Summary
Cellular processes are orchestrated by the precise coordination and regulation of molecular events in the cell. Fluorescent protein-based biosensors coupled with live-cell imaging have enabled the visualization of these events in real time and helped shape some of the current concepts of signal transduction, such as spatial compartmentation. The quantitative information produced by these tools has been incorporated into mathematical models that are capable of predicting highly complex and dynamic behaviors of cellular signaling networks, thus providing a systems level understanding of how pathways interact to produce a functional response. Finally, with technological advances in high throughput and in vivo imaging, these molecular tools promise to continually engender significant contributions to our understanding of cellular processes under normal and diseased conditions.
Introduction
In order to appropriately respond to environmental changes, cells need to accurately sense and translate extracellular cues into functional responses via highly organized intracellular signaling events. Understanding the mechanisms by which signaling information is transferred into the maintenance of proper cellular functions in changing environments is vital for the identification of the altered mechanisms responsible for dysfunction in disease states. While much information can be gained from identifying and characterizing the specific proteins and sequence of biochemical reactions involved in a given pathway, key information is also embedded in the spatial compartmentation and temporal coordination of signaling events (1). Recently, the development of fluorescent protein-based biosensors for live-cell imaging has yielded a better understanding of these dynamic processes by enabling the direct visualization and measurement of molecular events with remarkable spatial and temporal resolution.
Key characteristics of biosensors that have enabled the visualization of signaling activities in intact cells are versatility, sensitivity, specificity and targetability. These biosensors are constructed using various engineering schemes that include surrogate substrates of enzymes, binding domains of second messengers and full length proteins (2). The objective is to achieve sensitive detection of signaling activities with minimal perturbation and high specificity (A detailed discussion of biosensor technology is reviewed elsewhere in this section on ‘Molecular Imaging’ (3)). Being genetically encoded, these biosensors are expressed in situ by cellular machinery, bypassing the need for introducing them into cells via membrane permeabilization. For the same reason, they can be easily targeted to various subcellular loci, such as the nucleus and mitochondria, via the incorporation of known signal sequences, providing sensitive detection of cellular events within specific compartments of the cell. The continuously expanding collection of fluorescent protein-based biosensors includes probes of kinase and phosphatase activity, second messenger dynamics, metabolites such as glucose, and receptor-effector coupling (4–6). These molecular tools have led to new discoveries and greatly enhanced our understanding of the mechanisms of spatiotemporal regulation of events that pervade signaling networks.
Here we highlight current examples in which the use of fluorescent protein-based biosensors has yielded a better understanding of the regulation of signaling systems that govern biological functions.
Resolution of temporal coordination of signaling pathways
Fluorescent protein-based biosensors are powerful tools for resolving molecular events on the time scale of hundreds of milliseconds (4). These highly sensitive probes have shed light on the intricate coordination of events in live-cells, demonstrating that information can be transmitted through temporal patterns in signaling activity.
