Abstract
Although the glossopharyngeal nerve (IX) is mainly a sensory nerve, it innervates stylopharyngeus and some other pharyngeal muscles, whose excitations would likely improve upper airway patency since electrical IX stimulation increases pharyngeal airway size. As acute intermittent hypoxia (AIH) induces hypoglossal and genioglossal long-term facilitation (LTF), we hypothesized that AIH induces glossopharyngeal LTF, which requires serotonin 5-HT2 and NMDA receptors. Integrated IX activity was recorded in anesthetized, vagotomized, paralyzed and ventilated rats before, during and after 5 episodes of 3-min isocapnic 12% O2 with 3-min intervals of 50% O2. Either saline, ketanserin (5-HT2 antagonist, 2 mg/kg) or MK-801 (NMDA antagonist, 0.2 mg/kg) was (i.v.) injected 30–60 min before AIH. Both phasic and tonic IX activities were persistently increased (both P<0.05) after AIH in vehicle, but not ketanserin or MK-801, rats. Hypoxic glossopharyngeal responses were minimally changed after either drug. These data suggest that AIH induces both phasic and tonic glossopharyngeal LTF, which requires activation of 5-HT2 and NMDA receptors.
Keywords: Glossopharyngeal nerve, Long-term facilitation, Serotonin receptors, NMDA receptors, Respiratory control, Plasticity
1. Introduction
Coordinated activation of different pharyngeal muscles is important in maintaining upper airway patency. A decrease in pharyngeal dilator muscle activity during sleep can cause recurrent upper airway collapse in obstructive sleep apnea (OSA) patients, which in turn generates intermittent hypoxia and hypercapnia. The glossopharyngeal nerve (i.e., the ninth cranial nerve) is primarily a sensory nerve, but it also innervates stylopharyngeus and jointly innervates levator veli palatini, upper pharyngeal constrictor and cricopharyngeus through the pharyngeal plexus (Nishio et al., 1976; Furusawa et al., 1991; Williams, 1995). These pharyngeal muscles, when co-activated, can improve upper airway patency. For example, stimulation of the glossopharyngeal nerves increased the velopharyngeal and oropharyngeal cross-sectional areas in cats (Kuna and Brennick, 2002), whereas anesthetizing this nerve caused dorsal velopharyngeal collapse and inspiratory obstruction during exercise in horses (Tessier et al., 2004). Our recent, preliminary data also showed that transection of this nerve increased pharyngeal collapsibility throughout the breathing cycle in rats (Cao and Ling, 2007). These results suggest that efferent motor output of the glossopharyngeal nerve may play an important role in the neuromuscular control of upper airway patency.
Acute intermittent hypoxia (AIH, 3–10 episodes of short-term hypoxia) induces a persistent increase in respiratory activity, known as long-term facilitation (LTF), and pretreatment with chronic intermittent hypoxia (in some forms) enhances the AIH-induced LTF (Ling et al., 2001; McGuire et al., 2003; Peng and Prabhakar, 2003; Zabka et al., 2003; McGuire and Ling, 2005a). LTF has been identified in many animal species (Millhorn et al., 1980a; Cao et al., 1992; Hayashi et al., 1993; Turner and Mitchell., 1997; Mitchell et al., 2001b; Sokolowska and Pokorski, 2006; Terada et al., 2008), and manifested in forms of respiratory nerve and muscle activity in anesthetized animals, and ventilation in awake animals (cf. Mitchell et al., 2001a; Ling, 2008). Since LTF was also elicited in snorers and OSA patients during non-rapid eye movement (NREM) sleep (Babcock and Badr, 1998; Aboubakr et al., 2001), some have speculated that LTF may help stabilize upper airway in OSA patients after repeated apneas and/or hypopneas (Bach and Mitchell., 1996; Babcock and Badr, 1998; Ling et al., 2001; Bocchiaro and Feldman, 2004; Mahamed and Mitchell, 2007, 2008). However, nearly all LTF studies focused on major inspiratory muscles and/or their innervating nerves.
So far, all tested LTF is serotonin-dependent (cf. Mitchell et al., 2001a; Ling, 2008). More specifically, AIH-induced ventilatory and phrenic LTF is prevented by 5-HT2 receptor antagonism with ketanserin (Kinkead et al., 1998, 1999; Ling et al., 2001; McGuire et al., 2004). Both LTF also requires NMDA receptors (McGuire et al., 2005b, 2008). Serotonin receptors are required for induction, but not maintenance, of phrenic LTF (Fuller et al., 2001). However, NMDA receptors are necessary for both formation and maintenance of ventilatory LTF (McGuire et al., 2008). We believe that LTF might be a general property of the respiratory control system and that 5-HT2 and NMDA receptors might be required for induction and maintenance of all AIH-induced LTF (cf. Ling, 2008). Therefore the present study was designed to test whether AIH induces glossopharyngeal LTF, and if so, to determine whether this LTF requires 5-HT2 and NMDA receptors. We hypothesized that AIH would induce LTF in the efferent motor output of this mixed sensory and motor nerve, and this LTF requires activation of both serotonin 5-HT2 and NMDA receptors.
2. Methods
Experiments were conducted on adult male Sprague-Dawley rats (250–350 g, colony CDIGS, Charles River, Wilmington, MA). All experimental procedures described herein were approved by the Harvard Medical Area Standing Committee on Animals. The present study involved four series of experiments. The first series (n=7) tested if AIH induces glossopharyngeal LTF. The second series (n=5) was the time control group without AIH. The third series (n=6) explored the role of serotonin 5-HT2 receptors in glossopharyngeal LTF by examining the LTF following systemic injection of the selective serotonin 5-HT2 receptor antagonist ketanserin. The fourth series (n=6) explored the role of NMDA receptors in this LTF using the NMDA receptor antagonist MK-801. Both ketanserin and MK-801 (Sigma, USA) are widely used antagonists, which easily dissolve in saline and cross the blood-brain barrier.
2.1. Experimental preparation
The rats were anesthetized initially with isoflurane in a closed chamber and then injection of urethane (~1.6 g/kg in distilled water, i.p.). The depth of anesthesia was adjusted so that the animal failed to show a reflex withdrawal of the hind paw to a strong pinch. The trachea was cannulated and the rat was mechanically ventilated (Harvard Apparatus Inc, Holliston, MA, USA). The inspired gas mixture was 50% oxygen balanced by nitrogen during baseline and other non-hypoxia periods to improve the tolerance to experimental stresses and prolong the viability of the preparation. Bilateral vagotomy was performed at the mid-cervical level. A femoral venous catheter was inserted for fluid administration. A femoral arterial catheter was also placed to allow blood sample withdrawal for arterial blood gases and pH analysis. Pancuronium bromide (2.5 mg/kg, i.v., followed by additional doses as needed) was given to obtain neuromuscular blockade. About 30 min before AIH protocol, a supplemental dose of urethane (0.16 g/kg) was given to provide stable anesthesia for the remainder of the experiment. A slow infusion of sodium bicarbonate (5%) and lactated Ringer solution (50: 50, ~1.7 ml/kg/h) was initiated ~1 h after induction of anesthesia to maintain fluid and acid-base balance. Rectal temperature was maintained at 37.0–37.5 °C with a servo-controlled heat blanket and a heating lamp.
