific types (reviewed in Kreek et al., 2005). There is now no doubt that even in healthy volunteers, when there are one or two copies of the A118G variant with resulting mu-opioid receptor changes, there are profound alterations in stress responsivity. This may be due in part to the lowered presentation of the mu-opioid receptors on cell surface ready for activation, but with stronger signal transduction after binding of beta-endorphin. This would be a system which under stress could lead to a relative endorphin deficiency. We have reviewed this variant and many other gene variants extensively (Kreek et al., 2005; Kreek and LaForge, 2007; Kreek, 2008).
We further predicted that this variant would be associated with opiate addiction, but like any gene variant associated with heroin addiction and alcoholism, or any specific addictive disease, we predicted that it would play a modest role in the overall risk to develop an addiction. To conduct these molecular genetics studies in a population with limited admixture, we performed our research in central Sweden, in the Stockholm area, where over 80% of the population are Swedes with Swedish ancestry and no admixture for over 500 years, and less than 20% are from other places, but, to date, with very little admixture between the Swedish and non-Swedish populations. In two separate, large studies conducted in collaboration with the group of Heilig et al. at the Karolinska Institute, we found strong association of the A118G variant with a) heroin addiction and b) alcoholism. These highly significant associations with heroin addiction or with alcoholism pertained either with those of Swedish heritage only, or with the entire population studied, because of the very low admixture. Further, with the collaboration of the statistical geneticist Jurg Ott and his colleagues, we were able to determine that a very high “attributable risk” for developing heroin addiction rests with this one functional variant alone (Bart et al., 2004; Bart et al., 2005). Some other studies have corroborated these findings, and, in particular, in studies of Han Chinese, which is an only modestly admixed population (reviewed in Kreek et al., 2005). However, negative results have been frequently found, especially in populations with significant admixture, such as in the general population of the United States or the general population of Europe (as reviewed in Kreek et al., 2005).
Further, we predicted that the presence of one or two copies of the A118G variant would predict a more favorable response to treatment of alcoholism with naltrexone, since we had found that alcoholics seek a modest activation of the HPA axis, which may be effected by alcohol or a specific opioid antagonist such as naltrexone (Oslin et al., 2003; O’Malley et al., 2004; Kreek et al., 2004; Anton et al., 2008)
It has been found that non-human primates have a natural variant of the mu-opioid receptor which is very similar to that of the A118G variant in humans though in a slightly different location. It similarly results in an amino acid change and similarly alters beta-endorphin binding and stress responsivity (Miller et al., 2004; Barr et al., 2008; Vallender et al., 2008). Very recently, a group has successfully created a transgenic mouse with an analogous but not identical mutation to this A118G variant in humans. This A112G, placed into a mouse model by Blendy and her colleagues, has begun to be studied, with some extraordinarily exciting findings, including some gender differences in behavioral responsivity (Mague et al., 2009). Therefore, we have two animal models, non-human primates and rodent models for further studies in addition to our ability to conduct many more studies in both healthy humans and humans with specific addictive diseases.
Since we have shown that the mu-opioid receptor plays many roles in stress responsivity and, in particular, tonic inhibition of apparently the hypothalamic-pituitary components of the HPA axis, we have proceeded to study other variants of other genes that are directly part of this HPA axis, two of which will be discussed below.
7. POMC Variants
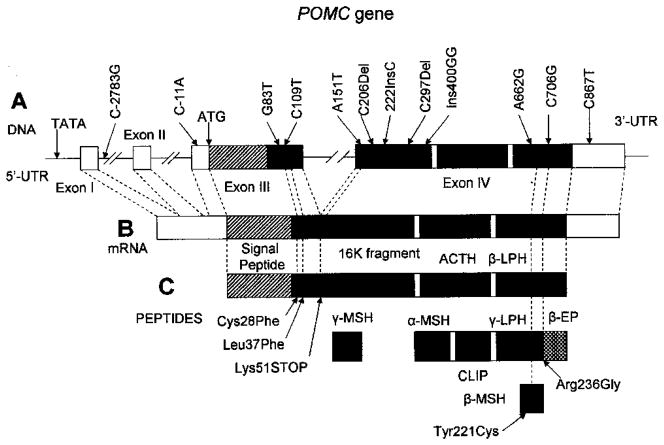
Proopiomelanocortin (POMC) (Fig. 4) is a source of a number of peptides, including ACTH, that play a key role in regulation of the HPA axis. These peptides that are derived from POMC are released by tissue-specific proteolitic processing (e.g., Bertagna, 1994) and play an important role in a number of functions including skin pigmentation and control of adrenal growth and function. Processing of POMC takes place in several steps. The first step of POMC processing results in the formation of beta-lipotropin (β-LPH), ACTH and gamma-melanotropin (γ-MSH). ACTH, in turn, is processed to alpha-melanotropin (α-MSH) and a corticotropin-like intermediary peptide (CLIP). β-LPH is processed to beta-endorphin, and also gamma-lipotropin (γ-LPH), which is processed to beta-melanotropin (β-MSH). POMC is highly expressed in the arcuate nucleus of the hypothalamus and in the anterior pituitary. Congenital deficiency of POMC results in a hypoadrenalism syndrome, severe obesity, and altered skin and hair pigmentation.
