Abstract
Purpose
To evaluate the outcomes of cyclophosphamide therapy for non-infectious ocular inflammation.
Design
Retrospective cohort study
Participants
Two hundred fifteen patients with non-infectious ocular inflammation observed from initiation of cyclophosphamide.
Methods
Patients initiating cyclophosphamide, without other immunosuppressive drugs (other than corticosteroids), were identified at four centers. Dose of cyclophosphamide, response to therapy, corticosteroid-sparing effects, frequency of discontinuation and reasons for discontinuation were obtained by medical record review of every visit.
Main Outcome Measures
Control of inflammation, corticosteroid-sparing effects, discontinuation of therapy.
Results
The 215 patients (381 involved eyes) meeting eligibility criteria carried diagnoses of uveitis (20.4%), scleritis (22.3%), ocular mucous membrane pemphigoid (45.6%) or other forms of ocular inflammation (11.6%). Overall, approximately 49.2% (95% confidence interval (CI): 41.7%-57.2%) gained sustained control of inflammation (for at least 28 days) within 6 months, and 76% (95% CI: 68.3%-83.7%) within 12 months. Corticosteroid-sparing success (sustained control of inflammation while tapering prednisone to 10 mg or less among those not meeting success criteria initially) was gained by 30.0% and 61.2% by six and 12 months respectively. Disease remission leading to discontinuation of cyclophosphamide occurred at the rate of 0.32/person-year (95% CI: 0.24 -0.41), and the estimated proportion with remission at or prior to 2 years was 63.1% (95% CI: 51.5%-74.8%). Cyclophosphamide was discontinued by 33.5% of patients within one year because of side effects-usually of a reversible nature.
Conclusions
Our data suggest that cyclophosphamide is effective for the majority of patients for controlling inflammation and allowing tapering of systemic corticosteroids to 10 mg of prednisone or less, although a year of therapy may be needed to achieve these goals. Unlike with most other immunosuppressive drugs, disease remission was induced by treatment in the majority of patients who were able to tolerate therapy. In order to titrate therapy properly and to minimize the risk of serious potential side effects, a systematic program of laboratory monitoring is required. Judicious use of cyclophosphamide appears beneficial for severe ocular inflammation cases where the potentially vision-saving benefits outweigh the substantial potential side effects of therapy, or when indicated for associated systemic inflammatory diseases.
Corticosteroids, first introduced for ophthalmic use in 1951,1 remain a mainstay of treatment for ocular inflammation.2 However, dose dependent side-effects from chronic use (particularly with systemic corticosteroids) and sometimes inadequate response are limitations of such therapy.3 In these settings, and/or for diseases which have shown better response to early initiation of immunosuppression, immunosuppressive agents are indicated for the management of ocular inflammatory diseases.3
Cyclophosphamide, an alkylating agent developed for cancer chemotherapy, was first introduced in 1952 for treatment of uveitis of unknown etiology,4 and has been used subsequently for various forms of ocular inflammation.3 It acts by exerting a cytotoxic effect on rapidly proliferating cells, by alkylating nucleophilic groups on DNA bases— particularly the 7-nitrogen position of guanine. This leads to cross-linking of DNA bases, abnormal base pairing, or DNA strand breakage, damaging cells when they undergo mitosis. This action profoundly suppresses the function of both T cells and B cells, broadly inhibiting the immune system.5, 6 Cyclophosphamide can be administered both orally (1-2mg/kg/day) and intravenously (750mg-1g/m2 body surface area every 3 to 4 weeks).5
Cyclophosphamide has been reported as effective for the treatment of ocular manifestations of systemic autoimmune diseases including Wegener's granulomatosis,7-14 rheumatoid vasculitis,15, 16 polyarteritis nodosa,17, 18 systemic lupus erythematosus,19, 20 and mucous membrane pemphigoid,21-26 as well as for primary ocular inflammatory conditions including Mooren's ulcer,27 Behçet's disease,28-30 and Vogt- Koyanagi-Harada syndrome. 31, 32 Most of these reports, however, have been based on series with small numbers of patients, resulting in imprecise estimates of success and of side effects.
To provide more information regarding the use of cyclophosphamide for ocular inflammatory diseases, we here report the outcomes of 215 patients followed from the point of initiation of cyclophosphamide at four ocular inflammation referral centers in the United States.
Methods
Study Population
The Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study is a multi-center cohort study for identifying long-term treatment adverse events, whose methods have been described previously.33 For this report, all patients at three academic subspecialty centers with non-infectious ocular inflammation since the inception of the center and an approximate 40% random sample, of such patients from a fourth center were potentially eligible. Sampling was done because of logistical constraints; to avoid selection bias, we used computer generated random numbers with a probability of selection based on the site of inflammation (such that conditions with greater likelihood of using immunosuppression—the primary focus of the study—were over-sampled). Patients from a fifth center participating in the study were not included in this analysis because the center's co-management approach to treatment produced a bias in ascertaining time-to-treatment success, because most visits were conducted at partner centers—both delaying the time-to-ascertainment of treatment success, and reducing the likelihood that successfully managed patients would return.
Patients observed to start cyclophosphamide during follow-up were eligible for inclusion in the present analysis. Patients who were on another immunosuppresant in addition to cyclophosphamide were excluded in order to better isolate the effects of cyclophosphamide therapy, but patients were not excluded if they used corticosteroids— systemic corticosteroid-sparing effects were a primary outcome of the study. Because patients had to have had at least one visit in which they were not taking cyclophosphamide, one when they started cyclophosphamide and at least one or two additional visits to ascertain outcomes (depending on the outcome), effectively patients had to have at least three visits to be included in analyses of outcomes (see below). Patients were followed either until discontinuation of cyclophosphamide, addition of a second immunosuppressive drug, cessation of patient visits at the study clinic, or the end of data collection, whichever occurred first.
Data collection
A database developed in Access (Microsoft Corporation, Redmond, Washington) for the SITE Cohort Study with an extensive suite of real-time quality control checks was used to collect information on every eye of every patient at every visit by trained expert reviewers.33 Information on demographic characteristics, ophthalmologic examination findings, presence or absence of systemic illnesses, all medications in use at every clinic visit (including all use of corticosteroids and immunosuppressive drugs), and reasons for stopping cyclophosphamide were utilized for this analysis.
Main outcome measures
The main outcomes studied were measures of effectiveness (control of inflammation and corticosteroid-sparing effects) and of toxicity leading to discontinuation of cyclophosphamide therapy. Inflammatory status was categorized as “active,” “slightly active,” or “inactive” for every eye at every visit, according to the clinician's judgment at the time of each visit, where “slightly active” inflammation reflected “activity that is minimally present, described also by terms such as mild, few, or trace cells, etc.” and “inactive” indicated there was no active inflammation, also expressed by words such as “quiet,” “quiescent,” or ”controlled.” Control of inflammation was evaluated as the transition from either “active” or “slightly active” to “inactive.” A sensitivity analysis evaluating transition from “active” to either “slightly active” or “inactive” also was performed. The time-to-success in reducing prednisone dose to 10mg, 5mg, or 0mg without recurrence of ocular inflammation activity was evaluated in patients who did not meet these success criteria at the beginning. When corticosteroids other than prednisone were used, their equivalent doses were calculated for evaluation of corticosteroid sparing success.34 For study of time-to-discontinuation of cyclophosphamide, the dates and the reasons for discontinuation of cyclophosphamide were noted.