End-tidal CO2 partial pressure (PET, CO2) was monitored in the expired line of the ventilator circuit using a flow-through capnograph (Novametrix; Wallingford, CT, USA) with sufficient response time (< 75 ms). The CO2-apneic threshold was defined as the PET, CO2 at which respiratory rhythmic activity resumed from hypocapnic silence in glossopharyngeal nerve activity recording. PET, CO2 was maintained at 5 mmHg above the threshold throughout the experiment by manipulating the inspired CO2, ventilator's rate and/or tidal volume. Setting PET, CO2 at 5 mmHg (not usual 2–3 mmHg) above the threshold was to make the glossopharyngeal rhythmic signal more evident and stable. PET, CO2 values obtained with this method closely approximated CO2 partial pressure in arterial blood (Pa, CO2) (usually within 1.2 mmHg). At the end of the experiments, rats were sacrificed by a lethal dose of urethane (~3.2 g/kg).
2.2. Glossopharyngeal nerve recording
The left glossopharyngeal nerve was dissected via a dorsal approach, cut distally [to avoid damaging the carotid sinus nerve (CSN)] and prepared for recording with a bipolar silver wire electrode. The glossopharyngeal nerve activity was filtered (300–10,000 Hz) and amplified (×10,000, BMA-200AC/DC Bioamplifier, CWE Inc., Ardmore, PA, USA). The amplified signal was full-wave rectified and integrated (Paynter Filter, BAK Electronics, Inc., Mount Airy, MD, USA; time constant=100 ms). The integrated glossopharyngeal nerve signals were digitized and acquired with computer software (LabView 8.0, National Instruments Corporation, USA), and analyzed with a customized program developed in our laboratory. This program determined the amplitude and timing of integrated glossopharyngeal nerve bursts, from which the minute glossopharyngeal activity could be calculated. This program also calculated the magnitude of tonic integrated glossopharyngeal nerve activity (during expiration).
2.3. Experimental protocols
The first baseline (B1) glossopharyngeal nerve activity was measured ~60 min after completion of the surgical preparation. Then vehicle (saline), ketanserin (2 mg/kg) or MK-801 (0.2 mg/kg) was systemically administrated (all ~1 ml, i.v.). The CO2-apneic threshold was measured again ~20 min after the drug administration and the second baseline (B2, PET, CO2 also set at 5 mmHg above the threshold) was measured. Rats were then exposed to AIH [5 episodes of 3-min isocapnic hypoxia (12% O2), separated by 3-min intervals of hyperoxia (50% O2)]. The integrated glossopharyngeal nerve activity was measured during and up to 60 min after AIH. In some rats, arterial blood samples (~0.3 ml) were drawn during B2, and at 30, 45 and/or 60 min post-hypoxia to ensure an isocapnic condition. At the end of the protocol, the glossopharyngeal nerve responses to hypercapnia (PET, CO2 = 90–95 mmHg) were recorded to obtain a measure of approximately maximal glossopharyngeal activity.
The time control experiments were conducted in separate rats (n=5), in which the naturally occurring fluctuation in glossopharyngeal nerve activity was examined. In these experiments, the same glossopharyngeal nerve activity measurement was conducted, except that the AIH protocol was not employed.
2.4. Data analysis
Integrated glossopharyngeal nerve activity was averaged in ~1 min bins at 11 time points (i.e., B1, B2, 5 hypoxia episodes and 4 post-hypoxia time points; note only one baseline in vehicle and time control rats). The hypoxic data were collected during the last minute of the 3-min hypoxia exposure when amplitude of integrated glossopharyngeal nerve activity had reached a plateau, and were averaged over five hypoxia episodes, since there was no significant difference among these episodes (the activity did not progressively increase during those intervals either). Two sets of data were calculated from these recordings: 1) those recorded during five hypoxia episodes to determine hypoxic glossopharyngeal response (HGR); and 2) those recorded at 15, 30, 45 and 60 min post-hypoxia time points to determine glossopharyngeal LTF (see Fig. 1).
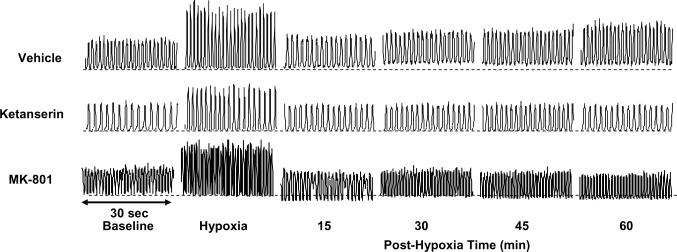
Fig. 1.
Representative tracings of the integrated glossopharyngeal nerve neurogram. These tracings were recorded before (baseline), during (hypoxia) and after acute intermittent hypoxia (AIH, 5 episodes of 3-min isocapnic 12% O2) in 3 rats, injected with vehicle (saline), ketanserin (2 mg/kg) and MK-801 (0.2 mg/kg), respectively. The horizontal dotted lines under the neurogram recordings represent the baseline magnitude of tonic integrated glossopharyngeal nerve activity. LTF exists in both phasic and tonic glossopharyngeal activity in the vehicle rat, but not in the ketanserin or MK-801 rat. There also appears to be tonic activity reduction in some post-hypoxia time points in the MK-801 rat, but this is not typical in the group data (see Fig. 5).
Variables determined in each time point included 3 phasic variables: the peak amplitude of integrated glossopharyngeal nerve activity (arbitrary units), glossopharyngeal nerve burst frequency (bursts/min) and minute glossopharyngeal nerve activity (peak amplitude × burst frequency), and one tonic variable: the magnitude of integrated glossopharyngeal nerve activity during expiration. Changes from baseline in the peak amplitude and minute activity were normalized as a percentage of their baseline (%baseline) and hypercapnic values (%maximum; to minimize normalization artifacts caused by variable baseline activities). Changes from baseline in the burst frequency used absolute units (bursts/min), while changes from baseline in the magnitude of expiratory integrated glossopharyngeal nerve activity were normalized as a percentage of the baseline (phasic) peak amplitude (%baseline peak amplitude). Thus for phasic variables, baseline changes were calculated by the equation: (B2-B1)/B1; HGR was calculated by (hypoxia-B2)/B2; and LTF at each time point was calculated by (post hypoxia-B2)/B2 (but frequency changes were absolute differences without normalization). For the tonic magnitude, HGR and LTF were also calculated by the same 2 equations except that the denominator B2 was baseline (phasic) peak amplitude instead of baseline tonic activity.
For LTF, both the within-group increases from baseline and the between-group differences in the post-hypoxia peak amplitude (also in the burst frequency, minute activity and magnitude of tonic activity) were statistically analyzed by a two-way ANOVA with repeated measures, followed by the Student-Newman-Keuls post-hoc test (SigmaStat version 3.1, Jandel Corporation, San Rafael, CA, USA). For baseline, HGR, apneic threshold and ratio of baseline to hypercapnic activity, a one-way ANOVA was used to statistically analyze the differences between and within groups unless otherwise stated. P<0.05 was considered significant. All values are expressed as means ± SE.
3. Results
3.1. Baseline setting
The CO2-apneic threshold for glossopharyngeal nerve activity in vehicle rats (PET, CO2: 41.4 ± 1.2 mmHg, n=7) was only measured after saline injection, and was not significantly different (all P>0.5; t-test) from that in time control (40.8 ± 1.0, n=5), ketanserin (before injection: 39.2 ± 1.2, n=6; after injection: 39.8 ± 1.0, n=5) or MK-801 (before: 39.3 ± 0.8, n=6; after: 40.3 ± 0.8, n=6) rats. The threshold was not significantly changed before and after ketanserin injection (n=5, P=0.305, paired t-test), but was slightly increased after MK-801 injection (n=6, P=0.041, paired t-test).