Figure 4. The structure of the POMC gene and location of the selected polymorphisms.
A- The structure of the POMC gene with selected polymorphisms. The position of the ATG initiation codon is indicated. The numbering of the polymorphism position is based upon a system that gives value +1 to the first nucleotide of this codon. The protein coding region is indicated in black.
B- The structure of POMC mRNA. The protein coding region is indicated in black.
C- POMC peptides. Selected functional polymorphisms are indicated.
Thus, missense variant Tyr221Cys (position A8067G according to the system described by Takahashi et al. (1983) or position A662G according to the system that assigns value +1 to the first nucleotide of the ATG codon) located in the region encoding β-MSH was reported to be significantly associated with early onset obesity (Biebermann et al., 2006; Lee et al., 2006). A number of other polymorphisms, including Cys28Phe (G83T) and Leu37Phe (109T) located in the amino-terminal region of POMC, also have been associated with early onset obesity (Creemers et al., 2008). The other variant, Arg236Gly (C8086G, C706G), was found in Caucasians with severe obesity from childhood (Challis et al., 2002). This variant disrupts the cleavage site between β-MSH and beta-endorphin. The resulting fusion protein binds to melanocortin 4 receptor (MC4R) with an affinity similar to natural ligands but fails to activate the receptor (Challis et al., 2002). Several other variants of POMC, including translation initiation mutation C3804A (C-11A), missense substitution A6851T (A151T), a deletion 6996del (C297Del) and a two-nucleotide insertion 7100+2G (Ins400GG), were found in homozygosity or compound heterozygosity in patients with adrenal insufficiency having early-onset severe obesity (Krude et al., 2003). Rare POMC variant C6906del (C206Del) results in complete loss of POMC-derived peptides and was found in a person with severe obesity and ACTH deficiency (Farooqi et al., 2006). Genotypes of C8246T (C867T, rs1042571) and C1032G (C2783G, rs1009388) have been reported to be associated with body fat distribution (waist-to-hip ratio) (Baker et al., 2005). A recent report on a patient homozygous for 6922InsC (222InsC) showed alterations in somatotropic, gonadotropic and thyroid axis, necessitating hormonal replacement and also absence in abnormality of pigmentation (Clément et al., 2008).
We hypothesize that dysregulation of the HPA axis caused by variants of POMC also might influence vulnerability to the initiation of drug addiction. Therefore, we consider the POMC gene as one of the primary candidates in our studies of association with drug addiction.
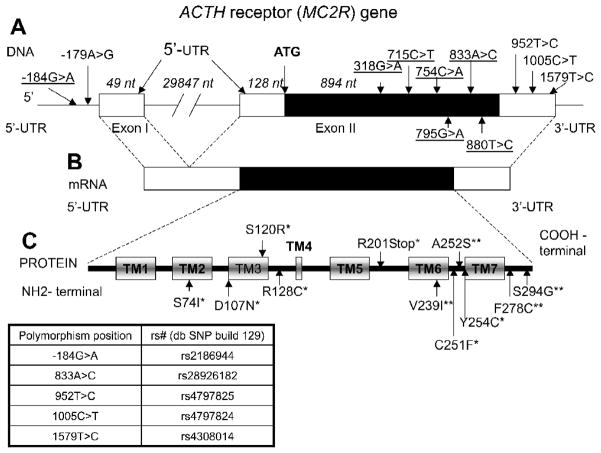
8. Melanocortin 2 Receptor (MC2R) Variants
One of the major peptides that derives from POMC processing is ACTH (Fig. 4). ACTH regulates adrenal glucocorticoid and androgen synthesis in the zona fasciculata and zona reticularis, respectively, in the adrenal cortex. ACTH binds to its specific melanocortin 2 receptor (MC2R or ACTH receptor, Fig. 5). Being involved in regulation of adrenal cortisol secretion, MC2R is important in the physiological response to stressors. MC2R is expressed mostly in the adrenal cortex and is also found in human skin (Slominski et al., 1996), ovarian steroid cell tumor (Lin et al., 2000), as well as in rodent anterior pituitary (Wachira et al., 2007) and some rodent embryonic tissue, including brain, spinal cord, lungs and testes (Nimura et al., 2006; Johnston et al., 2007). The functional expression of MC2R requires melanocortin 2 receptor accessory protein (MRAP) that is involved in the trafficking of the MC2R from the endoplasmic reticulum to the cell surface (Webb et al., 2009). Recently, another homolog of MRAP, MRAP2, expressed in the brain and adrenal gland and able to interact with all five mineralocortin receptors, has been identified (Chan et al., 2009).
Figure 5. The structure of the MC2R gene and location of the polymorphisms.
A- The structure of the MC2R gene with selected polymorphisms. The position of the ATG initiation codon is indicated. The numbering of the polymorphism position is based upon a system that gives value +1 to the first nucleotide of this codon. The protein coding region is indicated in black. The lengths of gene parts are in italics.