Statistical Methods
Statistical analyses used SAS version 9.1 (SAS Corporation, Cary, North Carolina). The distribution of demographic and clinical characteristics at the outset of therapy was tabulated. Control of inflammation and corticosteroid-sparing effects were evaluated according to the time-to-success using survival analysis. In order to avoid counting a transient improvement as a success, these outcomes were not accepted unless they were observed over 2 or more visits spanning 28 days. Sensitivity analyses evaluating time-to-success observed at a single visit also were performed, to allow comparisons with other reports using various immunosuppressive drugs which have used that approach. Discontinuation of therapy was assesed using a simple time-to-discontinuation approach. Kaplan-Meier methods were used to summarize the occurrence of success and failure, by-person and/or by-eye. Factors potentially associated with success or failure, such as demographic characteristics, anatomic location of inflammation, dosage, and prior usage of immunosuppressive therapies were evaluated by multiple regression analysis using Cox proportional hazards models.35
Results
Two hundred fifteen patients (77.2% with bilateral ocular inflammation—381 eyes) were identified who started cyclophosphamide as a single immunosuppressive agent during follow-up, with or without local or systemic corticosteroids and non-steroidal anti-inflammatory drugs. The demographic and clinical characteristics of this cohort are summarized in Table 1. The overall median age was 61.3 years (range 11.5-91.4). The majority of the patients were Caucasian (83.3%) and female (58.1%). The patients with uveitis were younger than the patients with other forms of ocular inflammation. Mucous membrane pemphigoid (MMP) was the most common diagnosis in affected eyes (45.6%) followed by scleritis (22.3%) and uveitis (20.4%). A total of 86 patients (40.0%) had received some form of immunosuppressive therapy prior to starting cyclophoshamide; 161 eyes (42.3%) had a visual acuity of 20/50 or worse at presentation.
Table 1.
Presenting characteristics of patients with ocular inflammation at the time of starting cyclophosphamide
| Characteristic | Uveitis | Scleritis | MMP | Other | Total |
|---|---|---|---|---|---|
| Person Specific Characteristics | |||||
| Patient Number | 44 | 48 | 98 | 25 | 215 |
| Median age, years (range) | 43.3 (11.5-76.4) | 56.3 (21.2-81.4) | 68.7 (42.8 -91.4) | 61.3 (30.3 -82.1) | 61.3 (11.5-91.4) |
| Gender, % Female | 27 (61.4%) | 33 (68.8%) | 50 (51.0%) | 15 (60.0%) | 125 (58.1%) |
| Race, % Caucasian | 33 (75.0%) | 36 (75.0%) | 87 (88.8%) | 23 (92.0%) | 179 (83.3%) |
| Race, % Black | 7 (15.9%) | 6 (12.5%) | 6 (6.1%) | 1 (4.0%) | 20 (9.3%) |
| Race, % Other | 4 (9.1%) | 6 (12.5%) | 5 (5.1%) | 1 (4.0%) | 16 (7.4%) |
| Duration of Inflammation,years (range) | 3.2 (0.0 -35.5) | 0.7 (-1.4-21.5) | 1.0 (0.0-18.1) | 0.7 (0.0 - 9.8) | 1.0 (-1.4-35.5) |
| Bilateral Inflammation | 37 (84.1%) | 28 (58.3%) | 91 (92.9%) | 10 (40.0%) | 166 (77.2%) |
| Prednisone Dose ≤10mg/day | 14 (31.8%) | 20 (41.7%) | 33 (33.7%) | 14 (56.0%) | 81 (37.7%) |
| Maximum Cyclophosphamide Dose ≤ 75 mg/day | 19 (43.2%) | 21 (43.8%) | 38 (38.8%) | 12 (48.0%) | 90 (41.9%) |
| 75 < Maximum Cyclophosphamide Dose ≤ 100 mg/day | 4 (9.1%) | 8 (16.7%) | 13 (13.3%) | 5 (20.0%) | 30 (14.0%) |
| 100 < Maximum Cyclophosphamide Dose ≤ 150 mg/day | 10 (22.7%) | 7 (14.6%) | 30 (30.6%) | 6 (24.0%) | 53 (24.7%) |
| 150 mg/day ≥ Maximum Cyclophosphamide Dose | 11 (25.0%) | 12 (25.0%) | 17 (17.3%) | 2 (8.0%) | 42 (19.5%) |
| Oral Cyclophosphamide | 32 (72.7%) | 40 (83.3%) | 89 (90.8%) | 21 (84.0%) | 182 (84.7%) |
| Prior Cyclophosphamide | 3 (6.8%) | 6 (12.5%) | 3 (3.1%) | 1 (4.0%) | 13 (6.0%) |
| Prior Antimetabolites (Other than Cyclophosphamide) | 18 (40.9%) | 18 (37.5%) | 28 (28.6%) | 7 (28.0%) | 71 (33.0%) |
| Prior Alkylating | 4 (9.1%) | 1 (2.1%) | 1 (1.0%) | 0 (0.0%) | 6 (2.8%) |
| Prior Tcell | 14 (31.8%) | 6 (12.5%) | 1 (1.0%) | 2 (8.0%) | 23 (10.7%) |
| Prior Biologics | 4 (9.1%) | 1 (2.1%) | 0 (0.0%) | 1 (4.0%) | 6 (2.8%) |
| Prior Immunosupressive | 25 (56.8%) | 23 (47.9%) | 30 (30.6%) | 8 (32.0%) | 86 (40.0%) |
| Eye Specific Characteristics | |||||
| Number of affected eyes | 81 | 76 | 189 | 35 | 381 |
| 20/50 or Worse | 44 (54.3%) | 23 (30.3%) | 76 (40.2%) | 18 (51.4%) | 161 (42.3%) |
| 20/200 or Worse | 27 (33.3%) | 8 (10.5%) | 41 (21.7%) | 12 (34.3%) | 88 (23.1%) |
| Ocular complications, affected eyes, % | 31 (38.3%) | 18 (23.7%) | 38 (20.1%) | 8 (22.9%) | 95 (24.9%) |
| Overall Activity - Inactive | 64 (79.0%) | 48 (63.2%) | 120 (63.5%) | 25 (71.4%) | 257 (67.5%) |
| Overall Activity - Slightly Active | 7 (8.6%) | 6 (7.9%) | 17 (9.0%) | 2 (5.7%) | 32 (8.4%) |
| Overall Activity - Active | 10 (12.3%) | 22 (28.9%) | 52 (27.5%) | 7 (20.0%) | 91 (23.9%) |
| Overall Activity - Missing | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) | 1 (0.3%) |
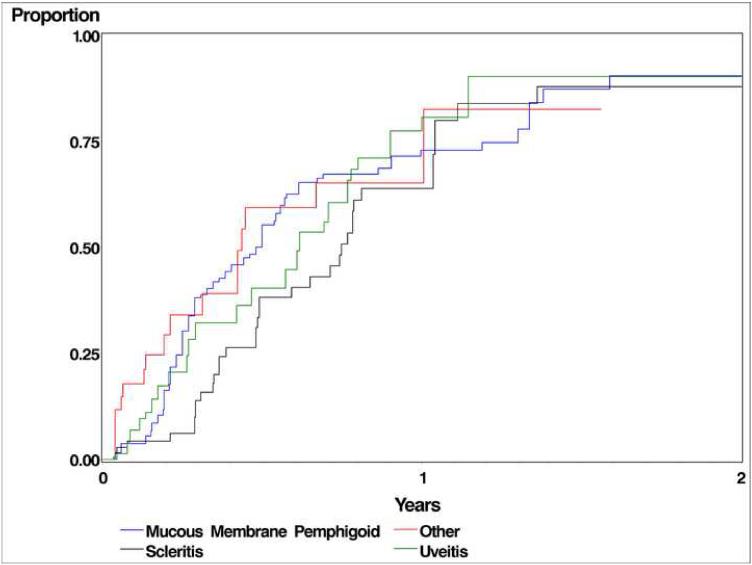
Table 2 and Figure 1 summarize the therapeutic outcomes of cyclophosphamide therapy, by patient, using Kaplan-Meier estimates. Within 6 months, complete control of inflammation (“inactive”), sustained over at least two visitis spanning at least 28 days, was observed in 50.2%_patients with uveitis, 53.3% patients with scleritis, 43.0% patients with ocular mucous membrane pemphigoid (MMP) and 72.0% patients with other forms of ocular inflammation. When the success criterion was eased to count either completely inactive or “slightly active” by 6 months as a success, the percentage of improvement changed to 52.5%, 61.5%, 56.4%, and 78.0% respectively for uveitis, scleritis, MMP, and other forms of ocular inflammation. .Success continued to improve through 12 months, by which time sustained, complete inactivity was observed in 81.3% patients with uveitis, 82.2% patients with scleritis, 68.7% patients with MMP and 89.5% patients with other forms of ocular inflammation. In a sensitivity analysis omitting the requirement that control of inflammation be sustained for at least 28 days, the proportion achieving success increased by approximately 10%. Outcomes were similar within subgroups of uveitis patients, although results for anterior and intermediate uveitis were imprecise because small numbers of patients were treated.