In vehicle rats, the ratio of baseline peak amplitude to hypercapnic peak amplitude was 52.4 ± 2.1% (n=7), which was relatively higher compared to our previous studies (Zhang et al., 2003; McGuire et al., 2005b), due likely to the fact that baseline PET, CO2 was set at 5 mmHg (not usual 2–3 mmHg) above the CO2-apneic threshold. This ratio was not significantly changed after ketanserin injection (52.5 ± 5.1%, n=5, P=0.949; t-test) or MK-801 injection (61.6 ± 7.5%, n=6, P=0.394; t-test).
3.2. Glossopharyngeal responses to AIH
HGR
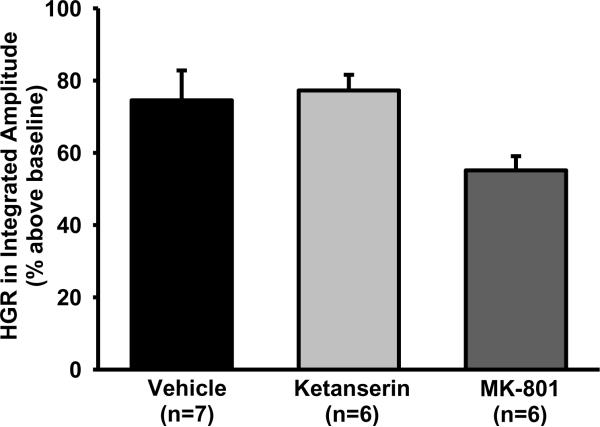
The glossopharyngeal responses (including peak amplitude, burst frequency, minute activity and tonic magnitude) to short-term isocapnic hypoxia were not significantly different among five hypoxia episodes in any group (all P>0.103) and were therefore averaged. In vehicle rats (n=7), average HGR was 74.5 ± 8.3% above baseline in the peak amplitude (Fig. 2), 6.1 ± 2.1 bursts/min above baseline (37.7 ± 6.8 bursts/min) in the burst frequency and 99.4 ± 11.7% above baseline in the minute activity (all P<0.02). However, average HGR in the tonic magnitude was only 1.1 ± 1.1% above baseline (n=7, P=0.834). These results suggest that during hypoxia, all 3 phasic variables were significantly increased above baseline, but the tonic variable was unchanged.
Fig. 2.
The hypoxic glossopharyngeal response (HGR) in the peak amplitude of integrated glossopharyngeal nerve activity. This HGR is the average response to 5 episodes of 3-min isocapnic hypoxia (12% O2) and is expressed as a percentage increase above baseline (% above baseline). Vehicle (saline), ketanserin (5-HT2 antagonist) or MK-801 (NMDA antagonist) was systemically injected ~30 min before hypoxia. Data are presented as means ± SE. There is no significant difference in the amplitude HGR among these 3 groups (1-way ANOVA, F2,18=3.655; P=0.064).
LTF
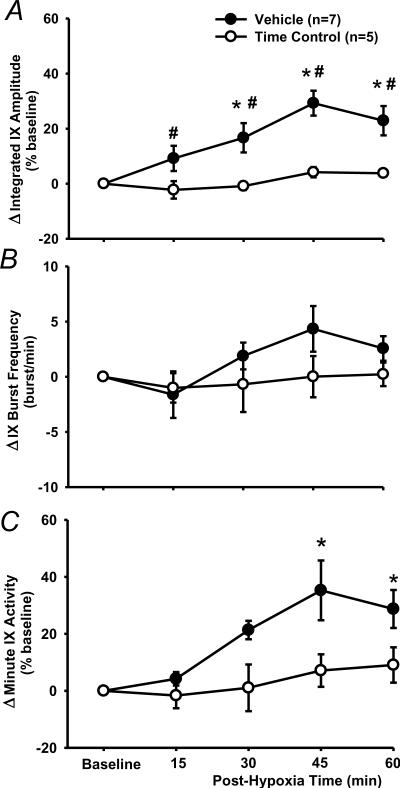
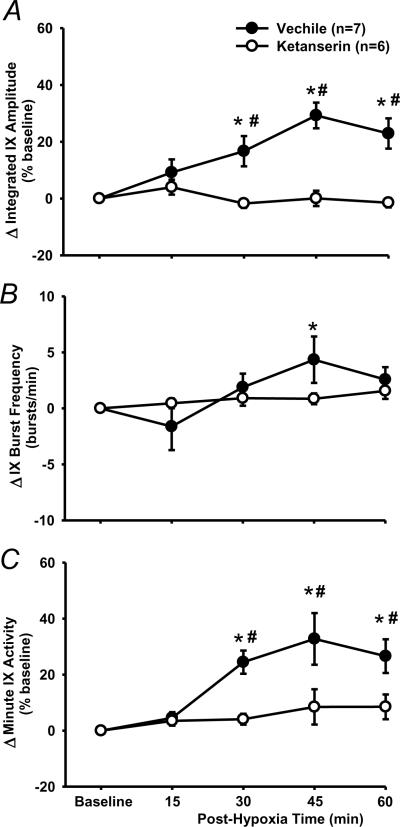
In vehicle rats (n=7), the peak amplitude progressively increased after AIH and reached its maximal value at 45 min post-hypoxia (Figs. 1, 3A, 4). The two-way ANOVA revealed a significant interaction effect (F4,40=3.394; P=0.018) between the group factor (2 levels: vehicle vs. time control) and time factor (5 levels: baseline, 15, 30, 45 and 60 min post-hypoxia) in peak amplitude (%baseline) data (Fig. 3A). The post-hypoxia peak amplitude (%baseline) at 30, 45 and 60 min post-hypoxia were significantly increased from baseline in vehicle rats (all P<0.05) and all post-hypoxia time points were larger than those corresponding ones in time control rats (all P<0.05, Fig. 3A). In the time control group, the peak amplitude was not significantly different from baseline at any post-hypoxia points (all P>0.499, Fig. 3A).
Fig. 3.
LTF of phasic glossopharyngeal nerve (IX) activity (%baseline). (A) Average changes from baseline in peak amplitude of integrated IX activity, normalized as a percentage of the baseline (%baseline). (B) Changes from baseline in IX burst frequency (bursts/min). (C) Changes from baseline in minute IX activity, normalized as a %baseline. These data were obtained before and after acute intermittent hypoxia (AIH, 5 episodes of 3-min isocapnic 12% O2) in vehicle (n=7) and time control (n=5) rats. Data are expressed as means ± SE. * Significant difference from baseline. # Significant difference from time control group (P< 0.05).
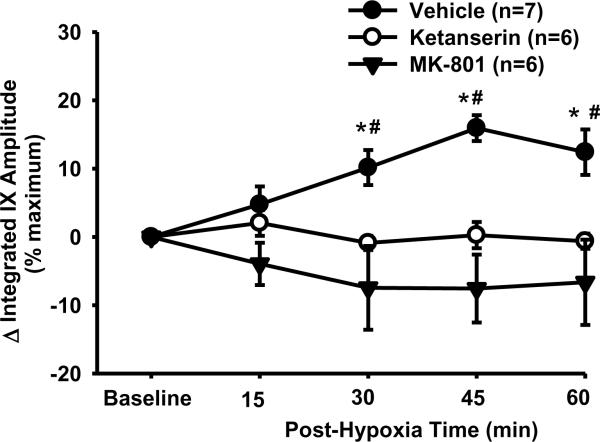
Fig. 4.
