Abstract
Risperidone is an atypical antipsychotic drug that is widely prescribed to young patients with different psychotic disorders. The long-term effects of this antipsychotic agent on neuronal receptors in developing brain remain unclear and require further investigation. In this study, we examined the effects of long-term treatment of risperidone on two serotonin receptor subtypes in brain regions of juvenile rat. Levels of 5-HT1A and 5-HT2A receptors in forebrain regions of juvenile rats were quantified after 3 weeks of treatment with three different doses of risperidone (0.3, 1.0 and 3.0 mg/kg). Findings were compared to previously reported changes in 5-HT receptors after risperidone treatment (3.0 mg/kg) in adult rat brain. The three doses of risperidone selectively and dose-dependently increased levels of 5-HT1A receptors in medial prefrontal and dorsolateral frontal cortices of juvenile animals. The higher doses (1.0 and 3.0 mg/kg) of risperidone also increased 5-HT1A receptor binding in hippocampal CA1 region of juvenile but not adult rats. In contrast, the three doses of risperidone significantly reduced 5-HT2A labeling in medial prefrontal and dorsolateral frontal cortices in juvenile as well as in adult animals in an equipotent fashion. 5-HT1A and 5-HT2A receptors in other forebrain regions were not altered by repeated risperidone treatment. These findings indicate that there are differential effects of risperidone on 5-HT1A and 5-HT2A receptors in juvenile animals, and that the 5-HT system in developing animals is more sensitive than adults to the long-term effects of risperidone.
Keywords: Autoradiography, Caudate-putamen, Childhood-onset schizophrenia, Frontal cortex, Risperidone, Serotonin receptors
1. Introduction
Childhood-onset schizophrenia is an uncommon, but severe form of psychotic illness that shares many features of the adult-onset disorder (Gordon et al., 1994; Nicolson and Rapoport, 1999). It occurs about 50-times less frequently than adult-onset schizophrenia (Beitchman 1985) when diagnosed by the same criteria applied to adults (Green et al., 1992; Gordon et al., 1994; Spencer and Campbell 1994). Children with schizophrenia can show severe impairments in social and motor development as well as significant premorbid language-delays than those with onset in adolescence (Russell et al., 1989; Alaghband-Rad et al., 1995). Genetic studies confirmed elevated risk of schizophrenia-spectrum disorders in relatives of index cases of childhood-onset schizophrenia compared to families of normal controls or adult-onset schizophrenia patients (Jacobsen and Rapoport, 1998; Nicolson and Rapoport, 1999). Cytogenetic abnormalities are increased in childhood-onset schizophrenia more than in adult-onset schizophrenia, consistent with a neurodevelopmental risk model of the early-onset disorder (Nicolson et al., 1999).
Risperidone (RSP) is widely used for treatment of early onset schizophrenia and other psychotic disorders despite the limited investigation of its efficacy and safety in children and adolescents (Findling and McNamara, 2004). Among the few studies that examined the clinical effects of RSP in pediatric patients, one study found that RSP improved both positive and negative psychotic symptoms in adolescents with schizophrenia (Armenteros et al., 1997), while another study reported that RSP improved cognitive functions in adolescents diagnosed with primary psychotic disorders (Grcevich et al., 1996). RSP was also effective in improving hyperactivity, unstable mood, aggression and self-injurious behaviors in children with pervasive developmental disorders (Perry et al., 1997; Barnard et al., 2002; McCracken et al., 2002; Erickson et al., 2005), and in reducing the severity of motor and vocal tics in patients with Tourette’s syndrome (Bruggeman et al., 2001).
Clinical studies have also indicated that pediatric patients are at a higher risk for adverse neurological, hormonal and metabolic effects of RSP than in adult patients. Therefore, defining the optimal therapeutic dose of RSP in young patients is essential to achieve maximal therapeutic efficacy with minimal adverse side effects. In addition, the bioavailability, absorption and metabolism of RSP in children have not been reported, and studies describing the pharmacokinetics of RSP in children and adolescents compared with adults are rare (Grothe et al., 2000; Frazier et al., 2003; McConville and Sorter, 2004). Well-designed clinical trials of RSP and other newer atypical antipsychotic agents are needed to determine their optimal doses, therapeutic effects, and long-term safety in pediatric or adolescent patients diagnosed with idiopathic neuropsychiatric disorders.
RSP targets multi receptor systems with different affinities (Schotte et al., 1996). Similar to other atypical antipsychotic agents, RSP displays a greater affinity for serotonin 5-HT2A than dopamine (DA) D2 receptors. It also has substantial affinity for DA D3 and D4 receptors, adrenergic α1 and α2 receptors and histamine H1 receptors (Schotte et al., 1996). A substantial number of studies have reported on the pharmacological, neurochemical and behavioral properties of RSP (reviewed in Arnt and Skarsfeldt, 1998; Waddington and Casey, 2000). In addition, we quantified the long-term effects of RSP and other antipsychotic drugs on 5-HT receptor subtypes in adult rat brain and reported differential effects on cortical 5-HT1A vs. 5-HT2A receptors (Tarazi et al., 2002). However, the repeated effects of RSP on 5-HT receptors in developing rat brain require investigation to further elucidate the developmental effects of RSP and its influence on brain maturation. Accordingly, we quantified the binding levels of two major 5-HT receptor subtypes (5-HT1A and 5-HT2A) that are known to mediate, at least in part, the actions of modern antipsychotic agents (Baldessarini and Tarazi, 2005), in different forebrain regions after long-term administration of multiple doses of RSP. We also compared the findings to previously reported effects of RSP-induced changes in same 5-HT receptors in adult rat brain (Tarazi et al., 2002).