B- The structure of MC2R mRNA. The protein coding region is indicated in black.
C- The seven transmembrane domain structure of the MC2R protein. *Selected functional polymorphisms; **novel polymorphisms that change the protein structure discovered in our study (Proudnikov et al., 2008). Transmembrane domains (TM1-TM7) are indicated in rectangles.
Variants in the MC2R gene in most human genetics studies have been linked to familial glucocorticoid deficiency (Tsigos et al., 1993; Elias et al., 1999). Recent studies showed possible involvement of MC2R in altered stress mechanisms. Substitution of A to G in -179A>G (-2T>C) of the MC2R gene results in lower promoter activity of this gene in vitro and was associated with impaired cortisol response to ACTH stimulation in vivo (Slawik et al., 2004). A clinical study with a six-hour ACTH stimulation test showed that subjects with the -179AA genotype have a higher dehydroepiandrosterone (DHEA) response than -179GG subjects, while baseline DHEA concentrations did not differ between groups (Reisch et al., 2005).
Recent studies on the functionality of MC2R revealed that ACTH1-16 (shortened form of natural ACTH 1-39) is the minimal peptide that is required for binding ACTH and signaling (Chen et al., 2007); mutations in transmembrane domains 2, 3 and 4 significantly decrease ACTH binding and signaling. In another study (Yang et al., 2007) the replacement of cysteine residues with serines showed that substitutions C21S in the N-terminus and also C245S, C251S, and C253S in the extracellular loop 3 decrease both receptor expression and function. Similar substitutions of cysteine with serine placed in all five other positions do not alter ACTH binding potency.
In our studies (Proudnikov et al., 2008) we found an experiment-wise significant association of the minor allele A of the polymorphism -184G>A of the MC2R gene and the haplotype AACT bearing a -184A allele with a protective effect from the development of heroin addiction in Hispanics. We also found a strong effect in association of individual genotype frequencies of this polymorphism or combination GA+AA vs. GG with a protective effect from heroin addiction, providing genetic evidence supporting our hypothesis that dysregulation of the HPA axis contributes to the development of drug addiction.
We have or are currently studying other genes that are involved in the molecular neurobiological bases of specific addictive diseases. For instance, we are methodically studying each one of the genes of the endogenous opioid system. We are also studying other components of the stress-responsive systems, as well as other neuropeptide and neurotransmitter systems. In addition, we have (despite the enormous expense) been able to conduct a few “genome-wide studies” or studies in which large groups of variants selected by hypotheses or findings are present on a single array, constructed to be of interest for studies of addiction and the affective disorders (Levran et al., 2008; Nielsen et al., 2008; Levran et al., 2009). It has been of interest to us that different variants of the mu-opioid receptor, which are present on these different arrays, have been found to be associated with one or more specific addictions or drug abuse. This suggests that indeed mu-opioid receptor gene variants are associated with substance abuse and specific addictions, but that multiple variants, and perhaps specific haplotypes blocks, may be involved. We have also found that gene variants of other components of the opioid system are also associated with specific addictive diseases. Therefore we have found that the endogenous opioid system, as well as the HPA axis or other stress-responsive systems in other parts of the brain, as briefly reviewed herein, may be of great importance in the neurobiological bases of specific addictive diseases and may contribute to the molecular genetic basis of these diseases.
Conclusion
It has now been well-established that alterations of stress responsivity are caused both by drugs of abuse and also by functional gene variants, which may be present in humans (or in non-human primates). In future, genomics and genetic studies will bring more understanding on altered stress responsivity in the development and perpetuation of and relapse to specific addictive diseases.