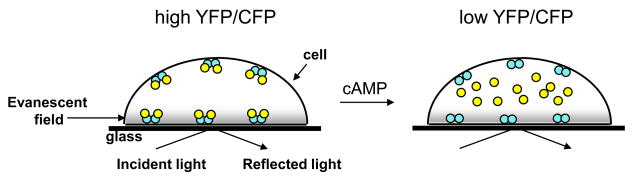
One such example involves the discovery and characterization of synchronized calcium ion (Ca2+) and cyclic 3′,5′ adenosine monophosphate (cAMP) oscillations in insulin-secreting pancreatic beta cells (7). Pancreatic beta cells secrete insulin in response to increases in blood glucose levels, thus playing an integral role in glucose homeostasis. Insulin secretion is dependent on increases in cytoplasmic Ca2+ concentrations as a result of changes in plasma membrane potential. Electrophysiology has shown these intracellular Ca2+ increases are often oscillatory (8). Cyclic AMP is another regulator of insulin secretion and has been shown to potentiate Ca2+-mediated secretion (9). Cyclic AMP is produced upon stimulation of G protein-coupled receptors (GPCRs) and subsequent activation of adenylyl cyclases. The classic effector protein, Protein Kinase A (PKA), consists of a heterotetramer of 2 regulatory subunits and 2 catalytic subunits, which dissociates upon cAMP binding to the regulatory subunits. PKA served as a building block for both a first generation cAMP biosensor (10) and a recently developed biosensor to study cAMP oscillations in beta cells. Using a truncated PKA regulatory subunit tagged with cyan fluorescent protein and targeted to the plasma membrane (ΔRII-CFP-CAAX), and a yellow fluorescent protein labeled PKA catalytic subunit (Cα-YFP), cAMP dynamics were measured in INS-1 beta cells using ratiometric evanescent wave microscopy (11). Increases in intracellular cAMP results in the redistribution of the Cα-YFP portion of the bimolecular probe from the plasma membrane to the cytosol, which can be monitored using total internal reflection fluorescence (TIRF) (Figure 1). It was found that low concentrations (0.3–1.0 nM) of the insulinotropic hormone glucagon-like peptide 1(GLP-1) produced cAMP oscillations. Furthermore Ca2+ and cAMP oscillations are interlinked, since removal of Ca2+ attenuated [cAMP]i oscillations and elevations of [cAMP]i coincided with those of [Ca2+]i. Finally, only sustained cAMP levels and not [cAMP]i oscillations resulted in the translocation of PKA catalytic subunit to the nucleus, an event necessary for PKA-mediated regulation of gene transcription (7). Thus, the second messengers Ca2+ and cAMP are temporally coordinated and reinforce each other in INS-1 beta cells. This study characterizes a new signaling mode for cAMP such that oscillations in the second messenger can bias towards fast cytosolic events, while sustained cAMP elevations affect more long-term signaling such as regulation of gene transcription.
Figure 1. Evanescent wave microscopy and fluorescent protein-based biosensors.
Total internal reflection (TIRF) occurs when a beam of light traveling through a medium of high refractive index, such as glass, encounters one of lower refractive index, such as an adherent cell, at a large angle. TIRF produces a small evanescent wave of light about 100 nm into the cell illuminating molecules within and near the plasma membrane. When combined with a PKA-based biosensor of cAMP that is targeted to the plasma membrane, this evanescent wave microscopy can be used to monitor cAMP dynamics as a decrease of FRET (shown as a decrease of YFP/CFP) as the catalytic subunit of PKA (yellow circles) dissociate from the regulatory subunits (blue circles) upon cAMP binding. This sensitive detection has been used to resolve temporally regulated cAMP dynamics, such as cAMP oscillations in pancreatic beta cells.
Another example of the importance of temporally coordinated signaling involves the transmittance of information in spontaneous neural activity to biochemical reactions necessary for proper visual development. Retinal waves, or spontaneous waves of action potentials, are produced across the retinal ganglion cell layer during early stages of development and are important for the normal development of vision (12). Since both spontaneous neuronal activity and cAMP/PKA have been implicated in retinal development, Dunn et al. probed the connection between these neuronal processes using two biosensors, a cAMP biosensor ICUE2 (13) and a PKA activity biosensor AKAR2.2 (14) in neonatal retinal explants. They found that Ca2+-dependent oscillations in cAMP and PKA activity were evoked by spontaneous depolarization of retinal neurons, demonstrating a new link between spontaneous neuronal activity in developing networks with temporal oscillations in kinase activity (15).
Visualization of compartmentalized signals
The observation that not all cAMP-generating signals elicit the same functional effect in cardiac cells gave rise to the concept of compartmentalization of second messengers and signaling proteins (1). The use of fluorescent protein-based biosensors enables the elucidation of such intricate spatial regulation that is otherwise difficult to capture with traditional biochemical techniques. In this section, we discuss examples in which the application of these powerful tools has helped characterize spatially compartmentalized signaling events.