Table 2.
Therapeutic outcomes of cyclophosphamide therapy for inflammatory eye disease
| Outcome | Uveitis | Scleritis | Mucous Membrane Pemphigoid | Other | Total |
|---|---|---|---|---|---|
| Used as only immunosuppressive drug therapy | 44 | 48 | 98 | 25 | 215 |
| Treatment Success At Or Before 6 months, % (95% Confidence Interval(CI)) | |||||
| Controlled Inflammation - No Activity at 6 months | 50.2 (33.8 - 69.2) | 53.3 (37.4 - 71.0) | 43.0 (33.0 - 54.5) | 72.0 (49.1 - 91.0) | 49.2 (41.7 - 57.2) |
| Controlled Inflammation - No Activity or Slightly Active at 6 months | 52.5 (34.4 - 73.1) | 61.5 (45.1 - 78.1) | 56.4 (45.4 - 68.0) | 78.0 (55.0 - 94.3) | 58.9 (51.0 - 67.0) |
| Corticosteroid Sparing -Controlled Inflammation and Prednisone ≤ 10 mg/day | 30.9 (18.3 - 49.1) | 30.2 (17.8 - 48.1) | 25.6 (17.6 - 36.3) | 50.2 (29.1 - 75.7) | 30.1 (23.8 - 37.6) |
| Corticosteroid Sparing -Controlled Inflammation and Prednisone ≤ 5 mg/day | 29.4 (17.0 - 48.0) | 17.9 (8.91 - 34.1) | 19.7 (12.7 - 29.8) | 33.6 (17.3 - 58.6) | 22.8 (17.3 - 29.7) |
| Corticosteroid Sparing -Controlled Inflammation and Prednisone = 0 mg/day | 5.71 (1.46 - 21.0) | 0.00 (0.00 - 0.00) | 2.22 (0.56 - 8.60) | 10.7 (2.76 - 36.8) | 3.31 (1.50 - 7.23) |
| Treatment Success At Or Before 12 months, % (95% CI) | |||||
| Controlled Inflammation - No Activity at 12 months | 81.3 (63.6 - 93.8) | 82.2 (65.4 - 94.0) | 68.7 (57.0 - 80.1) | 89.5 (65.4 - 99.2) | 76. 4 (68.3 - 83.7) |
| Controlled Inflammation - No Activity or Slightly Active at 12 months | 88.9 (70.8 - 98.0) | 80.0 (62.5 - 92.9) | 80.8 (68.5 - 90.5) | 89.0 (64.2 - 99.1) | 83.4 (75.3 - 90.0) |
| Corticosteroid Sparing -Controlled Inflammation and Prednisone ≤ 10 mg/day | 64.8 (46.5 - 82.6) | 60.5 (44.0 - 77.5) | 58.5 (46.8 - 70.6) | 68.9 (43.6 - 90.7) | 61.2 (53.0 - 69.5) |
| Corticosteroid Sparing -Controlled Inflammation and Prednisone ≤ 5 mg/day | 49.1 (32.6 - 68.6) | 37.8 (23.7 - 56.7) | 48.4 (37.0 - 61.2) | 64.6 (40.7 - 87.2) | 47.8 (39.9 - 56.4) |
| Corticosteroid Sparing -Controlled Inflammation and Prednisone = 0 mg/day | 16.2 (7.0 - 34.9) | 15.9 (6.90 - 34.1) | 7.65 (3.49 - 16.3) | 23.5 (9.41 - 51.5) | 12.6 (8.30 - 18.9) |
Figure 1.
Time-to-complete control of ocular inflammation while taking cyclophosphamide
The overall corticosteroid-sparing success rate for complete, sustained control of inflammation at a prednisone dose of 10 mg/day or less within 6 months was 30.1% (95% confidence interval (CI): 23.8% - 37.6%), which improved to 61.2% (95% CI: 53.0% - 69.5%) by 12 months. The success in reducing corticosteroids to less than 5mg and 0 mg by 6 months while maintaining complete, sustained control of inflamation was 22.6% and 3.3% respectively, and 47.8% (95% CI: 39.9% - 56.4%) and 12.6% (95% CI: 8.3% - 18.9%) respectively by 12 months. As with control of inflammation, the proportion with corticosteroid-sparing success continued to improve over time. In the sensitivity analysis omitting the criterion that success be sustained, the overall proportion achieving corticosteroid sparing success within six months increased to 43.9%.
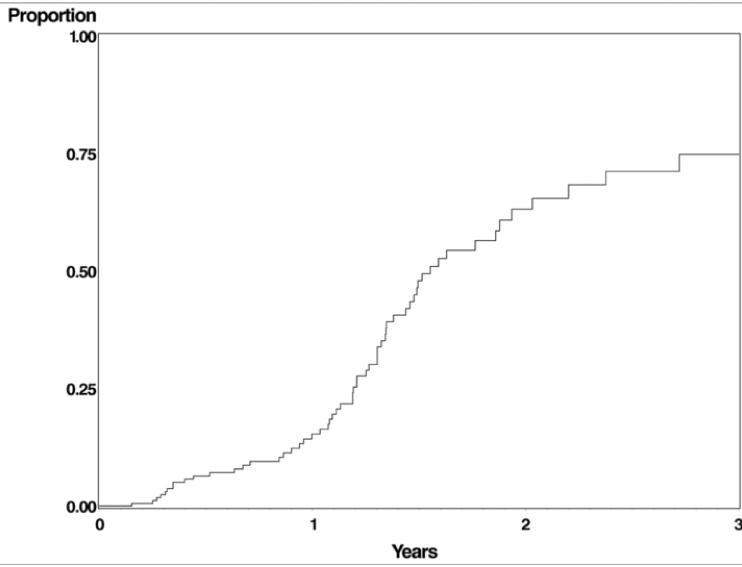
Disease remission leading to discontinuation of cyclophosphamide occurred in a large proportion of patients, given enough time (see Figure 2 and Table 4). The rate of remission was 0.32/person-year (95% CI 0.24 -0.41), mostly observed after the first year of therapy (clinicians typically continued therapy for a year before attempting discontinuation in controlled patients). The estimated proportion of patients with remission at or prior to two and three years respectively was 63.1% (51.5% - 74.8%) and 74.8% (61.6% - 86.3%). A Cox regression of time-to-remission found no relationship between maximum dose and the likelihood of remission (p=0.55). The mean follow-up of patients after remission was 6.2 years.