Acknowledgments
Funding support was received from the National Institutes of Health-National Institute on Drug Abuse Research Center P60-DA05130 (Kreek), and National Center for Research Resources UL1RR024143 (Coller). We thank Ms. Susan Russo for help in preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of μ-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM. Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J Neurochem. 1996;66:443–448. doi: 10.1046/j.1471-4159.1996.66020443.x. [DOI] [PubMed] [Google Scholar]
- Baker M, Gaukrodger N, Mayosi BM, Imrie H, Farrall M, Watkins H, Connell JM, Avery PJ, Keavney B. Association between common polymorphisms of the proopiomelanocortin gene and body fat distribution: a family study. Diabetes. 2005;54:2492–2496. doi: 10.2337/diabetes.54.8.2492. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proc Natl Acad Sci USA. 2008;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Heilig M, LaForge KS, Pollak L, Leal SM, Ott J, Kreek MJ. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry. 2004;9:547–549. doi: 10.1038/sj.mp.4001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, Kreek MJ, Ott J, LaForge KS, Proudnikov D, Pollak L, Heilig M. Increased attributable risk related to a functional mu-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology. 2005;30:417–422. doi: 10.1038/sj.npp.1300598. [DOI] [PubMed] [Google Scholar]
- Bart G, LaForge KS, Borg L, Lilly C, Ho A, Kreek MJ. Altered levels of basal cortisol in healthy subjects with a 118G allele in exon 1 of the mu opioid receptor gene. Neuropsychopharmacology. 2006;31:2313–2317. doi: 10.1038/sj.npp.1301128. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Kokoszka J, Peterson R, Long JC, Virkkunen M, Linnoila M, Goldman D. μ opioid receptor gene variants: lack of association with alcohol dependence. Mol Psychiatry. 1997;2:490–494. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- Bertagna X. Proopiomelanocortin-derived peptides. Endocrinol Metab Clin North Am. 1994;23:467–485. [PubMed] [Google Scholar]
- Biebermann H, Castañeda TR, van Landeghem F, von Deimling A, Escher F, Brabant G, Hebebrand J, Hinney A, Tschöp MH, Grüters A, Krude H. A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 2006;3:141–146. doi: 10.1016/j.cmet.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg L, Ho A, Peters JE, Kreek MJ. Availability of reliable serum methadone determination for management of symptomatic patients. J Addict Dis. 1995;14:83–91. doi: 10.1300/J069v14n03_06. [DOI] [PubMed] [Google Scholar]
- Borgland S, Taha S, Sarti F, Fields H, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Kuhn CM. Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther. 1991;256:204–210. [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, von Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, McRae AL, Moran-Santa Maria MM, DeSantis SM, Simpson AN, Waldrop AE, Back SE, Kreek MJ. Response to corticotropin-releasing hormone infusion in cocaine-dependent individuals. Arch Gen Psychiatry. 2009;66:422–430. doi: 10.1001/archgenpsychiatry.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein DM, Przewlocki R, Akil H. Effects of morphine treatment on proopiomelanocortin systems in rat brain. Brain Res. 1990;519:102–111. doi: 10.1016/0006-8993(90)90066-k. [DOI] [PubMed] [Google Scholar]
- Burstein Y, Grady RW, Kreek MJ, Rausen AR, Peterson CM. Thrombocytosis in the offspring of female mice receiving dl-methadone. Proc Soc Exp Biol Med. 1980;164:275–279. doi: 10.3181/00379727-164-40861. [DOI] [PubMed] [Google Scholar]
- Challis BG, Pritchard LE, Creemers JW, Delplanque J, Keogh JM, Luan J, Wareham NJ, Yeo GS, Bhattacharyya S, Froguel P, White A, Farooqi IS, O’Rahilly S. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet. 2002;11:1997–2004. doi: 10.1093/hmg/11.17.1997. [DOI] [PubMed] [Google Scholar]
- Chan LF, Webb TR, Chung TT, Meimaridou E, Cooray SN, Guasti L, Chapple JP, Egertová M, Elphick MR, Cheetham ME, Metherell LA, Clark AJ. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci U S A. 2009;106:6146–6151. doi: 10.1073/pnas.0809918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Aprahamian CJ, Kesterson RA, Harmon CM, Yang Y. Molecular identification of the human melanocortin-2 receptor responsible for ligand binding and signaling. Biochemistry. 2007;46:11389–11397. doi: 10.1021/bi700125e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology. 2006;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément K, Dubern B, Mencarelli M, Czernichow P, Ito S, Wakamatsu K, Barsh GS, Vaisse C, Leger J. Unexpected endocrine features and normal pigmentation in a young adult patient carrying a novel homozygous mutation in the POMC gene. J Clin Endocrinol Metab. 2008;93:4955–4962. doi: 10.1210/jc.2008-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers JW, Lee YS, Oliver RL, Bahceci M, Tuzcu A, Gokalp D, Keogh J, Herber S, White A, O’Rahilly S, Farooqi IS. Mutations in the amino-terminal region of proopiomelanocortin (POMC) in patients with early-onset obesity impair POMC sorting to the regulated secretory pathway. J Clin Endocrinol Metab. 2008;93:4494–4499. doi: 10.1210/jc.2008-0954. [DOI] [PubMed] [Google Scholar]
