Abstract
Although manganese (Mn) is an essential trace element for human development and growth, chronic exposure to excessive Mn levels can result in psychiatric and motor disturbances, referred to as manganism. However, there are no known mechanism(s) for efflux of excess Mn from mammalian cells. Here, we test the hypothesis that the cytoplasmic iron (Fe) exporter ferroportin (Fpn) may also function as a Mn exporter to attenuate Mn toxicity. Using an inducible human embryonic kidney (HEK293T) cell model, we examined the influence of Fpn expression on Mn-induced cytotoxicity and intracellular Mn concentrations. We found that induction of an Fpn-green fluorescent protein (GFP) fusion protein in HEK293T cells was cytoprotective against several measures of Mn toxicity, including Mn-induced cell membrane leakage and Mn-induced reductions in glutamate uptake. Fpn-GFP mediated cytoprotection correlated with decreased Mn accumulation following Mn exposure. Thus, Fpn expression reduces Mn toxicity concomitant with reduced Mn accumulation. To determine if mammalian cells may utilize Fpn in response to increased intracellular Mn concentrations and toxicity, we assessed endogenous Fpn levels in Mn exposed HEK293T cells and in mouse brain in vivo. We find that 6 hours of Mn exposure in HEK293T cells is associated with a significant increase in Fpn levels. Furthermore, mice exposed to Mn showed an increase in Fpn levels in both the cerebellum and cortex. Collectively, these results indicate that (1) Mn exposure promotes Fpn protein expression, (2) Fpn expression reduces net Mn accumulation and (3) reduces cytotoxicity associated with exposure to this metal.
Keywords: ferroportin, manganese, exporter, divalent metal transporter, cytotoxicity, iron
Introduction
Although manganese (Mn) is an essential trace element for development and multiple physiological functions (Erikson and Aschner 2003; Aschner and Aschner 2005; Golub et al., 2005), chronic exposure to excessive Mn levels can lead to a variety of psychiatric and motor disturbances, termed manganism (Cotzias et al., 1968; Olanow 2004; Aschner et al., 2007; Ellingsen et al., 2008). Generally, exposure to ambient Mn air concentrations in excess of 5 µg Mn/m3 can lead to Mn-induced symptoms. These exposure levels are encountered in occupational cohorts employed in welding (Bowler et al., 2006; Park et al., 2007), Fe and/or Mn smelting (Myers et al., 2003a), mining (Myers et al., 2003b) as well as the manufacturing of batteries (Bader et al., 1999).
Mn accumulation is modulated by numerous factors, including the brain’s Fe status (Erikson et al., 2002; 2004; Kim et al., 2005; Garcia et al., 2007). Given the essentiality of both Mn and Fe, their uptake and efflux are regulated at multiple levels by several shared transporters to assure optimalion concentrations within the brain (Jensen et al., 2009; Lee and Beutler, 2009). Experiments in animal models with inherent dysfunction in DMT1 (divalent metal transporter 1) have established the shared transporter characteristics of this transporter in regulating the levels of both Mn and Fe brain concentrations. For example, in both the Belgrade rat and in microcytic mice , both of which are characterized by loss-of-function of DMT-1, levels of both Mn and Fe are concomitantly reduced (Chua and Morgan, 1997; Fleming et al., 1999). In addition, iron deficiency (ID) alone (Erikson et al., 2002; 2004; Erikson and Aschner 2006; Kim et al., 2005) or ID coupled with high Mn levels (Garcia et al., 2007) results in enhanced Mn accumulation in brain, concomitant with increased expression of both DMT1 and TfR (transferrin receptor; Burdo et al., 2003; Erikson and Aschner 2006; Mims and Prchal, 2005 ).
Fe is an essential element for living organisms since it is required for activities of molecules responsible for a series of decisive physiological events, including oxygen transport, mitochondrial respiration and DNA synthesis (Hentze et al., 2004). However, mammals do not have a dedicated Fe secretory pathway and its metabolism and stores are largely regulated by intestinal Fe absorption, in part through hepcidin-Fpn interaction (Nemeth and Ganz, 2006; Darshan and Anderson, 2007; Wright and Andrews, 2008). Fpn is the receptor for hepcidin, a polypeptide hormone made by the liver in response to Fe stores and inflammation (Ganz and Nemeth, 2006). Binding of hepcidin to Fpn leads to the internalization and degradation of Fpn (Nemeth et al., 2004).