Figure 2.
Time-to-remission of ocular inflammation following cyclophosphamide therapy. Many clinicians do not attempt discontinuing cyclophosphamide until disease has been controlled off of corticosteroids for an extended period of time.3
Table 4.
Reasons for Discontinuation of Cyclophosphamide*
| Reason | No. of affected patients | Events per person-year (95% Confidence Interval (CI)) | Kaplan-Meier estimate for ≤1 year (95% CI) |
|---|---|---|---|
| Favorable Reasons | |||
| Remission | 61 (28%) | 0.32 (0.24, 0.41) | ** |
| Unfavorable Reasons | |||
| Ineffectiveness | 19 (8.8%) | 0.099 (0.060, 0.15) | 9.7 (5.7 - 16.4) |
| Discontinuation for side effects | 75 (35%) | 0.39 (0.31, 0.49) | 33.5 (26.8 - 41.4) |
| Low leukocyte count | 38 (18%) | 0.20 (0.14, 0.27) | 18.1 (12.7 - 25.3) |
| Low platelet count | 3 (1.4%) | 0.016 (0.0032, 0.046) | 1.7 (0.4 - 7.2) |
| Anemia | 7 (3.3%) | 0.036 (0.015, 0.075) | 3.6 (1.6 - 8.1) |
| Opportunistic infection | 5 (2.3%) | 0.026 (0.0084, 0.061) | 1.3 (0.3 - 5.2) |
| (Fatal pneumocystosis | 1 (0.5%) | 0.0052 (0.0001, 0.029) | 0.5 (0.1 - 3.5)) |
| Cystitis/blood in urine | 14 (6.5%) | 0.073 (0.040, 0.12) | 7.7 (4.1 - 14.2) |
| Sterility | 1 (0.5%) | 0.0052 (0.0001, 0.029) | 0.5 (0.1 - 3.5) |
| Malaise | 1 (0.5%) | 0.0052 (0.0001, 0.029) | 0.5 (0.1 - 3.6) |
| Gastrointestinal upset | 1 (0.5%) | 0.0052 (0.0001, 0.029) | 0.5 (0.1 - 3.6) |
| Liver problem | 1 (0.5%) | 0.0052 (0.0001, 0.029) | 0.5 (0.1 - 3.5) |
| Other side effects | 12 (5.6%) | 0.062 (0.032, 0.11) | 7.9 (4.5 - 13.6) |
| Reasons Unknown | 20 (9.3%) | 0.10 (0.064, 0.16) | 7.7 (4.3 - 13.3) |
| Total Stopping Cyclophosphamide for Any Reason | 164 (76%) | 0.85 (0.72, 0.99) | 50.5 (43.5 - 57.9) |
More than one cause could have been scored as contributing to discontinuation of the drug
In most cases clinicians do not attempt discontinuation on grounds of potential disease remission until disease has been quiescent for an extended period of time after discontinuation of corticosteroids.3 The Kaplan-Meier estimate for discontinuation on grounds of remission at 2 and 3 years respectively was 63.1% and 74.8%
Factors potentially affecting the likelihood of a favorable outcome were evaluated using multiple regression analysis (see Table 3). Compared with Caucasians, African-Americans had a similar chance of gaining control of inflammation with cyclophosphamide, but were less likely to achieve corticosteroid-sparing success to ≤10mg than whites (hazard ratio (HR)=0.38, 95%CI 0.19 - 0.76). A similar pattern was observed for corticosteroid-sparing success to ≤5mg (HR=0.47, 95%CI 0.23-0.97) and for discontinuation of all steroids (HR=0.29, 95%CI 0.12-0.73).
Table 3.
Cox Regression: Factors Associated With Successful Cyclophosphamide Therapy
| Characteristic Name | Variable | Crude Hazard Ratio (HR), Control of Inflammation (inactive), (95% Confidence Interval (CI) | Adjusted HR, Control of Inflammation (inactive), (95% CI) | Crude HR, Corticosteroid-sparing success (≤10 mg prednisone), (95% CI) | Adjusted HR, Corticosteroid-sparing success (≤10 mg prednisone), (95% CI) |
|---|---|---|---|---|---|
| Sex | Male | 1.46 (1.05 - 2.03) | 1.28 (0.88 - 1.86) | 1.33 (0.92 - 1.92) | 1.17 (0.77 - 1.78) |
| Race | White | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 0.64 (0.36 - 1.14) | 0.65 (0.38 - 1.12) | 0.38 (0.20 - 0.71) | 0.38 (0.19 - 0.76) | |
| Other | 0.61 (0.31 - 1.20) | 0.70 (0.34 - 1.41) | 0.57 (0.26 - 1.21) | 0.64 (0.28 - 1.43) | |
| Age | <18 years | 6.75 (3.89 - 11.72) | 3.17 (0.27 - 37.95) | 37.25 (17.91 - 77.46) | 5.95 (0.71 - 50.19) |
| 18-39 years | 1.00 | 1.00 | 1.00 | 1.00 | |
| 40-54 years | 1.25 (0.72 - 2.15) | 1.13 (0.57 - 2.26) | 2.09 (1.11 - 3.94) | 2.28 (1.07 - 4.86) | |
| 55-64 years | 1.08 (0.58 - 2.00) | 0.92 (0.42 - 2.01) | 2.08 (1.08 - 3.99) | 2.32 (1.05 - 5.17) | |
| 65 years or more | 0.93 (0.58 - 1.50) | 0.85 (0.44 - 1.65) | 2.05 (1.15 - 3.64) | 2.35 (1.11 - 4.99) | |
| Type of inflammation | Mucous Membrane Pemphigoid | 1.00 | 1.00 | 1.00 | 1.00 |
| Uveitis | 1.08 (0.71 - 1.64) | 1.07 (0.59 - 1.93) | 0.96 (0.60 - 1.54) | 1.49 (0.79 - 2.79) | |
| Scleritis | 0.97 (0.64 - 1.48) | 1.09 (0.70 - 1.69) | 0.71 (0.46 - 1.10) | 0.88 (0.56 - 1.37) | |
| Other | 1.58 (0.84 - 2.96) | 1.39 (0.67 - 2.86) | 1.25 (0.57 - 2.73) | 1.46 (0.64 - 3.35) | |
| Previous Cyclophosphamide | Yes | 0.82 (0.41 - 1.64) | 1.17 (0.50 - 2.73) | 1.15 (0.57 - 2.30) | 1.31 (0.56 - 3.06) |
| Antimetabolite(s) prior to treatment | Yes | 0.77 (0.53 - 1.11) | 0.84 (0.53 - 1.34) | 0.98 (0.66 - 1.46) | 0.84 (0.51 - 1.37) |
| Biologic(s) prior to treatment | Yes | 3.53 (0.89 - 13.96) | 4.82 (0.41 - 56.46) | 10.04 (2.59 - 38.87) | 7.52 (0.90 - 62.73) |
| Alkylating agent(s) prior to treatment | Yes | 0.82 (0.52 - 1.29) | 1.42 (0.67 - 3.03) | 0.56 (0.17 - 1.78) | 1.08 (0.43 - 2.71) |
| Route - Oral | Yes | 1.95 (1.18 - 3.20) | 1.55 (0.82 - 2.94) | 1.27 (0.76 - 2.14) | 0.90 (0.46 - 1.75) |
| Dosage, mg | ≤75 | 1.00 | 1.00 | 1.00 | 1.00 |
| 75 < Dose ≤ 100 | 1.31 (0.73 - 2.37) | 1.05 (0.57 - 1.95) | 1.12 (0.62 - 2.03) | 1.26 (0.67 - 2.36) | |
| 100< Dose ≤ 150 | 2.14 (1.33 - 3.45) | 1.86 (1.08 - 3.20) | 1.67 (1.00 - 2.77) | 1.68 (0.93 - 3.04) | |
| Dose>150 | 1.71 (1.04 - 2.82) | 1.63 (0.93 - 2.87) | 0.99 (0.56 - 1.73) | 1.18 (0.61 - 2.26) | |
| Systemic (extraocular) autoimmune disease | Yes | 1.12 (0.76 - 1.64) | 1.36 (0.86 - 2.17) | 1.01 (0.68 - 1.49) | 1.08 (0.67 - 1.76) |