LTF of phasic glossopharyngeal nerve (IX) activity (%maximum). Average changes from baseline in peak amplitude of integrated IX activity were normalized as a percentage of the hypercapnic response (%maximum). These data were obtained before and after acute intermittent hypoxia (AIH, 5 episodes of 3-min isocapnic 12% O2) in vehicle (n=7), ketanserin (n=6) and MK-801 (n=6) rats. There is a significant interaction (F8,56=5.04; P<0.001) between the group factor (3 groups) and time factor. Data are expressed as means ± SE. * Significant difference from baseline. # Significant difference from other two groups (P< 0.05).
The burst frequency appeared to be increased after AIH (Fig. 3B), but the interaction effect (F4,40=1.105; P=0.367) between the group and time factors, and the overall time effect (F4,40=1.941; P=0.122) were insignificant. Finally, in vehicle and time control rats, increases in the minute activity (%baseline) after AIH (Fig. 3C) were similar to those in the peak amplitude (Fig. 3A), although the between-group differences lost their statistical significance. Virtually the same conclusions can be drawn from the peak amplitude (%maximum) data, although the LTF values are numerically smaller than those %baseline ones (Fig. 4). This is also true for the minute activity (%maximum) data in vehicle rats, and all LTF (%maximum) data in ketanserin and MK-801 rats (see the peak amplitude data in Fig. 4). Thus only the %baseline data are presented in the present study except those in Fig. 4. Collectively, these results suggest that the AIH induces LTF in phasic glossopharyngeal nerve activity.
3.3. 5-HT2 receptor antagonism
Baseline and HGR
The baseline in peak amplitude was not significantly decreased after ketanserin injection (−6.4 ± 6.0%, n=6, P=0.229, paired t-test). The baseline in burst frequency (42.8 ± 2.8 bursts/min) and minute activity appeared to be decreased after ketanserin injection (frequency: −7.8 ± 3.7, minute activity: −19.7 ± 13.4%), but these decreases were not significant (both P>0.089, paired t-test). The average HGR was not significantly different between vehicle and ketanserin rats in peak amplitude (ketanserin: 77.2 ± 4.3%, n=6, P=0.785 vs. vehicle, t-test; Fig. 2), burst frequency (8.7 ± 3, P=0.488 vs. vehicle) or minute activity (129.3 ± 16.6%, P=0.16 vs. vehicle). These results suggest that systemic injection of ketanserin (2 mg/kg) has little effect on baseline glossopharyngeal activity and the HGR.
LTF
There was a significant interaction effect (F4,44=8.948; P<0.001) between the drug factor (vehicle vs. ketanserin) and time factor in peak amplitude data (Fig. 6A). The peak amplitude in ketanserin rats was not significantly different from baseline at any post-hypoxia time points (all P>0.428), but was significantly lower than the corresponding value at 30, 45 and 60 min in vehicle rats (all P<0.001; Fig. 6A). There was no significant interaction effect (F4,44=2.14; P=0.092) between the drug factor (vehicle vs. ketanserin) and time factor in the burst frequency (Fig. 6B). Finally, changes in the minute activity (Fig. 6C) were similar to those in the peak amplitude (Fig. 6A). These results suggest that phasic glossopharyngeal LTF was abolished by pretreatment with ketanserin (2 mg/kg).
Fig. 6.
The effect of 5-HT2 receptor antagonism on LTF in phasic glossopharyngeal nerve (IX) activity. (A) Average changes from baseline in peak amplitude of integrated IX activity, normalized as a percentage of the baseline (%baseline). (B) Changes from baseline in IX burst frequency (bursts/min). (C) Changes from baseline in minute IX activity, normalized as a %baseline. These values were obtained before and after 5 episodes of 3-min isocapnic hypoxia (12% O2) in vehicle (n=7) and ketanserin (n=6) rats. Data are expressed as means ± SE. * Significant difference from baseline. # Significant difference from ketanserin group (P< 0.05).
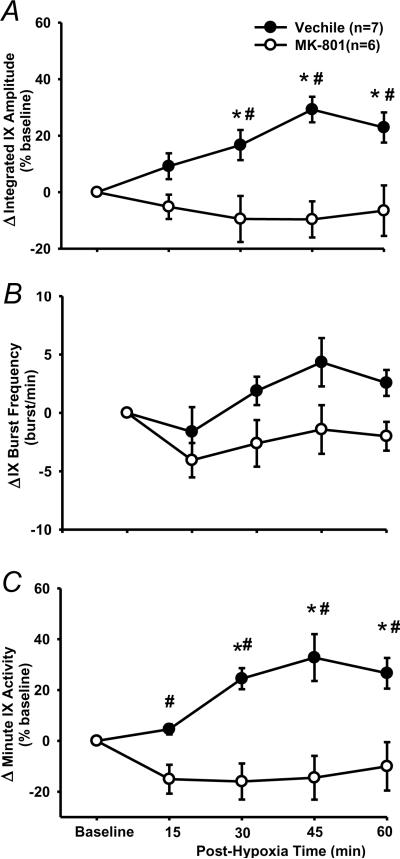
3.4. NMDA receptor antagonism
Baseline and HGR
After MK-801 injection, baseline was significantly decreased in peak amplitude (−27.7 ± 5.1%, P=0.003, paired t-test), but increased in burst frequency (15 ± 5.4 above the baseline 43.7 ± 2.7 bursts/min, P=0.038, paired t-test), leading to a minimal change in minute activity (−4.4 ± 10.8%, P=0.89, paired t-test). The average HGR appeared to be decreased but the decrease was not significant in peak amplitude (MK-801: 55.1 ± 3.9%, n=6, P=0.07 vs. vehicle, t-test; Fig. 2), burst frequency (5.9 ± 2.1 bursts/min, n=6, P=0.941 vs. vehicle, t-test) or minute activity (85.4 ± 17%, n=6, P=0.502 vs. vehicle, t-test). These data suggest that the systemic injection of MK-801 (0.2 mg/kg) reduced the peak amplitude of baseline glossopharyngeal activity but did not significantly change the HGR.
LTF
There was a significant interaction effect (F4,44=6.179; P<0.001) between the drug factor (vehicle vs. MK-801) and time factor in the peak amplitude data (Fig. 7). The peak amplitude in MK-801 rats was not significantly different from baseline at any post-hypoxia time points (all P>0.413; Figs 7A), but was significantly lower than the corresponding value at 30, 45 and 60 min post-hypoxia time points in the vehicle rats (all P<0.002; Fig. 7A). There was no significant interaction effect (F4,44=1.44; P=0.237) between the drug factor (vehicle vs. MK-801) and time factor in the burst frequency (Fig. 7B). Finally, changes in the minute activity (Fig. 7C) were similar to those in the peak amplitude (Fig. 7A). These results suggest that phasic LTF was abolished by pretreatment with MK-801 (0.2 mg/kg).
Fig. 7.