2. Experimental procedures
2.1. Materials and animal subjects
[±]-2-(N,N-di[2,3-3H]n-propylamino)-8-hydroxy-1,2,3,4-tetrahydronaphthalene ([3H]8-OH-DPAT; 154 Ci/mmol) was from Amersham (Arlington Heights, IL); and [ethylene-3H]ketanserin hydrochloride ([3H]ketanserin; 63.8 Ci/mmol) was from New England Nuclear-Perkin-Elmer Corp. (Boston, MA). Kodak biomax MR films and D-19 photographic developer and fixative were from Eastman-Kodak (Rochester, NY).
Janssen Pharmaceutica (Titusville, NJ) donated RSP. Methysergide maleate, pargyline hydrochloride, prazosin hydrochloride, and serotonin hydrochloride were from Sigma-RBI (Natick, MA). Cation hydrochlorides and tris-(hydroxymethyl)-aminomethane (Tris) hydrochloride were from Sigma Chemicals (St. Louis, MO).
Subjects were male Sprague-Dawley rats (Charles River Labs., Wilmington, MA) initially weighing 70–80 g at 22 d of age, weaned at 21 d, and maintained under artificial daylight (on, 07:00–19:00 h), in a temp.- and humidity-controlled environment with free access to standard rat chow and tapwater in a USDA-inspected, veterinarian-supervised, small-animal research facility of the Mailman Research Center of McLean Hospital. Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of McLean Hospital, in compliance with pertinent federal and local regulations.
2.2. In vitro serotonin receptor affinity
RSP was tested for affinity at the 5-HT1A and 5-HT2A receptors in juvenile (PD 30) and adult (PD 90) animals using whole rat brain preparations. Sprague-Dawley rats were sacrificed by decapitation. Brains were quickly removed and dissected on ice. Tissue was homogenized in 50 mM Tris-HCl buffer (pH 7.4) containing 150 mM NaCl, washed twice and resuspended in the same buffer. For the 5-HT1A receptor assay, homogenate was incubated with 800 pM [3H]8-OH-DPAT for 30 min at 30°C; nonspecific binding was defined with 10 μM 5-HT (Taylor et al., 1988). For the 5-HT2A receptor assay, homogenate was incubated with 400 pM [3H]ketanserin for 10–15 min at 30°C; nonspecific binding was defined with 10 μM 5-HT (Leysen et al., 1982). Binding was terminated by immersion in an ice bath. Tissue homogenates were rapidly separated from assay buffer mixtures on glass-fiber filter sheets (ISC BioExpress Co., Kaysville, UT) in a Brandel (Gaithersburg, MD) cell harvester, and washed with excess, ice-cold 150 mM saline. Samples on fiber sheets were punched out as discs and placed in minivials containing 4.5 ml Emulsifier-Safe (Packard Instruments, Meriden, CT), and samples were counted for tritium at 50% efficiency in a Beckman-Coulter liquid scintillation spectrophotometer (Fullerton CA). Assay included more than 10 different concentrations of RSP, in triplicate. IC50 ± SE was obtained with the ALLFIT program to fit percent inhibition of specific binding vs. drug concentration, and converted to Ki from the Cheng-Prusoff relationship, Ki = IC50/(1 + F/Kd), all as described previously (Kula et al., 1994).
2.3. Drug treatment and tissue preparation
Four groups of rats (N=6/group), at postnatal day 22 [PD 22]), received single, morning (10:00 h) intraperitoneal (i.p.) injections at 2 ml/kg body wt daily for 21 d. The four groups were given RSP in doses of 0.3, 1.0 or 3.0 mg/kg/d, or physiological saline (0.9% w/v) as a control. RSP doses were guided by molecular and in vivo occupancy studies in adult animals (Kusumi et al., 2000; Tarazi et al., 2002; Kapur et al., 2003). No significant changes in body weight were observed after repeated treatment of juvenile animals with three doses of RSP compared to saline-treated animals. After 3 weeks of treatment, juvenile rats were sacrificed 24 hrs after the last injection of RSP or saline (PD 43) by decapitation; brains were removed, quick-frozen in isopentane on dry ice, and stored at −80°C.
Frozen sections (10 μm) were prepared in a cryostat (Microm HM-505E) at −20°C, mounted on gelatin-coated, glass microscope slides, and stored at −80°C until use. Coronal brain sections were taken through medial prefrontal (MPC) and dorsolateral-frontal (DFC) cerebral cortex, nucleus accumbens (NAc), hippocampus (HIP), and medial and lateral caudate-putamen (CPu) [Fig. 1]. These cortical, limbic and extrapyramidal forebrain regions of interest are implicated in cognitive, emotional, and motor behaviors typically disturbed in young and adult patients with psychotic disorders (Baldessarini and Tarazi, 2005).
Fig. 1.