Fpn [also known as IREG1 (iron-regulated protein 1) or MTP1 (metal tolerance protein 1)] is the cytoplamic Fe exporter responsible for the entry of Fe into plasma, regulating its absorption and recycling (Donovan et al., 2000; McKie et al., 2000; Montosi et al., 2001) . Fpn is present on all physiologically relevant Fe-exporting tissues including placenta, macrophages, hepatocytes, and intestinal duodenum (Donovan et al., 2000; Donovan et al., 2005; Knutson et al., 2005; Yang et al., 2002). Fpn is ubiquitously expressed in neurons (Moos, et al., 2007; Moos and Rosengren Nielsen 2006) actively maintaining their Fe-homeostasis. In addition, some Fe is also exported in high molecular weight forms, such as heme or ferritin. Feline leukemia virus, subgroup C, receptor (FLVCR) has been shown to act as a heme exporter in mammals, and it is predicted to play a role in reducing excess heme levels in erythroid cell precursor and in heme release from macrophages (Keel et al., 2008). BCRP (breast cancer resistance protein)/Abcg2-ATP-binding cassette sub-family G member2, is another heme transport protein in the plasma membrane that facilitates heme efflux from immature erythroid cell (Krishnamurthy and Schuetz, 2006). Mutations in Fpn cause type VI hemochromatosis, commonly known as Fpn disease (Pietrangelo, 2004), which is predominantly characterized by Fe accumulation in reticuloendothelial macrophages. Fpn expression is responsive to Fe and inflammatory stimuli (Abboud and Haile, 2000; Zoller et al., 2001; Yang et al., 2002). It is regulated at several levels, including presumed transcriptional regulation in the duodenal mucosa and macrophages (McKie et al., 2000; Knutson et al., 2003), translational regulation by the Fe-responsive element/protein (IRE)/IRP regulatory system (Abboud and Haile, 2000; Liu et al., 2002; Lymboussaki et al., 2003) and posttranslational regulation by the action of hepcidin (Nemeth and Ganz, 2006).
Surprisingly, no studies have addressed the efflux of Mn from mammalian cells. Given the shared characteristics of Mn and Fe, the present study examined the hypothesis that Fpn, in addition to extracellularly transporting Fe, also mediates Mn efflux. The objectives of the study were to determine whether (1) an inverse relationship exists between Fpn protein expression levels and Mn-induced cytotoxicity and intracellular Mn levels, and (2) Mn treatment both in vivo and in vitro increases Fpn protein expression. Results from these studies demonstrate that increased Fpn protein expression in HEK293 cells is associated with decreased intracellular Mn concentration and attenuated cytotoxicity, characterized by the reversal of Mn-reduced glutamate uptake and diminished LDH leakage. To our knowledge, this is the first study to establish a role for Fpn in Mn efflux from mammalian cells.
Materials and Methods
Cells and treatments
WT HEK293T cells (ATCC, Manassas, VA, USA) were maintained in Dulbecco's modified Eagle's medium (DMEM) with 61.5 mg/L Penicillin, 100 mg/L Streptomycin and 10% fetal bovine serum (FBS). Experiments were carried out in a stable cell line HEK293T-Fpn (HEK293T cells transfected Fpn-GFP plasmid), expressing mouse Fpn with a C-terminal green fluorescent protein (GFP) under the control of the ecdysone-inducible promoter in the presence of the inducer, ponasterone A. The cells were a generous gift from Dr. Jerry Kaplan (University of Utah, Salt Lake City, UT), The HEK293T-Fpn-GFP cells were maintained in DMEM with 1g/L G418, 400 mg/L Zeocin, 1 mg/L Ciprofloxacin, 61.5 mg/L Penicillin, 100 mg/L Streptomycin, and 10% FBS. Cells were seeded to confluence for 24 h before treatments. Cells were treated for 6 hours with Mn at 100, 250, or 500 µM in OPTI-MEM [containing Fe(NO3)3·9H2O 4mg/L, 0.00248 mM; and no transferrin] based on our previous studies (Aschner and Aschner 2005; Milatovic et al., 2007; Stanwood et al., 2009).