The effect of NMDA receptor antagonism on LTF in phasic glossopharyngeal nerve (IX) activity. (A) Average changes from baseline in peak amplitude of integrated IX activity, normalized as a percentage of the baseline (%baseline). (B) Changes from baseline in IX burst frequency (bursts/min). (C) Changes from baseline in minute IX activity, normalized as a %baseline. These were obtained before and after 5 episodes of 3-min isocapnic hypoxia (12% O2) in vehicle (n=7) and M-801 (n=6) rats. Data are expressed as means ± SE. * Significant difference from baseline. # Significant difference from MK-801 group (P< 0.05).
Similar conclusions could be drawn when all four groups (vehicle, time control, ketanserin and MK-801) were analyzed together to determine the phasic LTF and the drug effects. For example, the interaction (F12,80=4.049; P<0.001) between the group (4 groups) and time factors, and the overall group effect (F3,80=10.482; P<0.001) were significant in the peak amplitude data, but the interaction (F12,80=1.14; P=0.341) in the burst frequency data was insignificant. Changes in the minute activity were similar to those in the peak amplitude, as both interaction (F12,80=2.813; P=0.003) and overall group effects (F3,80=13.653; P<0.001) were all very significant. In addition, the results of the post-hoc tests were all similar to those that were analyzed separately, except that there were significant differences in minute activity between the vehicle and time control rats at 30, 45 and 60 min, and no difference in minute activity between the vehicle and MK-801 rats at 15 min post-hypoxia. Collectively, these results suggest that the AIH induces LTF in phasic glossopharyngeal nerve activity and this LTF is abolished by pretreatment with ketanserin or MK-801.
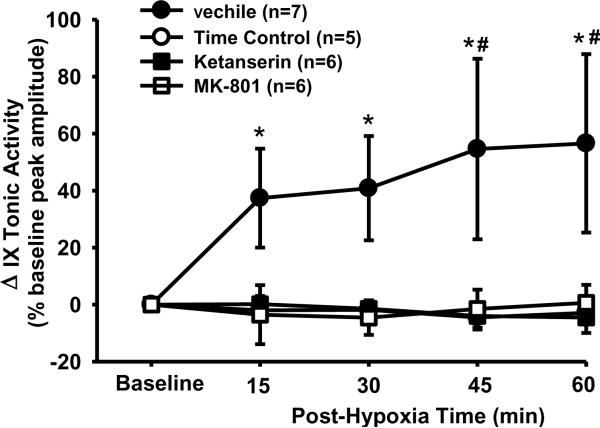
3.5. Tonic glossopharyngeal LTF
All four groups of data were also analyzed together to determine the tonic LTF and the drug effects. The interaction (F12,80=2.339; P=0.013) between the group (4 groups) and time factors, and the overall group effect (F3,80=3.278; P<0.042) were all significant. In vehicle rats, the tonic glossopharyngeal nerve activity was increased after AIH (Fig. 1A) and the tonic activity magnitude was significantly increased from baseline at all post-hypoxia time points (all P<0.001; Fig. 5). This increase, however, appeared to follow a different pattern; the tonic activity reached its plateau at 15 min post-hypoxia (Fig. 5). In contrast, the tonic activity magnitude in the time control, ketanserin or MK-801 rats was not significantly different from baseline at any post-hypoxia time points (all P>0.754), but was significantly lower than the corresponding value at 45 and 60 min post-hypoxia time point in vehicle rats (all P<0.026; Fig. 5). Collectively, these results suggest that AIH induces tonic glossopharyngeal LTF, which is abolished by pretreatment with ketanserin or MK-801.
Fig. 5.
LTF in tonic glossopharyngeal nerve (IX) activity with and without 5-HT2 or NMDA receptor antagonism. The magnitude of tonic integrated IX activity was obtained before and after 5 episodes of 3-min isocapnic hypoxia (12% O2) in vehicle (n=7), time control (n=5), ketanserin (n=6) and MK-801 (n=6) rats. Average changes from baseline in the tonic activity magnitude were normalized as a percentage of the baseline (phasic) peak amplitude (%baseline peak amplitude). There is a significant interaction (F12,80=2.339; P=0.013) between the group factor (4 groups) and time factor. These Data are expressed as means ± SE. * Significant difference from baseline. # Significant difference from all other three groups (P< 0.05).
3.6. Arterial blood gases
Arterial blood gases were measured in 10 rats (Table 1). Pa, O2 appeared to be decreased from baseline along the experiment in most rats, but all Pa, O2 remained above 150 mmHg (Table 1), indicating a consistent hyperoxic condition after AIH. All post-hypoxia Pa, CO2 closely approximated their baseline values (all differences ≤ 1.2 mmHg; Table 1), suggesting a consistent isocapnic condition throughout these experiments. In this subgroup of vehicle rats (n=4), Pa, CO2 value at post-hypoxia 45–60 min (44.7 ± 1.6 mmHg) was not significantly different (P=0.546, paired t-test) from baseline (44.4 ± 2.0; Table 1). The peak amplitude was increased by 3.1 ± 4.3% at 15 min, 15.4 ± 5.8% at 30 min, 27.1 ± 2.2 at 45% min and 24.2 ± 9.3% at 60 min after AIH (n=4). There was a significant interaction effect (F4,28=3.295; P=0.025) between the group factor (vehicle subgroup vs. time control) and time factor in peak amplitude data. The post-hypoxia peak amplitude at 30, 45 and 60 min post-hypoxia were all significantly increased from baseline (all P<0.032) and larger than the corresponding ones in time control rats (all P<0.004), indicating a peak amplitude LTF. In addition, when comparing this vehicle subgroup (n=4) with other no-blood-gas vehicle rats (n=3), the interaction between the group and time factors in the peak amplitude data was not significant (F4,20=0.574; P=0.685).
Table1.
Arterial blood gases in vehicle, ketanserin, MK-801 and time control rats
|
Pa,CO2 (mmHg) |
Pa,O2 (mmHg) |
|||||
|---|---|---|---|---|---|---|
| Baseline | P30 | P45 or P60 | Baseline | P30 | P45 or P60 | |
| Vehicle (n=4) | 46.5 | 47 | 46.8 | 193.9 | 201.6 | 201.9 |
| 45.3 | 46.1 | 44.4 | 224.9 | 178 | 175.3 | |
| 47 | 47.6 | 189.7 | 199.7 | |||
| 39.9 | 38.7 | 166.4 | 154.5 | |||
| Ketanserin (n=3) | 46.1 | 45.1 | 45.9 | 183.4 | 171.1 | 159.6 |
| 44.9 | 43.7 | 173.1 | 164 | |||
| 38.3 | 39.1 | 190.7 | 152.5 | |||
| MK-801 (n=2) | 44.2 | 45.1 | 197.5 | 184.3 | ||
| 45.5 | 44.9 | 182.4 | 176.6 | |||
| Time Control (n=1) | 47.4 | 46.6 | 46.5 | 201.2 | 197.5 | 190.2 |
P30, P45 and P60 denote post-hypoxia 30, 45 and 60 min time points. Values in each row were all obtained from one single rat.
4. Discussion
The present study demonstrated that AIH induced a persistent increase in both phasic and tonic glossopharyngeal nerve activity. Following systemic injection of the serotonin 5-HT2 receptor antagonist ketanserin (2 mg/kg) or the NMDA receptor antagonist MK-801 (0.2 mg/kg), both the phasic and tonic increases could no longer be induced by the same AIH, while the hypoxic glossopharyngeal response (HGR) was not significantly altered. These results suggest that AIH induces LTF in both phasic respiratory and tonic motor outputs of the glossopharyngeal nerve activity, whose manifestation requires activation of serotonin 5-HT2 and NMDA receptors.