Although patients with ages between 40 to 54 (HR=2.28, 95% CI 1.07 - 4.86), 55 to 64 (HR=2.32, 95%CI 1.05 - 5.17) and 65 or more years (HR=2.35, 95% CI 1.11 - 4.99) tended to have greater likelihood than young adults between 18 and 39 years of achieving corticosteroid-sparing success to ≤10mg, the effect was not consistent for corticosteroid sparing success to ≤5mg or 0mg, nor for control of inflammation. Neither the site of ocular inflammation, prior use of immunosuppression, nor the presence of autoimmune systemic diseases (not including mucous membrane pemphigoid, which was counted as synonymous with its associated cicatrizing conjunctivitis) were predictive of response to cyclophosphamide. Use of moderate to high dosages between 100 to 150mg every day was associated with significantly greater success in controlling inflammation (HR=1.86, 95%CI 1.08-3.20) than lower doses of cyclophosphamide (≤75mg), and was associated with a non-significant increase in corticosteroid-sparing success (HR=1.68, 95%CI 0.93-3.04). Comparing oral versus intravenous routes of administration, no statistically significant differences in time-to-control of inflammation (HR=1.55, 95% CI: 0.82-2.94) or in corticosteroid-sparing success ≤10 mg(HR=0.90, 95% CI: 0.46-1.75) were observed, although for control of inflammation, success tended to be greater with oral administration. All these results were similar in a sensitivity analysis where control of inflammation to either the “slightly active” or “inactive” level was considered a success. Cyclophosphamide was discontinued by 33.5% (95% CI: 25.9%-39.6%) of patients within one year because of side effects, usually of a reversible nature. Another 10.8% stopped cyclophosphamide for unknown reasons. Low white cell count and cystitis/blood in the urine were the most common toxicities leading to discontinuation in 18.1% and 7.7% respectively within the first year of therapy. Opportunistic infections led to discontinuation in 3.0% (95% CI: 1.2-7.1%) of the patients in the first year, including Pneumocystis carinii pneumonia leading to death in 1 (0.5%) patient, who had been managed in accordance with commonly accepted guidelines,2 but who had not taken pre-emptive Pneumocystis prophylaxis. Therapy was discontinued within one year in 9.7% (95% CI: 5.7%-16.4%) of the patients due to failure to control inflammation.
A search for factors affecting discontinuation of cyclophosphamide for toxicity using multiple regression analysis showed that African-Americans were less likely to discontinue cyclophosphamide due to side effects compared to white individuals (adjusted HR= 0.23, 95% CI 0.10-0.56). Patients receiving doses between 100 to 150 mg every day tended to discontinue cyclophosphamide for toxicity more often than patients receiving lesser doses (adjusted HR=1.81, 95%CI 0.94-3.48).
Discussion
This report confirms the beneficial effects of cyclophosphamide therapy for ocular inflammation. Cyclophosphamide was successful in achieving complete control of inflammation in 49.1% and 76.3% by 6 months and 12 months respectively. Similarly, corticosteroid-sparing success (sustained control of inflammation while tapering prednisone to 10 mg or less) was gained by 30.0% and 61.2% by 6 and 12 months respectively. At or before two years after initiation of treatment, 63% were able to discontinue therapy because of disease remission.
Other studies have suggested varying success rates of cyclophosphamide in treating different forms of ocular inflammation with small sample sizes, using different outcome definitions, which makes comparison between studies difficult.9, 22, 36-41 However, the success in terms of control of inflammation observed in this study seems lower than in some of these prior reports,36, 38, 39 likely because of our more stringent definition of “success,” our inclusion only of patients observed from the initiation of therapy who did not have the benefit of treatment prior to the initiation of observation time, and perhaps publication bias (particularly for the small case series reported). Although our conservative success criterion, requiring documentation of success at visits spanning at least 28 days may have resulted in a lower “success” rate, but is arguably a more satisfactory definition of success. Sensitivity analysis at which success in control of inflammation or corticosteroid-sparing omitted the requirement for sustained success indeed improved “success” to levels more similar comparable to some of the above studies (76%). While all centers participating in the study were tertiary centers, which tend to see more severe disease than less specialized centers, most other reports derive from tertiary centers as well. Nevertheless, all the available reports suggest that cyclophosphamide is effective for the control of most, but not all, patients with ocular inflammation.
Sixty-one patients discontinued cyclophosphamide after achieving remission at the rate of 0.32 remissions/person-year (PY), which is lower than the 0.50/PY (95% CI: 0.37-0.67/PY) rate in a report of an overlapping group of ocular pemphigoid patients,38 although the former reflects the rate of remission among all patients treated, and the latter is the rate of remission only among the 82.9% subset of patients initially controlled by cyclophosphamide. Thus, while our estimate of the remission rate is lower, it is unlikely that our result is different to a statistically significant degree. Two possible reasons why our study may have observed a lower remission rate include the possibility that some patients scored as discontinuing cyclophosphamide for toxicity may have gone on to have disease remission but not be counted as such, and our exclusion of patients who had the benefit of starting cyclophosphamide therapy prior to cohort entry—who may have reached remission sooner. In addition, our definition of remission was based on the reason for discontinuation of therapy, rather than using a definition based on follow-up after discontinuation,38 due to constraints of the data we had available. In any case, our results suggest that the substantial majority of patients able to continue therapy are likely to achieve medication-free remission in two years or less, a much higher rate of remission than we observed in our studies of methotrexate,42 azathioprine,43 mycophenolate mofetil (to be published separately), and cyclosporine (to be published separately).
”Full” doses in the range of 100 to 150 mg were more effective in controlling inflammation compared to doses of < 100mg, but were more likely to lead to dose-limiting toxicity, confirming that clinicians should use full dosing (1-2mg/kg) whenever it can be tolerated.5 Guidelines about how to implement such treatment are available.2
The observation that African-Americans tended to be less likely to achieve corticosteroid-sparing success compared to Caucasians, but had no significant difference with respect to control of inflammation, may be a random effect or a true difference and requires confirmation by supplemental studies. Neither the site of ocular inflammation nor prior use of immunosuppressive therapy appeared to affect the likelihood of success with cyclophosphamide.