Assessments of Mn-induced cytotoxicity
After 6-hour Mn treatment, cytotoxicity was evaluated with the lactate dehydrogenase (LDH) assay. The LDH assay (Sigma Chemical Co., Saint Louis, MO) were performed according to the manufacturer’s instructions. LDH release was normalized to controls (100%) adjusted to protein concentration.
SDS-PAGE and western blot analysis
Cells (HEK293T, HEK293T-Fpn-GFP with or without ponasterone A) were cultured for 16 hours and harvested with ice-cold lysis buffer containing: NaCl 150 mM, EDTA 10 mM, Tris-HCl 10 mM, 1% Triton X-100 and protease inhibitor mixture (Roche Applied Science, Indianapolis, IN). Thirty µg protein were loaded and electrophoresed on 10% SDS-PAGE. Western blot analysis was performed using either rabbit anti-GFP (1:1000, Abcam, Cambridge, MA) or rabbit anti-Fpn (1:400, Lifespan Biosciences, Seattle, WA) and detected by enhanced chemiluminescence technique (ECL) (Pierce, Rockford, IL).
Analysis of 3H-glutamate uptake
3H-glutamate uptake was examined as previously described (Allen et al., 2001). Briefly, cells in 12-well plates were washed with fresh sodium-HEPES buffer consisting of: 122 mM NaCl, 3.3 mM KCl, 0.4 mM MgSO4, 1.3 mM CaCl2, 1.2 mM KH2PO4, 10 mM glucose, and 25 mM HEPES adjusted to pH 7.4. In pre-incubation experiments, cells were pretreated in Na-HEPES buffer only, or with Na-HEPES buffer containing Mn (100, 250, or 500 µM) for 6 hours in a 37°C, 95% air/5% CO2 incubator. Next, the cells were washed with Na-HEPES buffer, followed by addition to each well of 0.5 ml of pre-warmed buffer containing 1 µCi/ml of L-[G-3H]-glutamate (American Radiolabeled Chemicals Inc. St. Louis, MO) in the presence of unlabeled glutamate (at a final concentration of 50 µM). Glutamate uptake was measured at 1 minute (a time period reflecting the ascending portion of cellular uptake) at room temperature. The reactions were terminated by aspirating the buffer, followed by washes with ice-cold mannitol buffer [290 mM of mannitol, 10 mM of Tris-nitrate, 0.5 mM of Ca(NO3)2; pH adjusted to 7.4 with KOH]. At the end of the experiment, cells were lysed in 1 ml of 1 M NaOH. An aliquot of 25 µl was used for protein determination by the bicinchoninic acid protein assay (Pierce, Rockford, IL). Cell lysates (750 µl) were combined with 75 µl of 10 M HCl and radioactivity was measured in a liquid scintillation counter (Tri-Carb 2900TR, Perkin Elmer Life Science, Waltham, MA). Radioactivity was corrected for the cellular protein content and the results were normalized to controls (100%).
Measurement of intracellular Mn concentration
Cellular Mn concentrations were measured with atomic absorption spectroscopy (Varian AA240, Varian, Inc., USA). Cell lysates were digested in ultra-pure nitric acid (1:2 dilution) for 48 hours in a sand bath (60° C). An aliquot of 100 µl of digested lysate was brought to 1 ml total volume with 2% nitric acid and analyzed for Mn content. Bovine liver (NBS Standard Reference Material, USDC, Washington, DC) (10 µg Mn/g) was digested in ultra-pure nitric acid and used as an internal standard for analysis (final concentration 5 µg Mn/L).