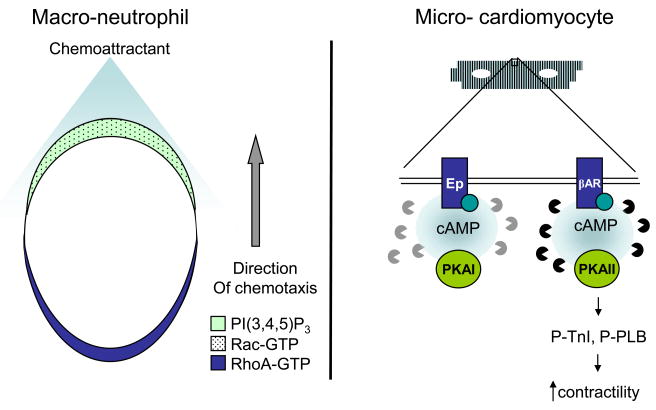
Chemotaxis, or the movement of a cell in response to a chemoattractant, plays an important role in physiological processes such as wound healing and inflammatory responses. Studies have revealed that this mechanochemical process is intricately regulated by the specific localization of various signaling molecules either to the leading or the trailing edge of the chemotaxing cell (16). In particular, a series of biosensors of lipid second messengers and small guanosine triphosphatases (GTPases) have been used to elucidate the molecular mechanisms underlying mammalian neutrophilic leukocyte (neutrophil) chemotaxis (17–20). Exposing differentiated HL-60 (neutrophil-like) cells to a gradient of neutrophil chemoattractant, N-formyl-Met-Leu-Phe (fMLP) resulted in the asymmetrical generation of phosphoinositide 3,4,5 triphosphate, PI(3,4,5)P3, at the leading edge, or pseudopods, of cells as detected by a PI(3,4,5)P3 translocation biosensor. The PI(3,4,5)P3 biosensor is composed a GFP-labeled plextrin homology (PH) domain from Akt (also know as PKB) that recognizes and binds these phosphoinositide molecules generated at the plasma membrane (17). Further examination of this steep internal gradient was performed using a Rac activity biosensor, comprised of a YFP-tagged p-21 binding domain (PBD) from p21-activated kinase (PAK) that recognizes and binds the GTP-activated form of Rac. Imaging with the Rac biosensor revealed that PI(3,4,5)P3 activates Rac, which in turn reciprocally stimulates PI(3,4,5)P3 production, defining a positive feedback mechanism necessary for the formation of the leading edge (18). More recently, these biosensors and a novel FRET-based biosensor of RhoA activity were used to paint a more complete picture of neutrophil polarization. RhoA activity was confined to the sides and trailing edge of the cell due to local PI(3,4,5)P3-mediated inhibition of RhoA activation at the leading edge (19). Thus, multiple biosensors aided in defining ‘frontness’ and ‘backness’ signals that are responsible for neutrophil polarity and direct movement (Figure 2).
Figure 2. Compartmentation of signaling components confer specificity on various scales.
Left: Distinct localization of lipid second messengers (PI(3,4,5)P3) and active small GTPases Rac and RhoA plays important roles in establishing neutrophil polarity and promoting chemotaxis. Right: Specific localization of PKA and PDE isoforms confer specificity of signaling through various GPCRs by establishing cAMP signaling microdomains within cardiomyocytes.