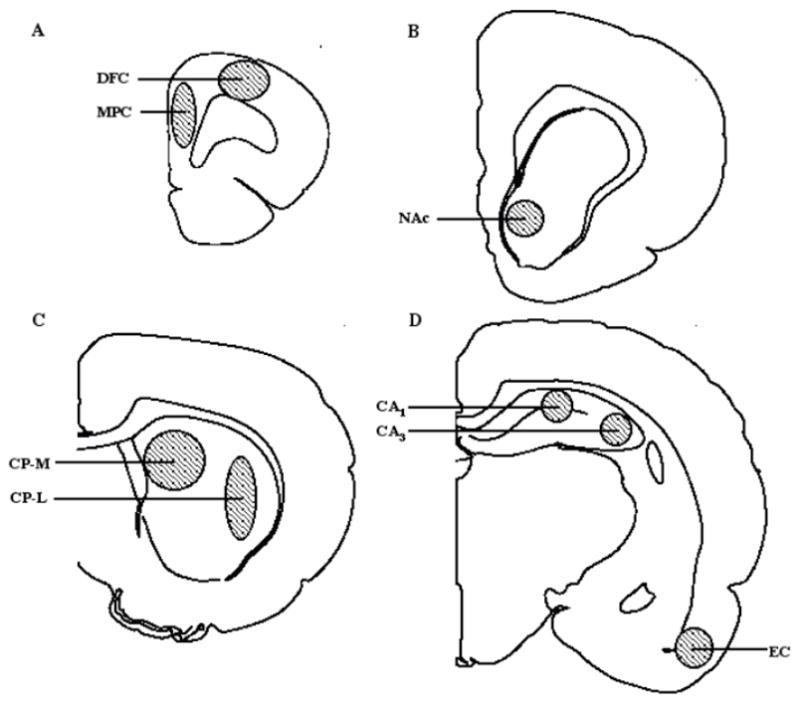
Sites of autoradiographic analyses of rat brain regions sampled in 10 μm coronal sections from: A (A 3.2–4.2), B (A 1.7–2.2), C (A 0.7–1.2), and D (A 0.2–0.7 mm anterior to bregma, according to Paxinos & Watson (1982). Abbreviations: NAc, nucleus accumbens septi; CPu, caudate-putamen (L, lateral and M, medial); DFC, dorsolateral-frontal cerebral cortex; EC, entorhinal cortex; CA1 and CA3, hippocampal regions; MPC, medial prefrontal cortex.
2.4. Receptor autoradiography
Brain sections from all drug- and saline-treated rats (total 864 sections) were evaluated at the same time in each receptor assay to minimize experimental variability. Sections were first preincubated for 1 h at room temperature (RT) in assay buffer to minimize the effects of endogenous 5-HT and potential interference with residual RSP. Preincubation step is effective in minimizing the effects of endogenous neurotransmitters and potential interference of residual drugs (Florijn et al., 1997).
2.4.1. 5-HT1A receptors
Sections were preincubated for 60 min at RT in 50 mM Tris-HCl buffer (pH 7.6) containing ascorbic acid (0.1%, w/v), CaCl2 (4 mM), and the monoamine oxidase (MAO) inhibitor pargyline-HCl (10 μM). Sections were then incubated for another 60 min at RT in buffer containing 2.0 nM [3H]8-OH-DPAT. Nonspecific binding was defined by 5-HT (1 μM). After incubation, slides were washed (2 × 5 min) in ice-cold buffer and dried under a stream of air (Simpson et al., 1996; Tarazi et al., 2002). The inclusion of Ca2+ and the exclusion Na+ and GTP selectively enhances the binding of [3H] 8-OH-DPAT to high-affinity agonist binding state of 5HT1A receptors (Gilman, 1987).
2.4.2. 5-HT2A receptors
5-HT2A receptors were assayed by our previously reported method (Florijn et al., 1997; Tarazi et al., 2002). [3H]ketanserin was used to label the total 5HT2A receptor population (Teitler et al., 1990). Brain sections were preincubated for 60 min at RT in 50 mM Tris-HCl buffer (pH 7.7), and then incubated for another 60 min at RT in fresh buffer containing 3.0 nM [3H]ketanserin and 1 μM prazosin (to block α1-adrenoceptors). Nonspecific binding was defined by methysergide (1 μM). After incubation, slides were washed (2 × 30 min) in ice-cold buffer, dipped in ice-cold water, and dried under a stream of air.
2.5. Autoradiography and image analysis
Radiolabeled sections on microscope slides, along with calibrated [3H]standards (Amersham), were exposed to Hyperfilm for 3–4 weeks at 4°C in light-tight cassettes. Films were developed in Kodak developer and fixative. Brain regions of interest were outlined and their optical density (OD) was quantified with a computerized densitometric image analyzer (MCID-M4, Imaging Research; St. Catharines, Ontario). OD was converted to nCi/mg of tissue with calibrated [3H]standards and, after subtracting nonspecific from total binding, specific binding was expressed as fmol/mg tissue (Tarazi et al., 2002).
2.6. Data analysis
Serotonin receptor binding data were analyzed first for overall effects of drug vs. vehicle for all receptor types and brain regions, using multiple regression modeling methods. Density measures were logarithmically transformed to achieve more Gaussian-like distributions prior to regression modeling. Model goodness-of-fit was checked using partial residual plot methods. Since individual brain specimens provided receptor density data for several brain regions, making for incomplete independence across observations, we used robust standard error estimates to adjust for this clustering effect. The estimation method permits relaxation of the assumption of independence of all observations and requires only that the observations be independent across specimens (Greene, 2000). Estimates of interaction effects were employed for post-hoc tests of drug effects for specific receptors and brain regions, with adjustment of p-values obtained from the regression analyses estimating these multiple comparisons by the standard method of Sidák (Winer et al., 1991). Data are presented as means ± SEM. Comparisons were considered significant at two-tailed p<0.05 based on N=6 rats/treatment group.
3. Results
Experiments with rat brain homogenates indicated that RSP exhibits low affinity for 5-HT1A receptors in juvenile and adult animals (Ki > 600 nM; Table 1). In contrast, RSP has very high affinity for 5-HT2A receptors in both aged groups (Ki < 1.7 nM; Table 1). No significant differences were observed in RSP’s affinity for either 5-HT1A or 5-HT2A receptors in developing vs. mature animals.
Table 1.