A consensus panel on immunosuppression for ocular disease concluded, based on previous available studies,41, 44 that pulsed cyclophosphamide therapy for uveitis is less effective than oral cyclophosphamide.3 A randomized clinical trial45 in Wegener's Granulomatosis patients concluded that pulse cyclophosphamide was as effective as oral cyclophosphamide in achieving initial remission and was associated with fewer side effects and lower mortality. However, in the long term, treatment with pulse cyclophosphamide did not maintain remission or prevent relapses as well as oral cyclophosphamide. In our experience, the likelihood of treatment success tended to be higher with oral cyclophosphamide, but not to a statistically significantly degree. Bladder toxicity and bladder cancer risk, some of the major toxicities of cyclophosphamide, may be reduced when the drug is administered intermittently via the intravenous route, compared to oral daily dosing.46, 47 Thus, while the available information suggests that oral administration might be more effective than intravenous administration of cyclophosphamide, considerations regarding the potentially lower risks of side effects with intravenous cyclophosphamide leave open the question as to which should be the preferred approach for ocular inflammation.
While cyclophosphamide was usually successful in controlling ocular inflammation— given enough time—a clinically important degree of side effects occurred, requiring discontinuation of therapy in a large minority of patients, and leading to seven (3.3%) opportunistic infections with one death. The most common side effects leading to drug discontinuation were leukopenia (18.1%) and cystitis/hematuria (7.7%). Various other studies have reported a higher incidence of the side effects in the range of 18 to 46% for leukopenia and 8 to 33% for hemorrhagic cystitis.25, 48-51 These differences in results probably derive from the fact that we only recorded problems resulting in discontinuation of therapy. Gonadal dysfunction has been observed in 60% of the patients after 6 months of treatment with cyclophosphamide.52 In our study only one patient discontinued due to sterility, although patients likely anticipated this risk when starting the medication. Ocular side effects including dry eyes, blurred vision and rise in intraocular pressure have been noted,53 which our study did not address. Based on our experience with opportunistic infections, we have adopted the routine use of trimethoprimsulfamethoxazole prophylaxis in our patients treated with cyclophosphamide. This approach is frequent, but not universal, among rheumatologists using cyclophosphamide for systemic inflammatory diseases.54
There is considerable evidence suggesting cyclophosphamide increases the risk of certain kinds of malignancy, and perhaps the risk of overall malignancy.47, 55, 56 Our study of ocular inflammation patients also has demonstrated that cyclophosphamide is not associated with a statistically significant increase in overall mortality (adjusted HR=1.14, p=0.45), but found that cancer mortality tended to be higher with respect to unexposed cohort (adjusted cancer mortality HR=1.61, p=0.17) and the U.S. general population (cancer specific SMR=1.42, p=0.056).57 Thus, our results could be consistent with a clinically important increase in overall cancer mortality-as suggested by a minority of reports based on the clinical experience in other fields.47, 58, 59 These toxicity considerations suggest that use of cyclophosphamide should be limited to the most vision-threatening cases of ocular inflammation, and cases where associated systemic disease provides an indication for the use of cyclophosphamide.
Cyclophosphamide also is teratogenic, causing skeletal and central nervous system abnormalities.60, 61 Therefore, use of effective contraception during cyclophosphamide therapy is required. Cyclophosphamide also can be excreted in breast milk, suggesting that mothers of infants should not breastfeed if cyclophosphamide therapy must be used.60, 62
Limitations of this retrospective, observational study include potential indications-for-treatment bias, missing data in chart notes, incomplete follow-up and potential referral bias. Alkylating agents typically have been reserved for severe cases, and it is possible that results would have been better if the drugs were used in milder cases. However, the side effect concerns suggest that limiting use to more severe cases is appropriate. The centers involved were selected in part because of their habits of maintaining complete records, in order to minimize missing data problems. Data were collected by trained expert ophthalmologist reviewers as per protocol in all the centers63 in order to minimize ascertainment bias. Ascertainment of treatment success and side effects was likely good because the patients typically are assessed every 4 to 6 weeks with monitoring blood work more often than that at all centers, although occasional successes and adverse effects may have been missed. The survival analysis approach assumes patients lost to follow-up are similar to patients continuing in the study; that patients starting a second immunosuppressive drug were censored may have resulted in a slight overestimation of benefits in the analyses of successes. Referral bias is a concern in studies from tertiary care centers, but our results should be generalizable to tertiary ocular inflammation centers where aggressive immunosuppression with cyclophosphamide typically is managed.
Strengths of the study include the large size of the cohort, assessment of the effects of cyclophosphamide as a single agent to avoid ascribing effects from second agents to cyclophosphamide, observation of patients from the time of initiation of therapy, and the ability to compare oral and intravenous therapies with respectable statistical power. Uniform data collection was promoted by quality control checks within the data system and employing data enters with extensive ophthalmologic clinical experience.33 We also carried out a more comprehensive analyses than have been employed by most prior reports, used a more realistic measure of treatment success than some prior reports, and conducted sensitivity analyses by changing our treatment success criteria to assess the robustness of our results.
In summary, these data suggest that—given enough time—cyclophosphamide is effective for the majority of patients with uveitis, scleritis, ocular mucous membrane pemphigoid, and other forms of ocular inflammation. Cyclophosphamide also has the advantage of a high rate of medication-free remission following treatment, which in our experience is not usually seen with alternative immunosuppressive treatments. However, the risk of side effects is substantially greater than with alternative agents, and requires very careful monitoring, and possibly pre-emptive anti-opportunistic infection prophylaxis.64, 65 This concern, along with the apparent increase in the risk of cancer following therapy, suggests that cyclophosphamide is best reserved for patients at high risk of substantial vision loss for whom other forms of treatment have failed or are unlikely to succeed. However, clinicians should not be hesitant to use cyclophosphamide in instances where underlying systemic inflammatory diseases require such therapy, where cyclophosphamide may be life-saving. If tolerable, doses in the 100-150 mg/day range appear more likely to succeed than lower doses.