4.1. Methodological consideration
Experiments were conducted in anesthetized, vagotomized, paralyzed and artificially ventilated rats, in which arterial blood gases and body temperature could be controlled, and episodes of isocapnic hypoxia could be relatively easily implemented. Since the glossopharyngeal nerve activity did not change throughout the experiment in the time control rats, our recordings were confirmed as stable and the naturally occurring, non-specific fluctuation could be ruled out as an important factor.
For technical reasons, arterial blood gases were not collected from every rat. However, we believe that our results presented above could not be explained by the possible changes in blood gases. First, our partial blood gas data (Table 1) were reasonable and were very much in keeping with previous results of this lab (Zhang et al., 2003, 2004; McGuire et al., 2005b). AIH also induced LTF in that vehicle subgroup with blood gas test. Second, maintenance of isocapnia by just monitoring and adjusting PET, CO2 has been shown to be practical in our experimental setting. Adjustment of ventilator rate based simply on discrepancy between PET, CO2 and Pa, CO2 almost never happened. Finally, the baseline PET, CO2 was set at 5 mmHg (instead of usual 2–3 mmHg) above the apneic threshold in the present study, thus the variables were more resistant to change, even if blood gas fluctuations did occur.
4.2. Glossopharyngeal Nerve
The glossopharyngeal nerve receives sensory fibers from the oropharynx, the oropharyngeal mucosa, the middle ear, the posterior 1/3 of the tongue and the carotid body-sinus. In addition, it also supplies motor fibres to stylopharyngeus muscle and jointly supplies motor fibers to levator veli palatini, upper pharyngeal constrictor and cricopharyngeus through the pharyngeal plexus (Nishio et al., 1976; Furusawa et al., 1991; Williams, 1995). The neuron cell bodies of these glossopharyngeal motor fibers are located in the nucleus ambiguus, in which respiratory motoneurons express NMDA receptors (Liu and Wong-Riley, 2005) and receive projections from the raphe serotonin neurons (Holtman, 1988). Both stylopharyngeus and levator palatini show phasic inspiratory and tonic expiratory activity. When activated, the stylopharyngeus muscle elevates the lateral pharyngeal wall and dilates the pharynx, while levator palatini pulls the soft palate in a posterosuperior direction and serves as the major elevator for the soft palate (Holcombe, 2006). As a result, changes in glossopharyngeal nerve activity can alter pharyngeal airway size and stiffness, thus affecting pharyngeal collapsibility.
4.3. Phasic glossopharyngeal LTF
Respiratory LTF was first elicited by repeated CSN stimulation in phrenic nerve activity (Millhorn et al., 1980a). LTF was later induced by AIH in several major inspiratory muscles (e.g., genioglossus, diaphragm and intercostals) and their innervating nerves (hypoglossal, phrenic and intercostal nerves) in anesthetized animals (Hayashi et al., 1993; Fregosi and Mitchell, 1994; Bach and Mitchell., 1996; Mateika and Fregosi, 1997; McKay et al., 2004; Fuller, 2005), and in ventilation in awake animals (Olson et al., 2001; McGuire et al., 2002), suggesting that LTF is a common feature shared by many inspiratory motoneurons responsible for ventilation. LTF was later also induced in non-major inspiratory muscles (nasal dilator and hyoglossus) (Mateika and Fregosi, 1997; Ryan and Nolan, 2009), respiratory pre-motor neurons (Morris et al., 2001) and reduced preparations, e.g., in vitro brainstem slice and ex vivo carotid body (Bocchiaro and Feldman, 2004; Peng et al., 2006). Recently LTF was elicited by AIH in the splanchnic sympathetic nerve activity, which was correlated with respiratory rhythm (Dick et al., 2007). The present results for the first time demonstrated that LTF exists in the efferent motor output of a primary sensory nerve. These data suggest that LTF might be a general property of all respiratory motoneurons in response to AIH.
This AIH-induced glossopharyngeal LTF was expressed primarily as an increase in amplitude (with minimal changes in burst frequency) following a progressively augmenting pattern, similar to other LTF forms in anesthetized, paralyzed, vagotomized and ventilated rats (cf. Mitchell et al., 2001a; Ling, 2008). However, this glossopharyngeal LTF may differ from others in some aspects. This LTF appeared to reach its plateau quicker (45 min) than others (~60 min) (Bach and Mitchell., 1996; Zhang et al., 2003, 2004; McGuire et al., 2005b). We also noticed that the size of HGR (~75%) appeared to be relatively smaller (McGuire et al., 2005b). Although this smaller size might result from different baseline PET, CO2 setting (5 mmHg above the CO2-apneic threshold vs. usual 2–3 mmHg), as HGR values are relative to baseline, we found that HGR was 55% while hypoxic phrenic response was 77% at least in one separate rat with both nerve activities being simultaneously recorded.
4.4. Tonic glossopharyngeal LTF
The tonic LTF data in the present study were not originally planned and were analyzed after our recent preliminary studies, which investigated upper airway mechanics, including the role played by the glossopharyngeal nerve. These preliminary data showed that: (1) the pharyngeal critical collapsing pressure (Pcrit, an index of pharyngeal airway collapsibility) was smaller (i.e., more negative or less collapsible) during inspiration vs. expiration (Cao et al., 2007b); (2) AIH induced a progressive and persistent decrease in both inspiratory and expiratory Pcrit (McGuire and Ling, 2006), which correlated inversely with genioglossal electromyogram activity LTF; and (3) bilateral hypoglossal nerve transection eliminated the AIH-induced decrease in inspiratory but not expiratory Pcrit, while hypoglossal plus glossopharyngeal nerve transections eliminated both AIH-induced decreases (Cao, Y., Ling, L., unpublished observations). These results strongly suggest the existence of tonic glossopharyngeal LTF, since an AIH-induced progressive increase in glossopharyngeal nerve activity (i.e., glossopharyngeal LTF) during expiration could explain the AIH-induced progressive decrease in expiratory Pcrit. This tonic glossopharyngeal LTF was indeed found when we re-examined the data.
In the present study, all phasic data (except frequency ones) were normalized as a percentage of their own baseline and maximum, whereas all tonic results were normalized as a percentage of the baseline (phasic) peak amplitude. Although both raw and integrated activities were recorded, the sample rate was set at 20 Hz. Raw data recorded at this rate are not adequate to determine the baseline tonic activity or to calculate tonic activity levels relative to the baseline. In contrast, the recorded integrated data can easily determine the phasic amplitude and frequency. However, since we did not measure the “original baseline” (i.e., the one without any respiratory activity), we were unable to obtain an absolute magnitude of tonic activity. We thus calculated the difference between post-hypoxia tonic activity levels and the baseline level, and normalized as a percentage of the baseline (phasic) peak amplitude in each rat. These normalized results were comparable between rats. Had we expected the tonic LTF, we would have sampled at a much higher frequency.