- Culpepper-Morgan JA, Inturrisi CE, Portenoy RK, Foley K, Houde RW, Marsh F, Kreek MJ. Treatment of opioid induced constipation with oral naloxone: A pilot study. Clin Pharm Ther. 1992;23:90–95. doi: 10.1038/clpt.1992.106. [DOI] [PubMed] [Google Scholar]
- Culpepper-Morgan JA, Kreek MJ. Hypothalamic-pituitary-adrenal axis hypersensitivity to naloxone in opioid dependence: A case of naloxone induced withdrawal. Metabolism. 1997;46:130–134. doi: 10.1016/s0026-0495(97)90289-4. [DOI] [PubMed] [Google Scholar]
- Cushman P, Kreek MJ. Some endocrinologic observations in narcotics addicts. In: Zimmerman E, George R, editors. Narcotics and the Hypothalamus. Raven Press; New York: 1974. pp. 161–173. [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole VP, Nyswander ME, Kreek MJ. Narcotic blockade. Arch Intern Med. 1966;118:304–309. [PubMed] [Google Scholar]
- Elias LL, Huebner A, Pullinger GD, Mirtella A, Clark AJ. Functional characterization of naturally occurring mutations of the human adrenocorticotropin receptor: poor correlation of phenotype and genotype. J Clin Endocrinol Metab. 1999;84:2766–2770. doi: 10.1210/jcem.84.8.5924. [DOI] [PubMed] [Google Scholar]
- Fang Y, Kelly MJ, Rønnekleiv OK. Proopiomelanocortin (POMC) mRNA expression: distribution and region-specific down-regulation by chronic morphine in female guinea pig hypothalamus. Brain Res Mol Brain Res. 1998;55:1–8. doi: 10.1016/s0169-328x(97)00348-3. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Drop S, Clements A, Keogh JM, Biernacka J, Lowenbein S, Challis BG, O’Rahilly S. Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes. 2006;55:2549–2553. doi: 10.2337/db06-0214. [DOI] [PubMed] [Google Scholar]
- Farren CK, O’Malley S, Grebski G, Maniar S, Porter M, Kreek MJ. Variable dose naltrexone induced HPA stimulation in abstinent alcoholics: a preliminary study. Alcohol Clin Exp Res. 1999;23:502–508. [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, Dileone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, Endres CJ, Dannals RF, Frost JJ. Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry. 2005;57:1573–1582. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML, Dannals RF, Frost JJ. Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology (Berl) 2008;200:475–486. doi: 10.1007/s00213-008-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Wand G, Luo X, Gelertner J, Kranzler HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) Am J Med Genet B Neuropsychiatr Genet. 2003;118B:60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Ignar DM, Kuhn CM. Effects of specific mu and kappa opiate tolerance and abstinence on hypothalamo-pituitary-adrenal axis secretion in the rat. J Pharmacol Exp Ther. 1990;225:1287–1295. [PubMed] [Google Scholar]
- Jingami H, Nakanishi S, Imura H, Numa S. Tissue distribution of messenger RNAs coding for opioid peptide precursors and related RNA. Eur J Biochem. 1984;142:441–447. doi: 10.1111/j.1432-1033.1984.tb08306.x. [DOI] [PubMed] [Google Scholar]
- Johnston H, King PJ, O’Shaughnessy PJ. Effects of ACTH and expression of the melanocortin-2 receptor in the neonatal mouse testis. Reproduction. 2007;133:1181–1187. doi: 10.1530/REP-06-0359. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142:3324–3331. doi: 10.1210/endo.142.8.8299. [DOI] [PubMed] [Google Scholar]
- Koob GF, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Kreek MJ, Raghunath J, Kleber HD. Cortisol levels during chronic naltrexone maintenance treatment in ex-opiate addicts. Biol Psychiatry. 1986a;21:217–220. doi: 10.1016/0006-3223(86)90150-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kreek MJ, Raghunath J, Kleber HD. A preliminary study of beta-endorphin during chronic naltrexone maintenance treatment in ex-opiate addicts. Life Sci. 1986b;39:55–59. doi: 10.1016/0024-3205(86)90437-6. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Medical safety and side effects of methadone in tolerant individuals. JAMA. 1973a;223:665–667. [PubMed] [Google Scholar]
- Kreek MJ. Plasma and urine levels of methadone. NY State J Med. 1973b;73:2773–2777. [PubMed] [Google Scholar]
- Kreek MJ. Medical complications in methadone patients. Ann NY Acad Sci. 1978;311:110–134. doi: 10.1111/j.1749-6632.1978.tb16769.x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Methadone disposition during the perinatal period in humans. Pharmacol Biochem Behav. 1979;ll(Suppl):1–7. [PubMed] [Google Scholar]
- Kreek MJ, Wardlaw SL, Friedman J, Schneider B, Frantz AG. Effects of chronic exogenous opioid administration on levels of one endogenous opioid (beta-endorphin) in man. In: Simon E, Takagi H, editors. Advances in Endogenous and Exogenous Opioids. Kodansha Ltd. Publishers; Tokyo: 1981. pp. 364–366. [Google Scholar]
- Kreek MJ, Schaefer RA, Hahn EF, Fishman J. Naloxone, a specific opioid antagonist, reverses chronic idiopathic constipation. Lancet Feb. 1983a;5:261–262. doi: 10.1016/s0140-6736(83)91684-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Wardlaw SL, Hartman N. Circadian rhythms and levels of beta-endorphin, ACTH, and cortisol during chronic methadone maintenance treatment in humans. Life Sci. 1983b;33:409–413. doi: 10.1016/0024-3205(83)90529-5. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Raghunath J, Plevy S, Hamer D, Schneider B, Hartman N. ACTH, cortisol and beta-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides. 1984;5:277–281. doi: 10.1016/0143-4179(84)90081-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Opiates, opioids and addiction. Mol Psychiatry. 1996a;1:232–254. [PubMed] [Google Scholar]