Affinity (Ki, nM ± S.E.) of risperidone at 5-HT1A and 5-HT2A receptors in juvenile vs. adult whole rat brain preparations
| Rat brain tissue | 5-HT1A | 5-HT2A |
|---|---|---|
| Juvenile animals (PD 30) | 750 ± 44 | 1.5 ± 0.4 |
| Adult animals (PD 90) | 620 ± 58 | 1.2 ± 0.2 |
Three weeks of daily injections of 0.3, 1.0 and 3.0 mg/kg of RSP to juvenile rats (from PD 22 to PD 42) significantly and dose-dependently increased binding of [3H]8-OH-DPAT to 5-HT1A receptors in MPC (by 22%, 49% and 75%, respectively; F [df =3; 20] = 11.8, p<0.001) and DFC (by 29%, 51% and 67%; F [df=3; 20] = 15.2, p<0.001) of juvenile rats (Table 2). In addition, repeated treatment with 1.0 and 3.0 mg/kg RSP increased levels of 5HT1A receptors in hippocampal CA1 region (by 38% and 57%; F [df=3; 20] = 16.1, p<0.001) of juvenile rats (Table 2).
Table 2.
5-HT1A receptor binding in juvenile rats (PD 42) after three weeks of daily administration of risperidone
| Adult Rats | Juvenile Rats | |||
|---|---|---|---|---|
| Brain region | Controls (3.0 mg/kg) | RSP (0.3 mg/kg) | RSP (1.0 mg/kg) | RSP (3.0 mg/kg) |
| Cerebral cortex | ||||
| Medial-prefrontal | 34.5 ± 1.0 (100) (152)* | 42.0 ± 2.3 (122)* | 51.3 ± 3.3 (149)* | 60.2 ± 2.0 (175)* |
| Dorsolateral | 21.5 ± 1.5 (100) (173)* | 27.8 ± 1.5 (129)* | 32.4 ± 2.1 (151)* | 36.0 ± 2.3 (167)* |
| Nucleus accumbens | 4.8 ± 0.3 (100) (119) | 4.7 ± 0.3 (98) | 5.0 ± 0.5 (104) | 5.1 ± 0.4 (106) |
| Caudate-putamen | ||||
| Medial | 4.9 ± 0.2 (100) (101) | 5.0 ± 0.2 (102) | 5.1 ± 0.3 (105) | 5.2 ± 0.2 (106) |
| Lateral | 5.4 ± 0.3 (100) (96) | 4.9 ± 0.1 (91) | 5.3 ± 0.3 (98) | 4.8 ± 0.2 (89) |
| Hippocampus | ||||
| CA1- Region | 67.7 ± 5.7 (100) (98) | 74.3 ± 6.7 (110) | 93.2 ± 7.8 (138)* | 106.1 ± 9.0 (157)* |
| CA3- Region | 32.4 ± 1.2 (100) (105) | 32.0 ± 1.5 (99) | 36.1 ± 2.0 (111) | 35.2 ± 1.3 (109) |
| Entorhinal cortex | 35.7 ± 2.5 (100) (99) | 34.2 ± 3.0 (96) | 34.2 ± 3.6 (96) | 35.1 ± 2.6 (98) |
Data are mean ± SEM values for binding (fmol/mg tissue, [% of control]), determined by quantitative autoradiography following daily i.p. injection of vehicle or risperidone (RSP) for 3 weeks, with significant differences from controls indicated in bold ([*] p<0.05, N=6 rats/group). Data (% of control) for RSP (3 mg/kg/d) in adult animals were reported previously (Tarazi et al., 2002) and are shown for comparison.
Long-term treatment with the three doses of RSP (0.3, 1.0 and 3.0 mg/kg) significantly decreased binding of [3H]ketanserin to 5HT2A receptors in an equipotent fashion in the MPC (by 41%, 38% and 36%, F [df=3; 20] = 17.4, p<0.001) and DFC (by 40%, 42% and 44%, F [df=3; 20] = 22.0, p<0.001) of juvenile rats (Table 3). There were no changes in 5-HT2A-selective labeling in other brain region analyzed after long-term administration of three doses of RSP (Table 3).
Table 3.
5-HT2A receptor binding in juvenile rats (PD 42) after three weeks of daily administration of risperidone
| ADULT RATS | JUVENILE RATS | |||
|---|---|---|---|---|
| Brain region | Controls (3.0 mg/kg) | RSP (0.3 mg/kg) | RSP (1.0 mg/kg) | RSP (3.0 mg/kg) |
| Cerebral cortex | ||||
| Medial-prefrontal | 80.9 ± 5.3 (100) (56)* | 48.1 ± 2.5 (59)* | 50.0 ± 2.7 (62)* | 51.8 ± 2.4 (64)* |
| Dorsolateral | 74.8 ± 4.7 (100) (69)* | 45.0 ± 2.4 (60)* | 43.4 ± 3.8 (58)* | 42.2 ± 2.3 (56)* |
| Nucleus accumbens | 20.0 ± 3.1 (100) (98) | 17.3 ± 1.4 (87) | 17.9 ± 1.9 (90) | 18.8 ± 2.9 (94) |
| Caudate-putamen | ||||
| Medial | 20.9 ± 3.5 (100) (87) | 21.5 ± 3.2 (103) | 22.4 ± 2.1 (107) | 22.2 ± 3.3 (106) |
| Lateral | 23.3 ± 10.6 (100) (92) | 24.6 ± 1.7 (106) | 22.2 ± 1.4 (95) | 22.8 ± 1.0 (98) |
| Hippocampus | ||||
| CA1- Region | 44.4 ± 3.1 (100) (96) | 45.4 ± 3.0 (102) | 46.2 ± 3.1 (104) | 45.7 ± 2.8 (103) |
| CA3- Region | 38.2 ± 1.7 (100) (96) | 36.0 ± 2.3 (94) | 36.2 ± 3.7 (95) | 34.7 ± 3.9 (91) |
| Entorhinal cortex | 34.0 ± 3.9 (100) (87) | 33.9 ± 2.0 (100) | 31.4 ± 1.6 (92) | 32.9 ± 1.6 (97) |
Data are mean ± SEM values for binding (fmol/mg tissue, [% of control]), determined by quantitative autoradiography following daily i.p. injection of vehicle or risperidone (RSP) for 3 weeks, with significant differences from controls indicated in bold ([*] p<0.05, N=6 rats/group). Data (% of control) for RSP (3 mg/kg/d) in adult animals were reported previously (Tarazi et al., 2002) and are shown for comparison.