In vivo animal studies
All experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committees. C57BL/6 female mice (Jackson Laboratory, Bar Harbor, ME) between 6 and 8 weeks of age were housed in a 12 h/12 h light/dark cycle at 21 ± 1°C/humidity 50 ± 10%. Mice had free access to water and food pellets (Rodent Laboratory Chow, Purina Mills Inc., St Louis, MO) containing 10 ppm Mn and 35 ppm Fe. One group of mice received a single subcutaneous (s.c.) injection at the scruff of the neck with 0 or 100 mg/kg Mn and a second group received three identical injections of 0 or 100 mg/kg on days 1, 4 and 7. Both groups (4–6 mice in each group) were sacrificed 24 hours after the last injection. Brains were removed and cortices and cerebella isolated on ice. Samples were homogenized and protein concentrations were assayed with the Bradford assay (Sigma, Saint Louis, MO). Fifty µg protein were loaded and applied to 10% SDS-PAGE following identical procedures to those described for HEK293T cells. The dose and route of Mn administration were based on a report by Dodd and co-workers (2005).
Statistical analysis
Differences between various treatment groups both in the in vitro and in vivo studies were analyzed by one-way analysis of variance (ANOVA), followed by Bonferroni's multiple comparison test with statistical significance set at p < 0.05. In vitro results were performed in triplicates from at least three independent cultures. In vivo values were derived from 4 to 6 mice in each group. All analyses were carried out with GraphPad Prism 4.02 for Windows (GraphPad Software, San Diego, CA, USA).
Results
Fpn expression in HEK cells
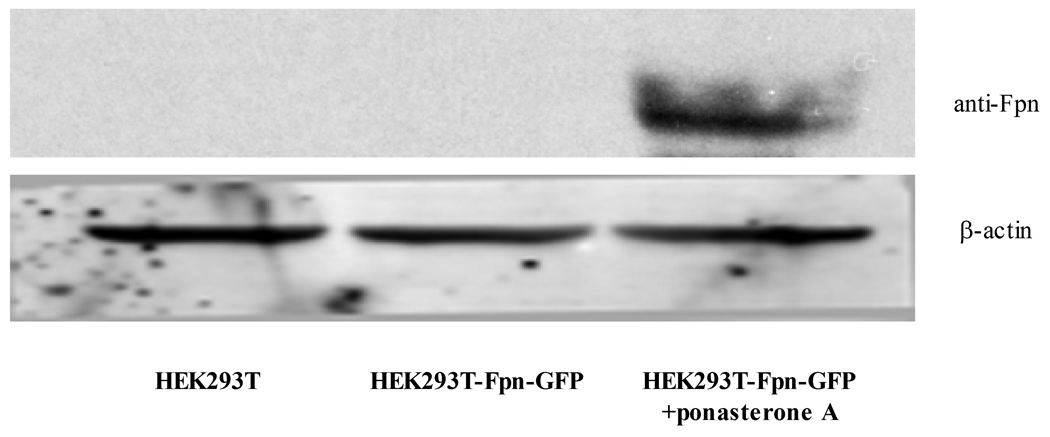
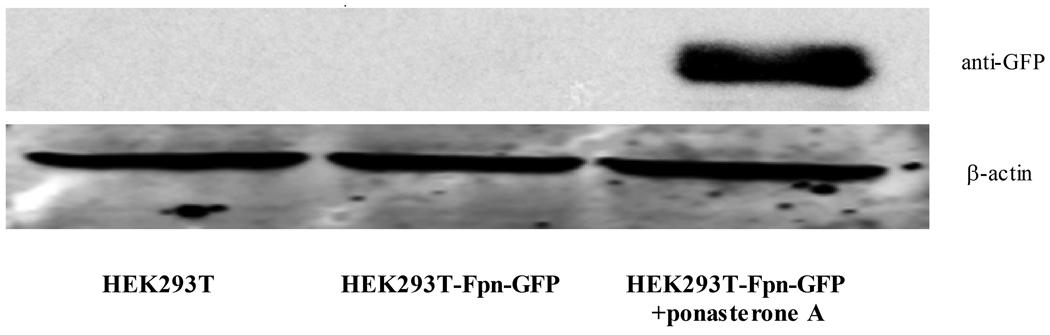
As shown in Figure 1, Fpn protein expression was absent in control wild-type (WT) HEK293T cells. In the absence of treatment with the inducer, ponasterone A, Fpn protein expression was undetected in Fpn-GFP transfected HEK cells. When ponasterone A was added to the media for 16 hours, high level of Fpn protein expression was detected with both anti-Fpn antibody and anti-GFP antibody from HEK293T-Fpn-GFP cell lysates (Fig. 1 A, B). Fpn protein expression was also visible in HEK293T-Fpn-GFP cells induced by ponasterone A under fluorescence microscope, but not in WT HEK293T cells or ponasterone A uninduced HEK293T-Fpn-GFP cells (Fig. 1 C).