Historically, LTF has always been identified in forms of amplitude of phasic respiratory activity and sometimes respiratory frequency as well (Bach and Mitchell., 1996; Turner and Mitchell., 1997; McGuire et al., 2008). Until very recently, tonic respiratory LTF (during expiration) has not been observed. To date, none of the CSN stimuli-elicited LTF (e.g., the phrenic, hypoglossal and intercostal nerve, and diaphragm, nasal dilator and genioglossus muscle LTF) exhibits tonic component. Repeated vagal stimuli also elicited phrenic LTF without tonic component (Zhang et al., 2003). For a long time, all AIH-induced LTF also showed no tonic component. Ryan and Nolan (2009) reported the first case of tonic LTF, in which AIH induced tonic but not phasic (peak amplitude) LTF in the hyoglossus muscle activity. Fuller (2005) also reported a trend towards an AIH-induced tonic LTF in both branches of the hypoglossal nerve, especially in the lateral branch activity. Interestingly, this lateral branch innervates the hyoglossus muscle. The present study is the first to demonstrate that AIH induces both (respiratory) phasic and tonic LTF in a mixed sensory and motor nerve.
4.5. 5-HT2 and NMDA receptors
In the present study, ketanserin injection (2 mg/kg, i.v.) did not significantly change baseline glossopharyngeal activity or HGR, which is consistent with previous studies on phrenic nerve activity (Kinkead and Mitchell, 1999). Therefore, the serotonin 5-HT2 receptor antagonist did not eliminate glossopharyngeal LTF via its action on the baseline or HGR. MK-801 injection (0.2 mg/kg, i.v.) decreased baseline peak amplitude, but increased baseline frequency, which is also consistent with previous studies on phrenic nerve activity (McGuire et al., 2005b). However, the MK-801 injection did not significantly change the HGR. Therefore, the NMDA receptor antagonist did not eliminate glossopharyngeal LTF via its action on the HGR. We are not particularly concerned about the baseline peak amplitude reduction because the reduced baseline theoretically should enlarge LTF magnitude, which is calculated as an increase above baseline, normalized to the baseline.
The first CSN stimuli-elicited phrenic LTF was found to be serotonin dependent shortly after its discovery (Millhorn et al., 1980b). Serotonin receptors were also required for LTF in the major nerves/muscles contributing to ventilation, the reduced preparations and the splanchnic sympathetic nerve activity mentioned above (Bach and Mitchell., 1996; Kinkead et al., 1998; Kinkead and Mitchell, 1999; Ling et al., 2001; Bocchiaro and Feldman, 2004; McGuire et al., 2004; Zhang et al., 2004; Peng et al., 2006; Dick et al., 2007). To our knowledge, thus far all LTF is dependent on activation of serotonin receptors. More specifically, all AIH-induced LTF in those major inspiratory muscles and their innervating nerves requires 5-HT2 receptors (Kinkead et al., 1998; Kinkead and Mitchell, 1999; Ling et al., 2001; McGuire et al., 2004). Serotonin 5-HT2 receptor antagonism with ketanserin before, but not after, AIH eliminates phrenic LTF, suggesting that 5-HT2 receptors are required for induction, but not maintenance, of phrenic LTF (Fuller et al., 2001).
Our recent studies demonstrated that microinjection of the NMDA receptor antagonist MK-801 into the phrenic motonucleus eliminated phrenic LTF in anesthetized rats (McGuire et al., 2005b). The other NMDA receptor antagonist APV also eliminated ventilatory LTF in awake rats, regardless of being (i.p.) injected before or after AIH (McGuire et al., 2008). These data suggest that both formation and maintenance of LTF require activation of NMDA receptors on the associated motoneurons. Since both ketanserin and MK-801 eliminated glossopharyngeal LTF, we speculate that 5-HT2 and NMDA receptors may be two basic mechanisms essential for all AIH-induced LTF.
4.6. Glossopharyngeal LTF and OSA
Although LTF was not elicited in normal humans during wakefulness (McEvoy et al., 1996; Jordan et al., 2002), it was evident in the presence of elevated CO2 levels (Harris et al., 2006; Wadhwa et al., 2008) or during NREM sleep (Chowdhuri et al., 2008; Pierchala et al., 2008). LTF was also elicited during NREM sleep in individuals with inspiratory flow limitation (e.g., snorers and OSA patients), mainly as a persistent decrease in upper airway resistance, a probable indication of upper airway dilatation due to motor output LTF of dilator muscles (Babcock and Badr, 1998; Aboubakr et al., 2001). In rats, LTF was even more easily induced during NREM sleep vs. wakefulness (Nakamura et al., 2006). LTF has thus been speculated to be an adaptive mechanism (Bach and Mitchell., 1996; Babcock and Badr, 1998; Ling et al., 2001; Bocchiaro and Feldman, 2004; McGuire et al., 2005b; Ling, 2008), which may help stabilize upper airway patency in OSA patients after repeated exposure to apneas and/or hypopneas.
If this hypothesis is correct, the present study and some other recent studies (Fuller, 2005; Ryan and Nolan, 2009) suggest that this protective mechanism is not only limited to major inspiratory muscles but can be extended to those pharyngeal and palatal muscles, which are not major contributors to ventilation but can change pharyngeal airway size and stiffness. It has been reported that in snorers and OSA patients, pharyngeal airway obstructions not only occur during inspiration but also may occur during expiration (Skatvedt, 1992; Schwab et al., 1993; Schwab, 1996; Woodson, 2003). Moreover, those pharyngeal airway obstructions and narrowing at end expiration make pharyngeal airway more vulnerable to suction collapse at the onset of subsequent inspirations (Horner, 1996; Woodson, 2003). Thus the tonic glossopharyngeal LTF may promote upper airway stability by maintaining an elevated muscle tone during expiration.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) grant HL64912. We wish to thank Dr. Julian Saboisky for his careful critique of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol. 2001;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- Babcock MA, Badr MS. Long-term facilitation of ventilation in humans during NREM sleep. Sleep. 1998;21:709–716. [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol. 1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Cao Y, Ling L. Glossopharyngeal nerve makes greater contribution than hypoglossal nerve to respiratory phasic changes in Pcrit. FASEB Journal 21: 918.25 In the meeting, the poster title was changed to: “Both glossopharyngeal and hypoglossal nerves contribute to respiratory phasic changes in Pcrit”. 2007 [Google Scholar]
- Cao Y, McGuire M, Ling WZ, Ling L. Mechanism of a lower Pcrit during inspiration vs. expiration. Am. J. Respir. Crit. Care. Med. (abstr. Issue) 2007;175:A70. [Google Scholar]
- Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol. 2008;160:65–75. doi: 10.1016/j.resp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol. 1994;477(Pt 3):469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. discussion 2000. [DOI] [PubMed] [Google Scholar]
- Fuller DD. Episodic hypoxia induces long-term facilitation of neural drive to tongue protrudor and retractor muscles. J Appl Physiol. 2005;98:1761–1767. doi: 10.1152/japplphysiol.01142.2004. [DOI] [PubMed] [Google Scholar]
- Furusawa K, Yamaoka M, Kogo M, Matsuya T. The innervation of the levator veli palatini muscle by the glossopharyngeal nerve. Brain Res Bull. 1991;26:599–604. doi: 10.1016/0361-9230(91)90101-o. [DOI] [PubMed] [Google Scholar]
- Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1111–1119. doi: 10.1152/ajpregu.00896.2005. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993;265:R811–819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Holcombe SJ. A Review of Upper Airway Anatomy and Physiology of the Horse. 8th AAEP Annual Resort Symposium; Rome, Italy. January 19 – 21, 2006.2006. [Google Scholar]