4. Discussion
4.1. Effects of risperidone treatment on 5-HT1A receptors
Developmental studies reported that the highest densities of 5-HT1A receptors were found in the cerebral cortex and hippocampus of the human fetal brain between the 16th and 22nd weeks of gestation. These levels were even higher than that reported in cortex and hippocampus of adult human brain (Bar-Peled O et al., 1991). Animal studies found that 5-HT1A receptor expression increased steadily in cerebral cortex, hippocampus and septum from birth till reaching adulthood levels around the third postnatal week (Daval et al., 1987). In our study, we found that long-term administration of 0.3, 1.0 and 3.0 mg/kg of RSP for 21 days significantly and dose-dependently increased 5-HT1A receptor binding in MPC (by 22%, 49% and 75%, respectively), and DFC (by 29%, 51% and 67%) of juvenile rats (age 43 days; Table 2). The significant increases in cortical 5-HT1A receptors in juvenile animals after repeated treatment with RSP parallel the observed increases in 5-HT1A receptors in adult animals after long-term treatment with 3.0 mg/kg of RSP (Table 2; Tarazi et al., 2002).
RSP displayed low affinity for cortical 5-HT1A receptors in both juvenile and adult animals (Ki = 750 nM and 620 nM, respectively, Table 1). This is in agreement with the reported low 5-HT1A receptor-potency of RSP (Ki = 490 nM; Bymaster et al., 1996). The relatively low 5-HT1A receptor-affinity of RSP in juvenile and adult animals suggests that RSP-induced increases in [3H]8-OH-DPAT binding to 5HT1A receptors in MPC and DFC reflect indirect mechanisms resulting from more potent effects of RSP on 5-HT2A (Table 2) and D2 receptors (Moran-Gates et al., 2007). Microdialysis study in adult animals suggested that RSP-induced increases in cortical DA release resulted from combined blockade of 5-HT2A and D2 receptors through a 5-HT1A-sensitive stimulatory mechanism and not from direct 5-HT1A receptor activation by RSP (Ichikawa et al., 2001). It is likely that such interactions also occur in juvenile animals and contribute to RSP-induced increases in cortical 5-HT1A receptors
Autoradiographic studies conducted on adult rat brain tissue found that 5-HT1A receptors are highly expressed in rat raphe nuclei, cerebral cortex, and hippocampus, with much lower levels in CPu and NAc (Pazos and Palacios 1985; Pompeiano et al., 1992). In frontal cortex, substantial levels of 5-HT1A receptors are found on neocortical glutamatergic pyramidal neurons that are implicated in cognitive and executive functions (Francis et al., 1992). 5-HT1A receptors have been suggested as neurotherapeutic targets that mediate the beneficial effects of atypical antipsychotic drugs (Millan, 2000). Our findings further support a role for 5-HT1A receptors in mediating actions of RSP and other antipsychotic drugs not only in adults but also in developing animals. It should be noted that postmortem studies reported significant increases in 5-HT1A receptors in frontal cerebral cortex of adult patients diagnosed with schizophrenia (Hashimoto et al., 1993; Burnet et al., 1996; Simpson et al., 1996; Sumiyoshi et al., 1996). Nonetheless, these findings were not reported in young schizophrenia patients, largely owing to the low prevalence of the early-onset disorder and lack of access to postmortem brain tissue from developing patients.
RSP, at 1.0 and 3.0 mg/kg, also increased the abundance of 5-HT1A receptors in the hippocampal CA1 region of juvenile animals (Table 2). This effect was unique to developing animals as a similar dose of RSP (3.0 mg/kg) failed to alter levels of 5-HT1A receptors in hippocampus of adult animals (Tarazi et al., 2002). This unique age-related receptor and neurochemical differences in RSP’s actions suggests that RSP can selectively modulate signal transduction cascades associated with hippocampal 5-HT1A receptors in juvenile and not adult animals.
4.2. Effects of risperidone treatment on 5-HT2A receptors
RSP displayed potent affinity for cortical 5-HT2A receptors in both juvenile and adult animals (Ki = 1.5 nM and 1.2 nM, respectively, Table 1). This is also in agreement with the reported high 5-HT2A receptor-potency of RSP (Ki = 0.6 nM; Bymaster et al., 1996). Three weeks of daily administration of 0.3, 1.0 and 3.0 mg/kg RSP significantly and equipotently decreased binding of [3H]ketanserin to 5HT2A receptors in MPC (by 41%, 38% and 36%, respectively) and DFC (by 40%, 42% and 44%, respectively), and DFC (by 29%, 51% and 67%) of juvenile rats (age 43 days; Table 2). These findings are in agreement with the observed decreases in 5-HT2A receptors in adult animals after continuous treatment with 3.0 mg/kg of RSP (Table 3; Tarazi et al., 2002). It is worthy to note that a low dose of RSP (0.3 mg/kg) reduced cortical 5-HT2A receptor levels in juvenile animals similar to that detected after treatment with a high dose of RSP (3.0 mg/kg) in adult animals (Table 3, Tarazi et al., 2002). These findings further support our hypothesis that developing animals are more sensitive than adults to the long-term neuronal effects of RSP.