While compartmentation can occur on a cellular scale such as in the establishment of the leading and trailing edge in neutrophils, the formation of microdomains is also seen at the microscopic level within cells. Since PKA has a multitude of substrate targets, it is believed that the precise regulation and localization of the PKA holoenzyme establishes substrate specificity. The two different isoforms of PKA, type I (RI) and type II (RII), have distinct biochemical properties and subcellular localization due to their association with different A kinase anchoring proteins (AKAPs) (21). In order to understand how spatially regulated pools of cAMP and PKA are coordinated to deliver a specific response, the specific AKAP-docking domains from PKA RI and RII were tethered to a FRET-based cAMP biosensor, thus cAMP generation upon various stimuli could be measured at the subcellular compartments of the different isoforms. Upon stimulation of different GPCRs and perturbation of AKAPs and phosphodiesterases (PDEs), these spatially localized probes revealed that each PKA isoform defines a distinct signaling compartment in cardiac myocytes defined by specific cAMP signals that are confined by a subset of cAMP degrading enzymes, PDEs. Specifically, the RII isoform is localized to the particulate fraction of cardiomyocytes where it is activated predominantly via a cAMP pool induced by isoproterenol, a β-adrenergic receptor agonist, which is primarily degraded by PDE4. Conversely, the RI isoform is more diffusible, can be activated by a cAMP pool generated via various GPCRs and degraded by PDE2 (22) (Figure 2). This elegant study provides a mechanistic explanation for the fundamental observation that prostaglandin E1 and isoproterenol lead to the phosphorylation of different PKA substrates resulting in distinct physiological responses (23).
Exemplified in the PKA isoform study, genetically encoded biosensors can easily be targeted to subcellular loci. These subcellular sites not only include organelles but also microdomains that are otherwise beyond the resolution of a standard light microscope. One such example is cholesterol-rich regions of the plasma membrane termed lipid rafts. These specialized regions of the membrane have been suggested to be major regulatory sites of signaling pathways (24). However due to their small size and heterogeneity, rafts are difficult to study and still the subject of controversy regarding their existence (25). Targeting a new reporter of Akt activity, AktAR, to raft and nonraft regions of the membrane revealed the presence of two pools of Akt activity at the plasma membrane in which the raft region exhibited faster and more robust Akt activity compared to nonraft regions. Furthermore, raft integrity was shown to be important for both insulin-like growth factor-1 (IGF-1) and platelet-derived growth factor (PDGF) signaling (26). The direct visualization of differential regulation of Akt signaling within the plasma membrane provides further support for the importance of membrane rafts as unique signaling platforms. Visualization of raft-dependent signaling using biosensors has also been documented for Src activation via epidermal growth factor (EGF) and pervanadate (27) and GPCR-mediated cAMP dynamics (28), demonstrating the wide applicability of this approach for studying the role of lipid rafts.
Fluorescent protein-based biosensors and mathematical modeling
Quantitative imaging with fluorescent protein-based biosensors has made an impact in the field of systems biology (29). With single-cell resolution, time-lapse data collection capability, and the ability to measure a pathway’s components (concentration, localization and posttranslational modifications) in a biological context, biosensors can help define parameters for building mathematical models as well as test their predictions. Such an approach provides quantitative information not only about individual components within a system but also about information flow through the system that cannot be determined by experimental approaches alone, leading to a more comprehensive understanding of signaling at the systems level.
In a clear example of the power of such an approach, the spatial regulation of protein tyrosine phosphatase 1B (PTP1B) catalytic activity was predicted by a mathematical model built upon quantitative imaging data. Using a novel approach termed enzyme substrate (ES) imaging, measurement of Michaelis-Menten enzyme kinetics of PTP1B revealed an ES gradient. The relatively slow establishment of the gradient was not consistent with the high catalytic activity of the enzyme in vitro, and the persistence of the gradient despite variations in concentrations of components did not correlate with a simple reaction-diffusion model which was sensitive to varying levels of phosphatase and kinase. On the other hand, a spatial regulation model was robust to variations in component concentrations and accounted for the apparent lower catalytic activity in vivo. Specifically, low PTP1B activity at the periphery of the cell maintains a low basal activity of the EGF receptor (EGFR) via dephosphorylation and allows signaling upon growth factor stimulation. Conversely, high PTP1B activity at the perinuclear region of the cell, where ligand-activated EGFR is transported upon endocytosis, terminates EGFR signaling (30). Such an approach enables the elucidation of the spatial regulation of a signaling component and could be extended to multiple components in the pathway.