Acknowledgments
Financial Support: This study was supported primarily by National Eye Institute Grant EY014943 (Dr. Kempen). Additional support was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. Dr Kempen is a Research to Prevent Blindness James S. Adams Special Scholar Award recipient. Drs. Jabs and Rosenbaum are Research to Prevent Blindness Senior Scientific Investigator Award recipients. Dr. Thorne is a Research to Prevent Blindness Harrington Special Scholar Award recipient. Dr. Levy-Clarke was previously supported by and Dr. Nussenblatt continues to be supported by intramural funds of the National Eye Institute. Dr. Suhler also received support from the Veterans' Administration. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; nor in the preparation, review, and approval of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon DM, McLean JM, Koteen H, et al. The use of ACTH and cortisone in ophthalmology. Am J Ophthalmol. 1951;34:1675–86. doi: 10.1016/0002-9394(51)90032-3. [DOI] [PubMed] [Google Scholar]
- 2.Foster CS, Vitale AT. Treatment of uveitis: overview. In: Foster CS, Vitale AT, editors. Diagnosis and Treatment of Uveitis. W.B. Saunders; Philadelphia, PA: 2002. p. 142. AQ: cannot verify if this is truly a multiauthored, edited work, chapter authors, or pagination. Photocopies of copyright and table of contents highlighting chapter. [Google Scholar]
- 3.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 4.Roda Perez E. Nitrogen mustard therapy of uveitis of unknown etiology [undetermined language] Rev Clin Esp. 1952;44:173–80. [PubMed] [Google Scholar]
- 5.Hemady R, Tauber J, Foster CS. Immunosuppressive drugs in immune and inflammatory ocular disease. Surv Ophthalmol. 1991;35:369–85. doi: 10.1016/0039-6257(91)90186-j. [DOI] [PubMed] [Google Scholar]
- 6.Gery I, Nussenblatt RB. Immunosuppressive drugs. In: Sears ML, editor. Pharmacology of the Eye. vol. 69. Springer-Verlag; Berlin: 1984. pp. 586–609. Handbook of Experimental Pharmacology. [Google Scholar]
- 7.Tervaert JW, Stegeman CA. Treatment of patients with Wegener's granulomatosis or ANCA-associated vasculitis [in Dutch] Ned Tijdschr Geneeskd. 2003;147:2265–7. [PubMed] [Google Scholar]
- 8.Leavitt RY, Fauci AS. Wegener's granulomatosis. Curr Opin Rheumatol. 1991;3:8–14. doi: 10.1097/00002281-199102000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Biswas J, Babu K, Gopal L, et al. Ocular manifestations of Wegener's granulomatosis: analysis of nine cases. Indian J Ophthalmol. 2003;51:217–23. [PubMed] [Google Scholar]
- 10.Charles SJ, Meyer PA, Watson PG. Diagnosis and management of systemic Wegener's granulomatosis presenting with anterior ocular inflammatory disease. Br J Ophthalmol. 1991;75:201–7. doi: 10.1136/bjo.75.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuende E, Mena AR, Andonegui J, et al. Ocular involvement in Wegener's granulomatosis responding to intravenous cyclophosphamide [letter] Rheumatology (Oxford) 2001;40:1066–8. doi: 10.1093/rheumatology/40.9.1066. [DOI] [PubMed] [Google Scholar]
- 12.Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 13.Reinhold-Keller E, Kekow J, Schnabel A, et al. Influence of disease manifestation and antineutrophil cytoplasmic antibody titer on the response to pulse cyclophosphamide therapy in patients with Wegener's granulomatosis. Arthritis Rheum. 1994;37:919–24. doi: 10.1002/art.1780370622. [DOI] [PubMed] [Google Scholar]
- 14.Rihova E, Havlikova M, Michalova K, Poch T. Diagnosis and therapy of Wegener's granulomatosis based on ocular changes [in Czech] Cesk Slov Oftalmol. 1997;53:223–8. [PubMed] [Google Scholar]
- 15.Jackson CG, Williams HJ. Disease-modifying antirheumatic drugs: using their clinical pharmacological effects as a guide to their selection. Drugs. 1998;56:337–44. doi: 10.2165/00003495-199856030-00003. [DOI] [PubMed] [Google Scholar]
- 16.Young A, Koduri G. Extra-articular manifestations and complications of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:907–27. doi: 10.1016/j.berh.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Colmegna I, Maldonado-Cocco JA. Polyarteritis nodosa revisited. Curr Rheumatol Rep. 2005;7:288–96. doi: 10.1007/s11926-005-0039-2. [DOI] [PubMed] [Google Scholar]
- 18.Pagnoux C, Guilpain P, Guillevin L. Microscopic polyangiitis [in French] Presse Med. 2007;36:895–901. doi: 10.1016/j.lpm.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 19.Sibilia J. Treatment of systemic lupus erythematosus in 2006. Joint Bone Spine. 2006;73:591–8. doi: 10.1016/j.jbspin.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Spertini F. New concepts for the therapy of systemic lupus erythematosus [in French] Rev Med Suisse. 2007;3:98–102. [PubMed] [Google Scholar]
- 21.Kirtschig G, Murrell D, Wojnarowska F, Khumalo N. Interventions for mucous membrane pemphigoid/cicatricial pemphigoid and epidermolysis bullosa acquisita: a systematic literature review. Arch Dermatol. 2002;138:380–4. doi: 10.1001/archderm.138.3.380. [DOI] [PubMed] [Google Scholar]
- 22.Saw VP, Dart JK, Rauz S, et al. Immunosuppressive therapy for ocular mucous membrane pemphigoid: strategies and outcomes. Ophthalmology. 2008;115:253–61. doi: 10.1016/j.ophtha.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 23.Elder MJ, Lightman S, Dart JK. Role of cyclophosphamide and high dose steroid in ocular cicatricial pemphigoid. Br J Ophthalmol. 1995;79:264–6. doi: 10.1136/bjo.79.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marx W, Reinhard T, Megahed M, Sundmacher R. Immunology-related chronic progressive cicatricial conjunctival diseases: diagnosis, therapy and prognosis [in German] Ophthalmologe. 2001;98:185–93. doi: 10.1007/s003470170182. [DOI] [PubMed] [Google Scholar]
- 25.Miserocchi E, Baltatzis S, Roque MR, et al. The effect of treatment and its related side effects in patients with severe ocular cicatricial pemphigoid. Ophthalmology. 2002;109:111–8. doi: 10.1016/s0161-6420(01)00863-6. [DOI] [PubMed] [Google Scholar]
- 26.Musette P, Pascal F, Hoang-Xuan T, et al. Treatment of cicatricial pemphigoid with pulse intravenous cyclophosphamide [letter] Arch Dermatol. 2001;137:101–2. [PubMed] [Google Scholar]
- 27.Tiev KP, Borderie VM, Briant M, et al. Severe Moorens ulcer: efficacy of monthly cyclophosphamide intravenous pulse treatment [in French] Rev Med Interne. 2003;24:118–22. doi: 10.1016/s0248-8663(02)00021-8. [DOI] [PubMed] [Google Scholar]
- 28.Kazokoglu H, Saatci O, Cuhadaroglu H, Eldem B. Long-term effects of cyclophosphamide and colchicine treatment in Behcet's disease. Ann Ophthalmol. 1991;23:148–51. [PubMed] [Google Scholar]
- 29.Mishima S, Masuda K, Izawa Y, et al. The eighth Frederick H. Verhoeff Lecture, presented by Saiichi Mishima, MD. Behcet's disease in Japan: ophthalmologic aspects. Trans Am Ophthalmol Soc. 1979;77:225–79. [PMC free article] [PubMed] [Google Scholar]
- 30.Oniki S, Kurakazu K, Kawata K. Treatment of Behcet's disease with cyclophosphamide [in Japanese] Nippon Ganka Gakkai Zasshi. 1973;77:508–15. [PubMed] [Google Scholar]
- 31.Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995;39:265–92. doi: 10.1016/s0039-6257(05)80105-5. [DOI] [PubMed] [Google Scholar]
- 32.Touitou V, Escande C, Bodaghi B, et al. Diagnostic and therapeutic management of Vogt-Koyanagi-Harada syndrome [in French] J Fr Ophtalmol. 2005;28:9–16. doi: 10.1016/s0181-5512(05)81020-4. [DOI] [PubMed] [Google Scholar]