Anatomical studies have located 5-HT2A receptors on local GABAergic neurons as well as on corticostriatal glutamatergic pyramidal neurons in frontal cortex (Francis et al., 1992; Wright et al., 1995). Long-term treatment with dissimilar atypical, and not typical, antipsychotic agents reduced 5-HT2A receptor labeling in rat frontal cerebral cortex (O’Dell et al., 1990; Florijn et al., 1997; Kuoppamaki et al., 1995; Kusumi et al., 2000; Tarazi et al., 2002). We have also found that repeated treatment with atypicals clozapine and olanzapine, and not typical agent fluphenazine, decreased 5-HT2A receptor binding in frontal cortex of juvenile animals [PD 43; Gardner et al., in preparation]. The high 5-HT2A receptor-affinity of RSP suggests that the observed decrease in 5-HT2A binding may reflect adaptations to direct 5-HT2A receptor blockade in both developing and mature animals. Alternatively, and based on the close functional interactions between 5-HT2A and glutamate NMDA receptors (Higgins et al., 2003), it is possible that RSP-induced downregulation of NMDA receptors (Choi et al., 2007) may alter 5-HT neurotransmission and lead to the observed reductions in cortical 5-HT2A receptors. Downregulation of 5-HT2A receptor after long-term treatment with antipsychotic drugs and other 5-HT2A antagonists is a paradoxical effect that contrasts the expected upregulation of neurotransmitter receptors after chronic blockade. Antagonist-induced internalization of 5-HT2A receptors is a possible explanation for this unique molecular phenomenon (Gray and Roth, 2001).
These findings suggest that 5-HT2A receptors constitute one of the common targets that mediate the actions of RSP in juvenile and adult animals. 5-HT2A receptor downregulation may help to compensate for the loss of dopaminergic function in the striatum due to RSP-induced D2 receptor upregulation and accordingly may lower the incidence of extrapyramidal adverse events and tardive dyskinesia associated with RSP and perhaps other atypical antipsychotics compared with typical antipsychotics in both young and adult patients (Tarsy et al., 2002; Baldessarini and Tarazi 2005). Selective changes in 5-HT2A receptors in cerebral cortex and not in other brain regions examined (Table 3) reflects the differential regional responses of 5-HT2A receptors in juvenile animals to repeated RSP treatment.
4.3. Conclusions
Long-term administration of multiple doses of RSP produced dose-dependent increases in 5-HT1A receptors in MPC and DFC of juvenile animals. Such increases were also observed in adult animals (Table 2). In contrast, repeated treatment with 1.0 and 3.0 mg/kg RSP increased 5-HT1A receptors in hippocampal CA1 region of juvenile and not adult animals (Table 2). Differences in concentrations of 5-HT1A receptors or magnitude of associated signal transduction cascades evelopmental may have contributed to age-related differences in hippocampal 5-HT1A receptor responses to long-term RSP treatment. Downregulation of cortical 5-HT2A receptors after repeated administration of RSP and other atypical antipsychotic agents suggest that these receptors are common targets that mediate, at least in part, the actions on RSP in developing and mature animals. However, the observed reduction in cortical 5-HT2A receptors in juvenile animals after treatment with a low dose of RSP (0.3 mg/kg) was similar to that observed after treatment with a high dose of RSP (3.0 mg/kg) in adult animals (Table 3). Together, these findings suggest that the 5-HT system in developing animals is more sensitive than adults to the long-term effects of RSP and perhaps other atypical antipsychotic agents.
Acknowledgments
Role of the funding source
Supported by Korea Research Foundation Grant funded by the Korean Government (KRF- 2006-214-C00057) [YKC], US NIH federal grants HD-052752 and Janssen Pharmaceutica [FIT].
Risperidone was generously donated by Janssen Pharmaceutica.