In addition to obtaining quantitative information of individual components, it is important to understand how a signal is propagated within a cell in time and space. Recently, a combined approach utilizing biosensor imaging, immunohistochemistry and mathematical modeling revealed that cellular geometry, along with network topology and kinetic parameters of key biochemical reactions, regulate and define the spatial information flow for the cAMP/PKA/B-Raf/MAPK1,2 network in rat hippocampal neurons (31). Specifically, this study demonstrated that the shape of dendrites and the resulting relatively high surface area favor local accumulation of signal at the membrane and the establishment of PKA and MAPK microdomains, as increasing dendritic diameter resulted in the dissipation of both microdomains. Interestingly, the upstream MAPKK (MEK) and MAPKKK (B-Raf) did not appear to be spatially regulated. Mathematical predictions and experimental confirmation revealed the spatial information was transferred from PKA to MAPK via protein tyrosine phosphatase activity.
Perspective and conclusion
As highlighted here, fluorescent protein-based biosensors have greatly shaped our current understanding of the signaling mechanisms underlying cellular processes. As the ‘molecular toolbox’ grows and imaging technology evolves, the application of these cellular probes promises to expand.
Drug discovery is a particular area that stands to benefit from the application of such biosensors. For example, ligand bias, the ability of ligands to selectively stabilize receptor conformations that stimulate or inhibit subsets of receptor activities, has become an emerging theme in receptor pharmacology. This concept was based upon the observations that receptor activities, such as desensitization, internalization and signaling, can be independently affected via the engagement of the receptor by agonists with distinct properties (32). As general inhibitors of GPCR-mediated signaling pathways exhibit undesirable side effects while having beneficial physiological effects, the discovery of biased ligands of GPCRs presents an exciting new direction for drug development. In fact, an assay using the combination of FRET-based biosensors of both cAMP and receptor internalization has been developed and utilized to identify clinical agonists of the β2-adrenergic receptor (β2-AR) that are biased towards β-arrestin signaling and receptor internalization instead of cAMP-mediated signaling (33). In another example, a FRET-based biosensor of Epac2, a guanine exchange factor for Rap1, was used to screen insulin secretagogues in pancreatic beta cells to identify activators of Epac2, a known promoter of insulin secretion. The antidiabetic sulfonylurea drugs, tolbutamide and glibenclamide, were found to be direct binders and activators of Epac2 leading to Rap1 activation, thus identifying a promising target for antidiabetic drug development (34). Furthermore, a high throughput platform for reading signaling activities, such as kinase activity, in live-cells has already been documented (35), where FRET-based biosensors have been utilized to identify modulators of the GPCR/cAMP/PKA pathway from a clinical compound library. Together, assays for activities or activation of drug targets coupled with a high throughput screening platform, mark the potential of fluorescent biosensors in drug discovery.
Another exciting frontier is to extend the application of these fluorescent biosensors into tissues and organisms. For instance, cAMP and PKA signaling dynamics have been successfully monitored in brain slices using these biosensors (36). More recently, endogenous activation of Cdc42 GTPase was measured in Drosophila embryos, revealing distinct spatial compartmentation of activity on both cellular and whole organism levels, as well as a time-dependent transition from inactive to active form of the protein after the first two-thirds of embryogenesis (37).
In conclusion, fluorescent protein-based biosensors have revolutionized the field of cell biology. In their ability to illuminate once elusive features of cell signaling, they have helped redefine signaling concepts, have provided important insights into cell physiology and promise to continue to make a considerable contribution to the development of novel therapeutics.