- 33.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol. 2008;15:47–55. doi: 10.1080/09286580701585892. [DOI] [PubMed] [Google Scholar]
- 34.Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed McGraw-Hill; 2006. pp. 1587–1612. [Google Scholar]
- 35.Cox DR, Oakes D. Analysis of Survival Data. Chapman & Hall; London: 1984. pp. xx–xx. Monographs on Statistics and Applied Probability 21. AQ: must provide specific inclusive pagination for cited material. [Google Scholar]
- 36.Foster CS. Cicatricial pemphigoid. Trans Am Ophthalmol Soc. 1986;84:527–663. [PMC free article] [PubMed] [Google Scholar]
- 37.Mondino BJ, Brown SI. Immunosuppressive therapy in ocular cicatricial pemphigoid. Am J Ophthalmol. 1983;96:453–9. doi: 10.1016/s0002-9394(14)77908-5. [DOI] [PubMed] [Google Scholar]
- 38.Thorne JE, Woreta FA, Jabs DA, Anhalt GJ. Treatment of ocular mucous membrane pemphigoid with immunosuppressive drug therapy. Ophthalmology. 2008;115:2146–52. doi: 10.1016/j.ophtha.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Durrani K, Papaliodis GN, Foster CS. Pulse IV cyclophosphamide in ocular inflammatory disease: efficacy and short-term safety. Ophthalmology. 2004;111:960–5. doi: 10.1016/j.ophtha.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Jampol LM, West C, Goldberg MF. Therapy of scleritis with cytotoxic agents. Am J Ophthalmol. 1978;86:266–71. doi: 10.1016/s0002-9394(14)76823-0. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbaum JT. Treatment of severe refractory uveitis with intravenous cyclophosphamide. J Rheumatol. 1994;21:123–5. [PubMed] [Google Scholar]
- 42.Gangaputra S, Newcomb CW, Liesegang TL, et al. Methotrexate for ocular inflammatory diseases. Ophthalmology. doi: 10.1016/j.ophtha.2009.04.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasadhika S, Kxxxxxxx J, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol. In press. AQ: second author missing; cannot confirm in press (not in PubMed, not listed as in press on journal website), please provide preprint or other confirmation. [Google Scholar]
- 44.Ozyazgan Y, Yurdakul S, Yazici H, et al. Low dose cyclosporin A versus pulsed cyclophosphamide in Behcet's syndrome: a single masked trial. Br J Ophthalmol. 1992;76:241–3. doi: 10.1136/bjo.76.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guillevin L, Cordier JF, Lhote F, et al. A prospective, multicenter, randomized trial comparing steroids and pulse cyclophosphamide versus steroids and oral cyclophosphamide in the treatment of generalized Wegener's granulomatosis. Arthritis Rheum. 1997;40:2187–98. doi: 10.1002/art.1780401213. [DOI] [PubMed] [Google Scholar]
- 46.Martin F, Lauwerys B, Lefebvre C, et al. Side-effects of intravenous cyclophosphamide pulse therapy. Lupus. 1997;6:254–7. doi: 10.1177/096120339700600307. [DOI] [PubMed] [Google Scholar]
- 47.Kempen JH, Gangaputra S, Daniel E, et al. Long-term risk of malignancy among patients treated with immunosuppressive agents for ocular inflammation: a critical assessment of the evidence. Am J Ophthalmol. 2008;146:802–12. doi: 10.1016/j.ajo.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neumann R, Foster CS. Corticosteroid-sparing strategies in the treatment of retinal vasculitis in systemic lupus erythematosus. Retina. 1995;15:201–12. [PubMed] [Google Scholar]
- 49.Akova YA, Jabbur NS, Foster CS. Ocular presentation of polyarteritis nodosa: clinical course and management with steroid and cytotoxic therapy. Ophthalmology. 1993;100:1775–81. doi: 10.1016/s0161-6420(93)31405-3. [DOI] [PubMed] [Google Scholar]
- 50.Foster CS, Forstot SL, Wilson LA. Mortality rate in rheumatoid arthritis patients developing necrotizing scleritis or peripheral ulcerative keratitis: effects of systemic immunosuppression. Ophthalmology. 1984;91:1253–63. doi: 10.1016/s0161-6420(84)34160-4. [DOI] [PubMed] [Google Scholar]
- 51.Messmer EM, Foster CS. Destructive corneal and scleral disease associated with rheumatoid arthritis: medical and surgical management. Cornea. 1995;14:408–17. doi: 10.1097/00003226-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 52.Fairley KF, Barrie JU, Johnson W. Sterility and testicular atrophy related to cyclophosphamide therapy. Lancet. 1972;1:568–9. doi: 10.1016/s0140-6736(72)90358-3. [DOI] [PubMed] [Google Scholar]
- 53.Fraunfelder FT, Meyer SM. Ocular toxicity of antineoplastic agents. Ophthalmology. 1983;90:1–3. doi: 10.1016/s0161-6420(83)34600-5. [DOI] [PubMed] [Google Scholar]
- 54.Gupta D, Zachariah A, Roppelt H, et al. Prophylactic antibiotic usage for Pneumocystis jirovecii pneumonia in patients with systemic lupus erythematosus on cyclophosphamide: a survey of US rheumatologists and the review of literature. J Clin Rheumatol. 2008;14:267–72. doi: 10.1097/RHU.0b013e31817a7e30. [DOI] [PubMed] [Google Scholar]
- 55.Park MC, Park YB, Jung SY, et al. Risk of ovarian failure and pregnancy outcome in patients with lupus nephritis treated with intravenous cyclophosphamide pulse therapy. Lupus. 2004;13:569–74. doi: 10.1191/0961203304lu1063oa. [DOI] [PubMed] [Google Scholar]
- 56.Asten P, Barrett J, Symmons D. Risk of developing certain malignancies is related to duration of immunosuppressive drug exposure in patients with rheumatic diseases. J Rheumatol. 1999;26:1705–14. [PubMed] [Google Scholar]
- 57.Kempen JH, Dxxxxxx E, Dunn JP, et al. Risk of overall and cancer mortality among patients with ocular inflammation treated with immunosuppressive therapy. Brit Med J. In press. AQ: second author missing; cannot confirm in press (not in PubMed, not listed as in press on journal website), please provide preprint or other confirmation. [Google Scholar]
- 58.Baltus JA, Boersma JW, Hartman AP, Vandenbroucke JP. The occurrence of malignancies in patients with rheumatoid arthritis treated with cyclophosphamide: a controlled retrospective follow-up. Ann Rheum Dis. 1983;42:368–73. doi: 10.1136/ard.42.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker GL, Kahl LE, Zee BC, et al. Malignancy following treatment of rheumatoid arthritis with cyclophosphamide: long-term case-control follow-up study. Am J Med. 1987;83:1–9. doi: 10.1016/0002-9343(87)90490-6. [DOI] [PubMed] [Google Scholar]
- 60.Ostensen M. Treatment with immunosuppressive and disease modifying drugs during pregnancy and lactation. Am J Reprod Immunol. 1992;28:148–52. doi: 10.1111/j.1600-0897.1992.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 61.Porter AJ, Singh SM. Transplacental teratogenesis and mutagenesis in mouse fetuses treated with cyclophosphamide. Teratog Carcinog Mutagen. 1988;8:191–203. doi: 10.1002/tcm.1770080403. [DOI] [PubMed] [Google Scholar]
- 62.Rubin B, Palestine AG. Complications of corticosteroid and immunosuppressive drugs. Int Ophthalmol Clin. 1989;29(3):159–71. doi: 10.1097/00004397-198902930-00006. Fall. [DOI] [PubMed] [Google Scholar]
- 63.Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data: results of the first international workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG, Dutch Co-trimoxazole Wegener Study Group Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener's granulomatosis. N Engl J Med. 1996;335:16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- 65.Chung JB, Armstrong K, Schwartz JS, Albert D. Cost-effectiveness of prophylaxis against Pneumocystis carinii pneumonia in patients with Wegner's granulomatosis undergoing immunosuppressive therapy. Arthritis Rheum. 2000;43:1841–8. doi: 10.1002/1529-0131(200008)43:8<1841::AID-ANR21>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]