Footnotes
Contributors
Yong-Kee Choi, Taylor Moran-Gates, Matthew P. Gardner, and Frank I. Tarazi
Conflict of interest
None of the authors has any financial or non-financial conflict of interest with the findings of this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaghband-Rad J, McKenna K, Gordon CT, Albus KE, Hamburger SD, Rumsey JM, Frazier JA, Lenane MC, Rapoport JL. Childhood-onset schizophrenia: the severity of premorbid course. J Am Acad Child Adolesc Psychiatry. 1995;34:1273–1283. doi: 10.1097/00004583-199510000-00012. [DOI] [PubMed] [Google Scholar]
- Armenteros JL, Whitaker AH, Welikson M, Stedge DJ, Gorman J. Risperidone in adolescents with schizophrenia: an open pilot study. J Am Acad Child Adolesc Psychiatry. 1997;36:694–700. doi: 10.1097/00004583-199705000-00021. [DOI] [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Tarazi FI. Pharmacotherapy of psychosis and mania. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 2005. pp. 461–500. [Google Scholar]
- Barnard L, Young AH, Pearson J, Geddes J, O’Brien G. A systematic review of the use of atypical antipsychotics in autism. J Psychopharmacol. 2002;16:93–101. doi: 10.1177/026988110201600113. [DOI] [PubMed] [Google Scholar]
- Bar-Peled O, Gross-Isseroff R, Ben-Hur H, Hoskins I, Groner Y, Biegon A. Fetal human brain exhibits a prenatal peak in the density of serotonin 5-HT1A receptors. Neurosci Lett. 1991;127:173–176. doi: 10.1016/0304-3940(91)90787-t. [DOI] [PubMed] [Google Scholar]
- Beitchman JH. Childhood schizophrenia. A review and comparison with adult-onset schizophrenia. Psychiatr Clin North Am. 1985;8:793–814. [PubMed] [Google Scholar]
- Bruggeman R, van der Linden C, Buitelaar JK, Gericke GS, Hawkridge SM, Temlett JA. Risperidone versus pimozide in Tourette’s disorder: a comparative double-blind parallel-group study. J Clin Psychiatry. 2001;62:50–56. doi: 10.4088/jcp.v62n0111. [DOI] [PubMed] [Google Scholar]
- Burnet PWJ, Eastwood SL, Harrison PJ. 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology. 1996;15:442–455. doi: 10.1016/S0893-133X(96)00053-X. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Choi YK, Gardner MP, Tarazi FI. Effects of risperidone on glutamate receptor subtypes in developing rat brain. Eur Neuropsychopharmacol. 2009;19:77–84. doi: 10.1016/j.euroneuro.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daval G, Vergé D, Becerril A, Gozlan H, Spampinato U, Hamon M. Transient expression of 5-HT1A receptor binding sites in some areas of the rat CNS during postnatal development. Int J Dev Neurosci. 1987;5:171–180. doi: 10.1016/0736-5748(87)90027-x. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Stigler KA, Posey DJ, McDougle CJ. Risperidone in pervasive developmental disorders. Expert Rev Neurother. 2005;5:713–719. doi: 10.1586/14737175.5.6.713. [DOI] [PubMed] [Google Scholar]
- Findling RL, McNamara NK. Atypical antipsychotics in the treatment of children and adolescents: clinical applications. J Clin Psychiatry. 2004;65 (Suppl 6):30–44. [PubMed] [Google Scholar]
- Florijn WJ, Tarazi FI, Creese I. Dopamine receptor subtypes: differential regulation after 8 months treatment with antipsychotic drugs. J Pharmacol Exp Ther. 1997;280:561–569. [PubMed] [Google Scholar]
- Francis PT, Pangalos MN, Pearson RCA, Middlemiss DN, Stratmann GC, Bowen DM. 5-Hydroxytryptamine-1A but not 5-hydroxytryptamine-2 receptors are enriched on neocortical pyramidal neurons destroyed by intrastriatal volkensin. J Pharmacol Exp Ther. 1992;261:1273–1281. [PubMed] [Google Scholar]
- Frazier JA, Cohen LG, Jacobsen L, Grothe D, Flood J, Baldessarini RJ, Piscitelli S, Kim GS, Rapoport JL. Clozapine pharmacokinetics in children and adolescents with childhood-onset schizophrenia. J Clin Psychopharmacol. 2003;23:87–91. doi: 10.1097/00004714-200302000-00012. [DOI] [PubMed] [Google Scholar]
- Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Gilman AG. G proteins: Transducers of receptor-generated signals. Ann Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Gordon CT, Frazier JA, McKenna K, Giedd J, Zametkin A, Zahn T, Hommer D, Hong W, Kaysen D, Albus KE. Childhood-onset schizophrenia: an NIMH study in progress. Schizophr Bull. 1994;20:697–712. doi: 10.1093/schbul/20.4.697. [DOI] [PubMed] [Google Scholar]
- Grcevich SJ, Findling RL, Rowane WA, Friedman L, Schulz SC. Risperidone in the treatment of children and adolescents with schizophrenia: a retrospective study. J Child Adolesc Psychopharmacol. 1996;6:251–257. doi: 10.1089/cap.1996.6.251. [DOI] [PubMed] [Google Scholar]
- Greene WH. Econometric Analysis. 4. Upper Saddle River, NJ: Prentice Hall; 2000. pp. 462–465. [Google Scholar]
- Green WH, Padron-Gayol M, Hardesty AS, Bassiri M. Schizophrenia with childhood onset: a phenomenological study of 38 cases. J Am Acad Child Adolesc Psychiatry. 1992;31:968–976. doi: 10.1097/00004583-199209000-00027. [DOI] [PubMed] [Google Scholar]
- Grothe DR, Calis KA, Jacobsen L, Kumra S, DeVane CL, Rapoport JL, Bergstrom RF, Kurtz DL. Olanzapine pharmacokinetics in pediatric and adolescent inpatients with childhood-onset schizophrenia. J Clin Psychopharmacol. 2000;20:220–225. doi: 10.1097/00004714-200004000-00015. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kitamura N, Kajimoto Y, Shirai Y, Shirakawa O, Mita T, Nishino N, Tanaka C. Differential changes in serotonin 5-HT1A and 5-HT2 receptor binding in patients with chronic schizophrenia. Psychopharmacology. 1993;112:S35–S39. doi: 10.1007/BF02245005. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and ‘impulsive-type’ behaviors produced by NMDA receptor antagonism. Psychopharmacology. 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Rapoport JL. Childhood-onset schizophrenia: implications of clinical and neurobiological research. J Child Psychol Psychiatry. 1998;39:101–113. [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Kula NS, Baldessarini RJ, Kebabian JW, Neumeyer JL. S(+)-Aporphines are not selective for human D3 dopamine receptors. Cell Mol Neurobiol. 1994;14:185–191. doi: 10.1007/BF02090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuoppamaki M, Palvimaki EP, Hietala J, Syvalahti E. Differential regulation of rat 5-HT2Aand 5-HT2C receptors after chronic treatment with clozapine, chlorpromazine and three putative atypical antipsychotic drugs. Neuropsychopharmacology. 1995;13:139–150. doi: 10.1016/0893-133X(95)00049-J. [DOI] [PubMed] [Google Scholar]