Acknowledgments
Work in the lab is funded by NIH (DK073368 and CA122673), the Young Clinical Scientist Award Program of the Flight Attendant Medical Research Institute, a Scientist Development Award from the American Heart Association, and 3M (to J. Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and annotations
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Buxton I, Brunton L. Compartmentation of cAMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983;258:10233–10239. [PubMed] [Google Scholar]
- 2.Miyawaki A. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev Cell. 2003;4:295–305. doi: 10.1016/s1534-5807(03)00060-1. [DOI] [PubMed] [Google Scholar]
- 3.Campbell review
- 4•.Willoughby D, Cooper DMF. Live-cell imaging of cAMP dynamics. Nat Meth. 2008;5:29–36. doi: 10.1038/nmeth1135. This is a comprehensive review of current collection of cAMP biosensor that includes the comparison of their individual properties. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Allen MD. FRET-based biosensors for protein kinases: illuminating the kinome. Mol Biosyst. 2007;3:759–765. doi: 10.1039/b706628g. [DOI] [PubMed] [Google Scholar]
- 6.Lalonde S, Ehrhardt DW, Frommer WB. Shining light on signaling and metabolic networks by genetically encoded biosensors. Curr Opin Plant Biol. 2005;8:574–581. doi: 10.1016/j.pbi.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyachok O, Isakov Y, Sagetorp J, Tengholm A. Oscillations of cyclic AMP in hormone-stimulated insulin-secreting β-cells. Nature. 2006;439:349–352. doi: 10.1038/nature04410. [DOI] [PubMed] [Google Scholar]
- 8.Dryselius S, Grapengiesser E, Hellman B, Gylfe E. Voltage-dependent entry and generation of slow Ca2+ oscillations in glucose-stimulated pancreatic β-cells. Am J Physiol. 1999;276:E512–E518. doi: 10.1152/ajpendo.1999.276.3.E512. [DOI] [PubMed] [Google Scholar]
- 9.Tengholm A, Gylfe E. Oscillatory control of insulin secretion. Mol Cell Endocrinol. 2009;297:58–72. doi: 10.1016/j.mce.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Zaccolo M, Pozzan T. Discrete Microdomains with High Concentration of cAMP in Stimulated Rat Neonatal Cardiac Myocytes. Science. 2001;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 11.Steyer JA, Almers W. A real-time view of life within 100 nm of the plasma membrane. Nature Rev Mol Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- 12.Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 13.Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ. beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem. 2008;283:2949–61. doi: 10.1074/jbc.M707009200. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Hupfeld CJ, Taylor SS, Olefsky JM, Tsien RY. Insulin disrupts beta-adrenergic signalling to protein kinase A in adipocytes. Nature. 2005;437:569–73. doi: 10.1038/nature04140. [DOI] [PubMed] [Google Scholar]
- 15.Dunn TA, Wang C, Colicos MA, Zaccolo M, DiPilato LM, Zhang J, Tsien RY, Feller MB. Imaging of cAMP levels and Protein Kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J Neurosci. 2006;26:12807–12815. doi: 10.1523/JNEUROSCI.3238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens L, Milne L, Hawkins P. Moving towards a better understanding of chemotaxis. Curr Biol. 2008;18:R485–R494. doi: 10.1016/j.cub.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 17.Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–1040. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srinivasan S, Wang F, Glavas S, Ott A, Hofmann F, Aktories K, Kalman D, Bourne HR. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Wang F, Van Keymrulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 20.Wong K, Pertz O, Hahn K, Bourne H. Neutrophil polarization: Spatiotemporal dynamics of RhoA activity support a self-organizing mechanism. Proc Natl Acad Sci USA. 2006;103:3639–3644. doi: 10.1073/pnas.0600092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skalhegg BS, Tasken K. Specificity in the cAMP/PKA signaling pathway. Differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 2000;5:D678–D693. doi: 10.2741/skalhegg. [DOI] [PubMed] [Google Scholar]