- Holtman JR., Jr. Immunohistochemical localization of serotonin- and substance P-containing fibers around respiratory muscle motoneurons in the nucleus ambiguus of the cat. Neuroscience. 1988;26:169–178. doi: 10.1016/0306-4522(88)90135-2. [DOI] [PubMed] [Google Scholar]
- Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol. 2002;93:2129–2136. doi: 10.1152/japplphysiol.00135.2002. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5-carboxamidotryptamine. Am J Physiol. 1999;277:R658–666. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Brennick MJ. Effects of pharyngeal muscle activation on airway pressure-area relationships. Am J Respir Crit Care Med. 2002;166:972–977. doi: 10.1164/rccm.200203-214OC. [DOI] [PubMed] [Google Scholar]
- Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr., Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci. 2001;21:5381–5388. doi: 10.1523/JNEUROSCI.21-14-05381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L. Serotonin and NMDA receptors in respiratory long-term facilitation. Respir Physiol Neurobiol. 2008;164:233–241. doi: 10.1016/j.resp.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Respiratory long-term facilitation: too much or too little of a good thing? Adv Exp Med Biol. 2008;605:224–227. doi: 10.1007/978-0-387-73693-8_39. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol. 1997;82:419–425. doi: 10.1152/jappl.1997.82.2.419. [DOI] [PubMed] [Google Scholar]
- McEvoy RD, Popovic RM, Saunders NA, White DP. Effects of sustained and repetitive isocapnic hypoxia on ventilation and genioglossal and diaphragmatic EMGs. J Appl Physiol. 1996;81:866–875. doi: 10.1152/jappl.1996.81.2.866. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Effect of hypoxic episode number and severity on ventilatory long-term facilitation in awake rats. J Appl Physiol. 2002;93:2155–2161. doi: 10.1152/japplphysiol.00405.2002. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Chronic intermittent hypoxia enhances ventilatory long-term facilitation in awake rats. J Appl Physiol. 2003;95:1499–1508. doi: 10.1152/japplphysiol.00044.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R334–341. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- McGuire M, Ling L. Ventilatory long-term facilitation is greater in 1- vs. 2-mo-old awake rats. J Appl Physiol. 2005a;98:1195–1201. doi: 10.1152/japplphysiol.00996.2004. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Phrenic long-term facilitation requires NMDA receptors in the phrenic motonucleus in rats. J Physiol. 2005b;567:599–611. doi: 10.1113/jphysiol.2005.087650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M, Ling L. Pharyngeal airway collapsibility progressively decreases for about 1 hour following acute intermittent hypoxia in rats. Sleep Med. 2006;7:S5–S6. [Google Scholar]
- McGuire M, Liu C, Cao Y, Ling L. Formation and maintenance of ventilatory long-term facilitation require NMDA but not non-NMDA receptors in awake rats. J Appl Physiol. 2008;105:942–950. doi: 10.1152/japplphysiol.01274.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol. 2004;557:13–18. doi: 10.1113/jphysiol.2004.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980a;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980b;42:171–188. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr. Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol. 2001a;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol. 2001b;124:117–128. doi: 10.1016/s0034-5687(00)00197-3. [DOI] [PubMed] [Google Scholar]
- Morris KF, Shannon R, Lindsey BG. Changes in cat medullary neurone firing rates and synchrony following induction of respiratory long-term facilitation. J Physiol. 2001;532:483–497. doi: 10.1111/j.1469-7793.2001.0483f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Wenninger JM, Olson EB, Bisgard GE, Mitchell GS. Ventilatory long-term facilitation following intermittent hypoxia is state-dependent in rats. J Physiol Sci. 2006;56(Suppl):S75. doi: 10.1152/japplphysiol.90778.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J, Matsuya T, Machida J, Miyazaki T. The motor nerve supply of the velopharyngeal muscles. Cleft Palate J. 1976;13:20–30. [PubMed] [Google Scholar]
- Olson EB, Jr., Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchell GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol. 2001;91:709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Prabhakar NR. Reactive oxygen species in the plasticity of respiratory behavior elicited by chronic intermittent hypoxia. J Appl Physiol. 2003;94:2342–2349. doi: 10.1152/japplphysiol.00613.2002. [DOI] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Jacono FJ, Kumar GK, Prabhakar NR. 5-HT evokes sensory long-term facilitation of rodent carotid body via activation of NADPH oxidase. J Physiol. 2006;576:289–295. doi: 10.1113/jphysiol.2006.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol. 2008;160:259–266. doi: 10.1016/j.resp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Ryan S, Nolan P. Episodic hypoxia induces long-term facilitation of upper airway muscle activity in spontaneously breathing anaesthetized rats. J Physiol. 2009;587:3329–3342. doi: 10.1113/jphysiol.2009.169680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab RJ, Gefter WB, Pack AI, Hoffman EA. Dynamic imaging of the upper airway during respiration in normal subjects. J Appl Physiol. 1993;74:1504–1514. doi: 10.1152/jappl.1993.74.4.1504. [DOI] [PubMed] [Google Scholar]
- Schwab RJ. Functional Properties of the Pharyngeal Airway. Sleep. 1996;19(10):S170–S174. doi: 10.1093/sleep/19.suppl_10.170. [DOI] [PubMed] [Google Scholar]
- Skatvedt O. Continuous pressure measurements in the pharynx and esophagus during sleep in patients with obstructive sleep apnea syndrome. Laryngoscope. 1992;102:1275–1280. doi: 10.1288/00005537-199211000-00014. [DOI] [PubMed] [Google Scholar]
- Sokolowska B, Pokorski M. Ventilatory augmentation by acute intermittent hypoxia in the rabbit. J Physiol Pharmacol. 2006;57(Suppl 4):341–347. [PubMed] [Google Scholar]
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- Tessier C, Holcombe SJ, Derksen FJ, Berney C, Boruta D. Effects of stylopharyngeus muscle dysfunction on the nasopharynx in exercising horses. Equine Vet J. 2004;36:318–323. doi: 10.2746/0425164044890553. [DOI] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol. 1997;499(Pt 2):543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? J Appl Physiol. 2008;104:1625–1633. doi: 10.1152/japplphysiol.01273.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. Alimentary system: tongue and pharynx. In: Bannister LH, editor. Gray's anatomy. Churchill Livingston; New York: 1995. pp. 1721–1733. [Google Scholar]
- Woodson BT. Expiratory pharyngeal airway obstruction during sleep: a multiple element model. Laryngoscope. 2003;113:1450–1459. doi: 10.1097/00005537-200309000-00004. [DOI] [PubMed] [Google Scholar]
- Zabka AG, Mitchell GS, Olson EB, Jr., Behan M. Selected contribution: chronic intermittent hypoxia enhances respiratory long-term facilitation in geriatric female rats. J Appl Physiol. 2003;95:2614–2623. doi: 10.1152/japplphysiol.00476.2003. discussion 2604. [DOI] [PubMed] [Google Scholar]
- Zhang Y, McGuire M, White DP, Ling L. Episodic phrenic-inhibitory vagus nerve stimulation paradoxically induces phrenic long-term facilitation in rats. J Physiol. 2003;551:981–991. doi: 10.1113/jphysiol.2003.048157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McGuire M, White DP, Ling L. Serotonin receptor subtypes involved in vagus nerve stimulation-induced phrenic long-term facilitation in rats. Neurosci Lett. 2004;363:108–111. doi: 10.1016/j.neulet.2004.03.067. [DOI] [PubMed] [Google Scholar]