- Kusumi I, Takahashi Y, Suzuki K, Kameda K, Koyama T. Differential effects of subchronic treatments with atypical antipsychotic drugs on dopamine D2 and serotonin 5-HT2A receptors in the rat brain. J Neural Transm. 2000;107:295–302. doi: 10.1007/s007020050024. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982;21:301–314. [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J, McDougle CJ, Posey D, Swiezy N, Kohn A, Scahill L, Martin A, Koenig K, Volkmar F, Carroll D, Lancor A, Tierney E, Ghuman J, Gonzalez NM, Grados M, Vitiello B, Ritz L, Davies M, Robinson J, McMahon D Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- McConville BJ, Sorter MT. Treatment challenges and safety considerations for antipsychotic use in children and adolescents with psychoses. J Clin Psychiatry. 2004;65:S20–S29. [PubMed] [Google Scholar]
- Millan MJ. Improving the treatment of schizophrenia: Focus on serotonin 5-HT1A receptors. J Pharmacol Exp Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- Moran-Gates T, Grady C, Park YS, Baldessarini RJ, Tarazi FI. Effects of risperidone on dopamine receptor subtypes in developing rat brain. Eur Neuropsychopharmacol. 2007;17:448–455. doi: 10.1016/j.euroneuro.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R, Giedd JN, Lenane M, Hamburger S, Singaracharlu S, Bedwell J, Fernandez T, Thaker GK, Malaspina D, Rapoport JL. Clinical and neurobiological correlates of cytogenetic abnormalities in childhood-onset schizophrenia. Am J Psychiatry. 1999;156:1575–1579. doi: 10.1176/ajp.156.10.1575. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46:1418–1428. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, La Hoste GJ, Widmark CB, Shapiro RM, Potkin SG, Marshall JF. Chronic treatment with clozapine or haloperidol differentially regulates dopamine and serotonin receptors in rat brain. Synapse. 1990;6:146–153. doi: 10.1002/syn.890060205. [DOI] [PubMed] [Google Scholar]
- Paxinos F, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in rat brain. I Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Perry R, Pataki C, Munoz-Silva DM, Armenteros J, Silva RR. Risperidone in children and adolescents with pervasive developmental disorder: pilot trial and follow-up. J Child Adolesc Psychopharmacol. 1997;7:167–179. doi: 10.1089/cap.1997.7.167. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of messenger RNA coding for 5-HT1A receptor in the rat brain–correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AT, Bott L, Sammons C. The phenomenology of schizophrenia occurring in childhood. J Am Acad Child Adolesc Psychiatry. 1989;28:399–407. doi: 10.1097/00004583-198905000-00017. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PFM, Gommeren W, Luyten WHML, Gompel PV, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology. 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Simpson MDC, Lubman DI, Slater P, Deakin JFW. Autoradiography with [3H]8-OH-DPAT reveals increases in 5-HT1A receptors in ventral prefrontal cortex in schizophrenia. Biol Psychiatry. 1996;39:919–928. doi: 10.1016/0006-3223(95)00026-7. [DOI] [PubMed] [Google Scholar]
- Spencer EK, Campbell M. Children with schizophrenia: diagnosis, phenomenology, and pharmacotherapy. Schizophr Bull. 1994;20:713–725. doi: 10.1093/schbul/20.4.713. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Stockmeier CA, Overholser JC, Dilley GE, Meltzer HY. Serotonin1A receptors are increased in postmortem prefrontal cortex in schizophrenia. Brain Res. 1996;708:209–214. doi: 10.1016/0006-8993(95)01361-x. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Zhang K, Baldessarini RJ. Long-term effects of olanzapine, risperidone, and quetiapine on serotonin 1A, 2A and 2C receptors in rat forebrain regions. Psychopharmacology. 2002;161:263–270. doi: 10.1007/s00213-002-1016-3. [DOI] [PubMed] [Google Scholar]
- Tarsy D, Baldessarini RJ, Tarazi FI. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs. 2002;16:23–45. doi: 10.2165/00023210-200216010-00003. [DOI] [PubMed] [Google Scholar]
- Taylor EW, Nikam SS, Lambert G, Martin AR, Nelson DL. Molecular determinants for recognition of RU 24969 analogs at central 5-hydroxytryptamine recognition sites: use of a bilinear function and substituent volumes to describe steric fit. Mol Pharmacol. 1988;34:42–53. [PubMed] [Google Scholar]
- Teitler M, Leonhardt S, Weisberg EL, Hoffman BJ. 4-[125I]iodo-(2,5-dimethoxy)phenyl-isopropylamine and [3H]ketanserin labeling of 5-hydroxytryptamine2 (5-HT2) receptors in mammalian cells transfected with a rat 5-HT2 cDNA: Evidence for multiple states and not multiple 5-HT2 receptor subtypes. Mol Pharmacol. 1990;38:594–598. [PubMed] [Google Scholar]
- Waddington JL, Casey D. Comparative pharmacology of classical and novel (second-generation) antipsychotics. In: Waddington JL, Buckley PF, editors. Schizophrenia and Mood Disorders. Butterworth-Heinemann; Oxford: 2000. pp. 1–13. [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. 3. New York: McGraw Hill; 1991. pp. 165–166. [Google Scholar]
- Wright DE, Seroogy KB, Lundgren KH, Davis BM, Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J Comp Neurol. 1995;351:357–373. doi: 10.1002/cne.903510304. [DOI] [PubMed] [Google Scholar]