Figure 1. Fpn protein overexpressed in ponasterone A induced HEK293T cells.
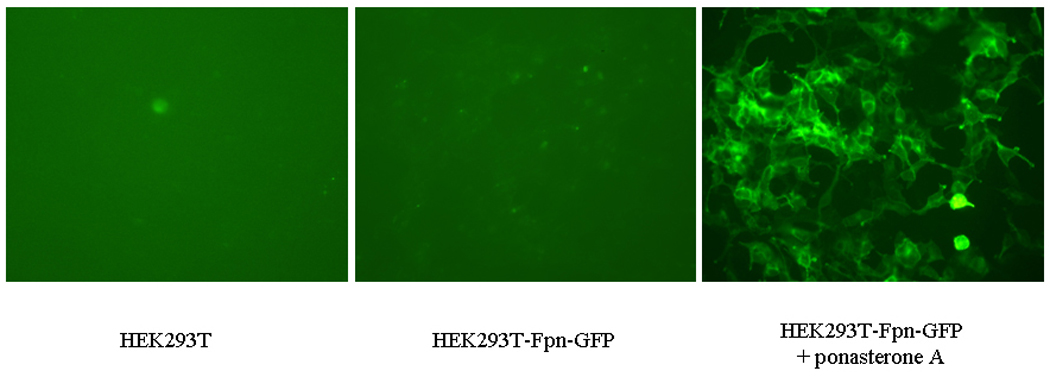
HEK293T cells were maintained in DMEM with the supplement of 10% FBS. FPN expression was undetectable in WT HEK293T cells and ponasterone A uninduced HEK293T-Fpn-GFP cells. With ponasterone A, HEK293T-Fpn-GFP cells expressed high level of Fpn protein, detected by anti-FPN (A) and anti-GFP (B), as well as visible under fluorescent microscope (C).
Fpn levels are inversely related with Mn-induced toxicity in HEK cells
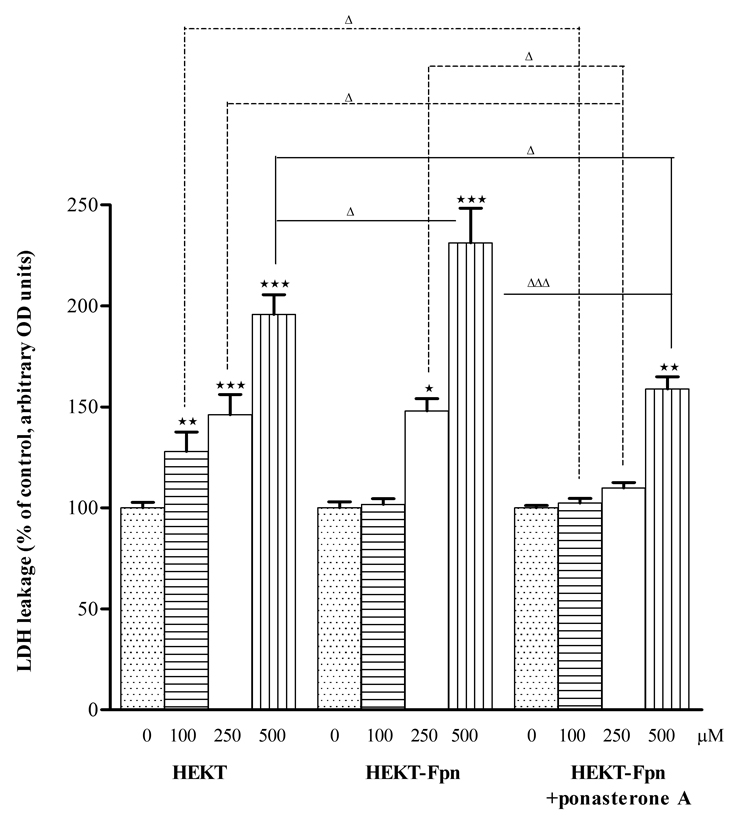
Mn-induced cytotoxicity was determined by the LDH release assay. Treatment (6 hours) of WT HEK293T and ponasterone A uninduced HEK293T-Fpn-GFP cells with Mn (100, 250, or 500 µM) led to a concentration-dependent increase (p<0.05 to 0.001) in LDH release. In contrast, in ponasterone A induced HEK293T-Fpn-GFP cells a significant (p<0.01) increase in LDH release occurred only at 500 µM Mn. Furthermore, the extent of LDH leakage was significantly lower in ponasterone A induced HEK293T-Fpn-GFP cells than in WT HEK293T (p<0.05) and in ponasterone A uninduced HEK293T-Fpn- GFP cells (p<0.05 or 0.001) (Fig. 2).