- 22••.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein Kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–44. doi: 10.1161/CIRCRESAHA.108.174813. This is an elegant study combining fluorescent biosensor technology with various additional assays including FRAP imaging and immunohistochemistry to elucidate the regulatory mechanisms that give rise to the dichotomous physiological effects of PGE1 and isoproterenol in cardiac myocytes that were first observed over 25 years ago. [DOI] [PubMed] [Google Scholar]
- 23.Hayes JS, Brunton LL, Mayer SE. Selective activation of particulate cAMP-dependent protein kinase by isoproterenol and prostaglandin E1. J Biol Chem. 1980;255:5113–5119. [PubMed] [Google Scholar]
- 24.Hanzal-Bayer MF, Hancock JF. Lipid rafts and membrane traffic. FEBS Lett. 2007;581:2098–104. doi: 10.1016/j.febslet.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Zhang J. Spatiotemporal analysis of differential Akt regulation in plasma membrane microdomains. Mol Biol Cell. 2008;19:4366–4373. doi: 10.1091/mbc.E08-05-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu S, Ouyang M, Seong J, Zhang J, Chien S, Wang Y. The spatiotemporal pattern of Src activation at lipid rafts revealed by diffusion-corrected FRET imaging. PLoS Comput Biol. 2008;4:1–11. doi: 10.1371/journal.pcbi.1000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiPilato LM, Zhang J. The role of membrane microdomains in shaping beta2-adrenergic receptor-mediated cAMP dynamics. Mol Biosyst. 2009;5:832–7. doi: 10.1039/b823243a. [DOI] [PubMed] [Google Scholar]
- 29•.Megason SG, Fraser SE. Imaging in systems biology. Cell. 2007;130:784–95. doi: 10.1016/j.cell.2007.08.031. This is a comprehensive review of the expanding role of molecular imaging in the field of systems biology. [DOI] [PubMed] [Google Scholar]
- 30•.Yudushkin IA, Schleifenbaum A, Kinkhabwala A, Neel BG, Schultz C, Bastiaens PI. Live-cell imaging of enzyme-substrate interaction reveals spatial regulation of PTP1B. Science. 2007;315:115–119. doi: 10.1126/science.1134966. Using a novel biosensor of PTP1B and mathematical modeling, the authors define the spatial regulation of the phosphatase that confers specificity of EGF receptor signaling. The novel, yet generalizeable, engineering scheme described in this study should be able to be extended to multiple enzymes. [DOI] [PubMed] [Google Scholar]
- 31.Neves SR, Tsokas P, Sarkar A, Grace EA, Rangamani P, Taubenfeld SM, Alberini CM, Schaff JC, Blitzer RD, Moraru II, Iyengar R. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell. 2008;133:666–680. doi: 10.1016/j.cell.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. β-arrestin-biased agonism at the β2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 33.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 34••.Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S. The cAMP sensor Eapc2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325:607–610. doi: 10.1126/science.1172256. In this study, a new mode of action of two sulfonylureas was identified. This exciting discovery has important implications for future antidiabetic drug development and screening. [DOI] [PubMed] [Google Scholar]
- 35.Allen MD, DiPilato LM, Rahdar M, Ren YR, Chong C, Liu JO, Zhang J. Reading dynamic kinase activity in living cells for high-throughput screening. ACS Chem Biol. 2006;1:371–376. doi: 10.1021/cb600202f. [DOI] [PubMed] [Google Scholar]
- 36.Gervasi N, Hepp R, Tricoire L, Zhang J, Lambolez B, Paupardin-Tritsch D, Vincent P. Dynamics of protein kinase A signaling at the membrane, in the cytosol, and in the nucleus of neurons in mouse brain slices. J Neurosci. 2007;27:2744–50. doi: 10.1523/JNEUROSCI.5352-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamiyama D, Chiba A. Endogenous activation patterns of Cdc42 GTPase within Drosophila embryos. Science. 2009;324:1338–1340. doi: 10.1126/science.1170615. [DOI] [PMC free article] [PubMed] [Google Scholar]