- Kreek MJ. Opioid receptors: Some perspectives from early studies of their role in normal physiology, stress responsivity and in specific addictive diseases. Journal of Neurochemical Research. 1996b;21:1469–1488. doi: 10.1007/BF02532387. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Opiate and cocaine addictions: Challenge for pharmacotherapies. Pharm Biochem Behav. 1997;57:551–569. doi: 10.1016/s0091-3057(96)00440-6. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Methadone-related opioid agonist pharmacotherapy for heroin addiction: History, recent molecular and neurochemical research and the future in mainstream medicine. Ann NY Acad Sci. 2000;909:186–216. doi: 10.1111/j.1749-6632.2000.tb06683.x. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schlussman SD, Bart G, LaForge KS, Butelman ER. Evolving perspectives on neurobiological research on the addictions: celebration of the 30th anniversary of NIDA. Neuropharmacology. 2004;47(Suppl 1):324–344. doi: 10.1016/j.neuropharm.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Bart G, Lilly C, LaForge KS, Nielsen DA. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol Rev. 2005;57:1–26. doi: 10.1124/pr.57.1.1. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS. Stress responsivity, addiction, and a functional variant of the human mu-opioid receptor gene. Molec Interv. 2007;7:74–78. doi: 10.1124/mi.7.2.7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Role of a functional human gene polymorphism in stress responsivity and addictions. Clin Pharm Ther. 2008;83:615–618. doi: 10.1038/clpt.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, Zhou Y, Butelman ER. Bidirectional translational research: Progress in understanding addictive diseases. Neuropharmacology. 2009;56:32–43. doi: 10.1016/j.neuropharm.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroslak T, LaForge KS, Gianotti RJ, Ho A, Nielsen DA, Kreek MJ. The single nucleotide polymorphism A118G alters functional properties of the human mu opioid receptor. J Neurochem. 2007;103:77–87. doi: 10.1111/j.1471-4159.2007.04738.x. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, Grüters A. Obesity due to proopiomelanocortin deficiency: three new cases and treatment trials with thyroid hormone and ACTH4-10. J Clin Endocrinol Metab. 2003;88:4633–4640. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential implications for addictions. Eur J Pharmacol. 2000;410:249–268. doi: 10.1016/s0014-2999(00)00819-0. [DOI] [PubMed] [Google Scholar]
- Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, Wraight V, Sims M, Vatin V, Meyre D, Shield J, Burren C, Ibrahim Z, Cheetham T, Swift P, Blackwood A, Hung CC, Wareham NJ, Froguel P, Millhauser GL, O’Rahilly S, Farooqi IS. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006;3:135–140. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Leri F, Zhou Y, Goddard B, Cummins E, Kreek MJ. Effects of high dose methadone maintenance on cocaine place conditioning, cocaine self-administration, and mu-opioid receptor mRNA expression in the rat brain. Neuropsychopharmacology. 2006;31:1462–1474. doi: 10.1038/sj.npp.1300927. [DOI] [PubMed] [Google Scholar]
- Leri F, Zhou Y, Goddard B, Levy A, Jacklin D, Kreek MJ. Steady-state methadone blocks cocaine seeking and cocaine-induced gene expression alterations in the rat brain. Eur Neuropsychopharmacol. 2009;19:238–249. doi: 10.1016/j.euroneuro.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction: a candidate-gene association study. Genes Brain Behav. 2008;7:720–728. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levran O, Londono D, O’Hara K, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Heroin addiction in African Americans: a hypothesis-driven association study. Genes Brain Behav. 2009;8:531–540. doi: 10.1111/j.1601-183X.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Jorge AA, Latronico AC, Marui S, Fragoso MC, Martin RM, Carvalho FM, Arnhold IJ, Mendonca BB. Origin of an ovarian steroid cell tumor causing isosexual pseudoprecocious puberty demonstrated by the expression of adrenal steroidogenic enzymes and adrenocorticotropin receptor. J Clin Endocrinol Metab. 2000;85:1211–1214. doi: 10.1210/jcem.85.3.6454. [DOI] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. Proc Natl Acad Sci U S A. 2009;106:10847–10852. doi: 10.1073/pnas.0901800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine “binge” alters basal dopamine extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272:652–657. [PubMed] [Google Scholar]
- Maiya R, Zhou Y, Norris EH, Kreek MJ, Strickland S. Tissue plasminogen activator modulates the cellular and behavioral response to cocaine. Proc Natl Acad Sci U S A. 2009;106:1983–1988. doi: 10.1073/pnas.0812491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Steroid hormones and the brain: cellular mechanisms underlying neural and behavioral plasticity. Psychoneuroendocrinology. 1980;5:1–11. doi: 10.1016/0306-4530(80)90003-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gould EA, Sakai RR. The vulnerability of the hippocampus to protective and destructive effects of glucocorticoids in relation to stress. Br J Psychiatry. 1992;15:18–23. [PubMed] [Google Scholar]
- Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Mol Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Ritter A, Costa E. Down-regulation of proopiomelanocortin synthesis and beta-endorphin utilization in hypothalamus of morphine-tolerant rats. J Mol Neurosci. 1989;1:33–38. doi: 10.1007/BF02896854. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashoto S, Khotib J, Miyatake M, Sakurai T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs. food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009;34:899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Ji F, Yuferov V, Ho A, Chen A, Levran O, Ott J, Kreek MJ. Genotype patterns that contribute to increased risk for or protection from developing heroin addiction. Mol Psychiatry. 2008;13:417–428. doi: 10.1038/sj.mp.4002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimura M, Udagawa J, Hatta T, Hashimoto R, Otani H. Spatial and temporal patterns of expression of melanocortin type 2 and 5 receptors in the fetal mouse tissues and organs. Anat Embryol (Berl) 2006;211:109–117. doi: 10.1007/s00429-005-0066-9. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2004;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Peles E, Kreek MJ, Kellogg S, Adelson M. High methadone dose significantly reduces cocaine abuse in Methadone Maintenance Treatment (MMT) patients. J Addict Dis. 2006;25:43–50. doi: 10.1300/J069v25n01_07. [DOI] [PubMed] [Google Scholar]
- Proudnikov D, Hamon S, Ott J, Kreek MJ. Association of polymorphisms in the melanocortin receptor type 2 (MC2R, ACTH receptor) gene with heroin addiction. Neurosci Lett. 2008;435:234–239. doi: 10.1016/j.neulet.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15–20. doi: 10.1016/s0166-4328(98)00073-4. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Perrotti LI, Mc Monagle J, Ho A, Kreek MJ. Ovarian hormone replacement affects cocaine-induced behaviors in ovariectomized female rats. Pharmacol Biochem Behav. 2000a;67:417–422. doi: 10.1016/s0091-3057(00)00381-6. [DOI] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Perrotti LI, Ho A, Jenab S, Schlussman SD, Franck J, Kreek MJ. Cocaine affects progesterone plasma levels in female rats. Pharmacol Biochem Behav. 2000b;66:449–453. doi: 10.1016/s0091-3057(00)00213-6. [DOI] [PubMed] [Google Scholar]
- Ragavan VV, Wardlaw SL, Kreek MJ, Frantz AG. Effect of chronic naltrexone and methadone administration on brain immunoreactive beta-endorphin in the rat. Neuroendocrinology. 1983;37:266–268. doi: 10.1159/000123556. [DOI] [PubMed] [Google Scholar]
- Reisch N, Slawik M, Zwermann O, Beuschlein F, Reincke M. Genetic influence of an ACTH receptor promoter polymorphism on adrenal androgen secretion. Eur J Endocrinol. 2005;153:711–715. doi: 10.1530/eje.1.02015. [DOI] [PubMed] [Google Scholar]
- Rosen MI, Kosten TR, Kreek MJ. The effects of naltrexone maintenance on the response to yohimbine in healthy volunteers. Biol Psychiatry. 1999;45:1636–1645. doi: 10.1016/s0006-3223(98)00259-5. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Ho A, Borg L, Porter M, Maniar S, Gunduz M, Perret G, King A, Kreek MJ. Nalmefene causes greater hypothalamic-pituitary-adrenal axis activation than naloxone in normal volunteers: Implications for the treatment of alcoholism. Alcohol Clin Exp Res. 1998;22:1430–1436. doi: 10.1111/j.1530-0277.1998.tb03931.x. [DOI] [PubMed] [Google Scholar]
- Schluger JH, Borg L, Ho A, Kreek MJ. Altered HPA axis responsivity to metyrapone testing in methadone maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacology. 2001;24:568–575. doi: 10.1016/S0893-133X(00)00222-0. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR. Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence. Am J Psychiatry. 2005;162:340–349. doi: 10.1176/appi.ajp.162.2.340. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Slawik M, Reisch N, Zwermann O, Maser-Gluth C, Stahl M, Klink A, Reincke M, Beuschlein F. Characterization of an adrenocorticotropin (ACTH) receptor promoter polymorphism leading to decreased adrenal responsiveness to ACTH. J Clin Endocrinol Metab. 2004;89:3131–3137. doi: 10.1210/jc.2003-032010. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudate-putamen. Mol Brain Res. 1993;19:323–327. doi: 10.1016/0169-328x(93)90133-a. [DOI] [PubMed] [Google Scholar]
- Spangler R, Zhou Y, Schlussman SD, Ho A, Kreek MJ. Behavioral stereotypies induced by “binge” cocaine administration are independent of drug-induced increases in corticosterone levels. Behav Brain Res. 1997;86:201–204. doi: 10.1016/s0166-4328(96)02257-7. [DOI] [PubMed] [Google Scholar]
- Stine SM, Burns B, Kosten T. Methadone dose for cocaine abuse. Am J Psychiatry. 1991;148:1268–1273. doi: 10.1176/ajp.148.9.1268a. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependent cocaine users. Psychopharmacology. 1994;116:401–406. doi: 10.1007/BF02247469. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Hakamata Y, Watanabe Y, Kikuno R, Miyata T, Numa S. Complete nucleotide sequence of the human corticotropin-beta-lipotropin precursor gene. Nucleic Acids Res. 1983;11:6847–6858. doi: 10.1093/nar/11.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. Febs Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Arai K, Hung W, Chrousos GP. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. J Clin Invest. 1993;92:2458–2461. doi: 10.1172/JCI116853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- Vallender EJ, Priddy CM, Chen GL, Miller GM. Human expression variation in the mu-opioid receptor is paralleled in rhesus macaque. Behav Genet. 2008;38:390–395. doi: 10.1007/s10519-008-9207-2. [DOI] [PubMed] [Google Scholar]
- Volavka J, Bauman J, Pevnick J, Reker D, James B, Cho D. Short-term hormonal effects of naloxone in man. Psychoneuroendocrinology. 1980;5:225–234. doi: 10.1016/0306-4530(80)90026-8. [DOI] [PubMed] [Google Scholar]
- Wachira SJ, Temoney S, Ramlochansingh C, Hughes-Darden CA. Prevalence of melanocortin system transcripts in rat salt homeostasis endocrine tissues. Cell Mol Biol (Noisy-le-grand) 2007;15:8–14. [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Webb TR, Chan L, Cooray SN, Cheetham ME, Chapple JP, Clark AJ. Distinct melanocortin 2 receptor accessory protein domains are required for melanocortin 2 receptor interaction and promotion of receptor trafficking. Endocrinology. 2009;150:720–726. doi: 10.1210/en.2008-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel B, Hoehe MR. The human mu opioid receptor gene: 5’ regulatory and intronic sequences. J Mol Med. 1998;76:525–532. doi: 10.1007/s001090050246. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Willenberg HS, Haase M, Papewalis C, Schott M, Scherbaum WA, Bornstein SR. Corticotropin-releasing hormone receptor expression on normal and tumorous human adrenocortical cells. Neuroendocrinology. 2005;82:274–281. doi: 10.1159/000093126. [DOI] [PubMed] [Google Scholar]
- Yang Y, Chen M, Kesterson RA, Jr, Harmon CM. Structural insights into the role of the ACTH receptor cysteine residues on receptor function. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1120–R1126. doi: 10.1152/ajpregu.00240.2007. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Zhou Y, Spangler R, Maggos CE, Ho A, Kreek MJ. Acute “binge” cocaine increases mu-opioid receptor mRNA levels in areas of the rat mesolimbic mesocortical dopamine system. Brain Res Bull. 1999;48:109–112. doi: 10.1016/s0361-9230(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Landthaler M, Schlussman SD, Yuferov V, Ho A, Tuschl T, Kreek MJ. Mu opioid receptor knockdown in the substantia nigra/ventral tegmental area by synthetic small interfering RNA blocks the rewarding and locomotor effects of heroin. Neuroscience. 2009;158:474–483. doi: 10.1016/j.neuroscience.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Steady-state methadone in rats does not change mRNA levels of corticotropin-releasing factor, its pituitary receptor or proopiomelancortin. Eur J Pharmacol. 1996a;315:31–35. doi: 10.1016/s0014-2999(96)00672-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and type 1 corticotropin releasing factor receptor messenger RNAs in rat brain and pituitary during “binge”-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996b;279:351–358. [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Maggos CE, Wang XM, Han JS, Ho A, Kreek MJ. Hypothalamic–pituitary–adrenal activity and pro-opiomelanocortin mRNA levels in the hypothalamus and pituitary of the rat are differentially modulated by acute intermittent morphine with or without water restriction stress. J Endocrinol. 1999a;163:261–267. doi: 10.1677/joe.0.1630261. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Schlussman SD, Ho A, Spangler R, Fienberg AA, Greengard P, Kreek MJ. Effects of chronic “binge” cocaine administration on plasma ACTH and corticosterone levels in mice deficient in DARPP-32. Neuroendocrinology. 1999b;70:196–199. doi: 10.1159/000054476. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Ho A, Kreek MJ. Increased CRH mRNA levels in the rat amygdala during acute withdrawal from chronic “binge” cocaine. Brain Res Mol Brain Res. 2003a;114:73–79. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of POMC and CRH-R1 mRNAs in the pituitary and hypothalamus of the rat during chronic “binge” cocaine and withdrawal. Brain Res. 2003b;964:187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Yuferov V, Schlussman SD, Ho A, Kreek MJ. Effects of selective D1- or D2-like dopamine receptor antagonists with acute “binge” pattern cocaine on corticotropin-releasing hormone and proopiomelanocortin mRNA levels in the hypothalamus. Mol Brain Res. 2004;130:61–67. doi: 10.1016/j.molbrainres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Yuferov V, Schlussman SD, Ho A, Kreek MJ. Amygdalar vasopressin mRNA increases in acute cocaine withdrawal: evidence for opioid receptor modulation. Neuroscience. 2005;134:1391–1397. doi: 10.1016/j.neuroscience.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi J, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague–Dawley rats. Neuroscience. 2008a;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Leri F, Cummins E, Hoeschele M, Kreek MJ. Involvement of arginine vasopressin and V1b receptor in heroin withdrawal and heroin seeking precipitated by stress and by heroin. Neuropsychopharmacology. 2008b;33:226–236. doi: 10.1038/sj.npp.1301419. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2:1225–1229. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]