Figure 2. Fpn protein expression inversely associated with Mn toxicity to HEK cells.
Six hour exposure of Mn (100, 200, or 500 µM) induced LDH leaking from cell membrane to media in a concentration-dependent manner in WT HEK293T control cells and ponasterone A uninduced HEK293T-Fpn-GFP cells; a significant increase in LDH release occurred only at 500 µM Mn treatment in ponasterone A induced HEK293T-Fpn-GFP cells. The extent of LDH leakage was significantly lower (p<0.05 or 0.001) in ponasterone A induced HEK293T-Fpn-GFP cells than in WT HEK293T and in uninduced HEK293T-Fpn-GFP cells. Data were analyzed by one-way analysis of variance (ANOVA), followed by Bonferroni's multiple comparison test with statistical significance set at p < 0.05. Values are mean ± SEM derived from three independent experiments conducted in triplicates. * p<0.05, ** p<0.01, *** p<0.001 compared to control; Δ p<0.05, ΔΔΔ p<0.001 compared between treatments at the same concentration in the different groups.
Fpn suppresses the Mn-dependent decrease in HEK cell glutamate uptake
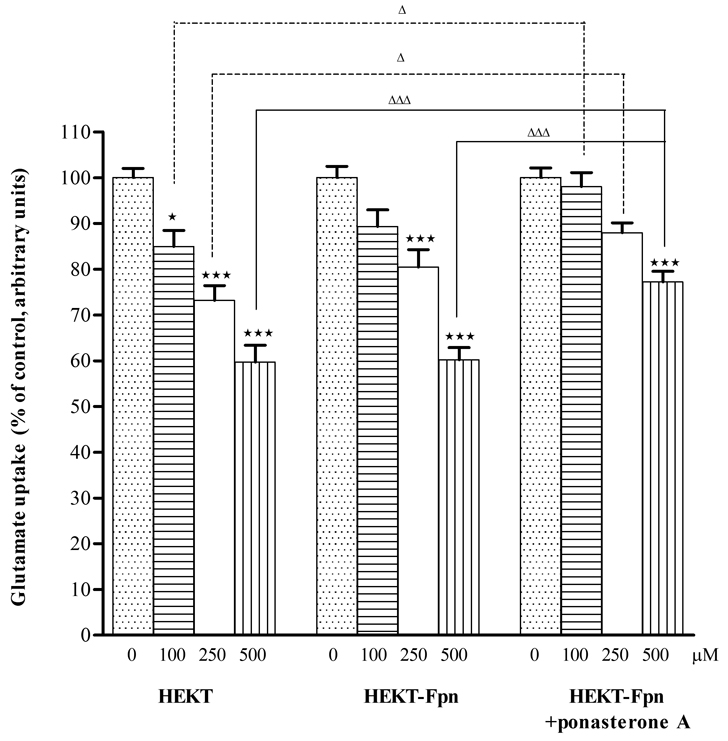
Mn neurotoxicity is, at least in part, caused by altered glutamate metabolism (Brouillet et al., 1993). Accordingly, studies were designed to test the effect of Mn on glutamate uptake in HEK293T cells and the relationship between increased Fpn protein expression and glutamate transport in these cells. As shown in Figure 3, Mn (100, 250, or 500 µM) significantly (p<0.05 or p<0.001) decreased the 1-minute glutamate uptake in a concentration-dependent manner in WT HEK293T and ponasterone A uninduced HEK293T-Fpn-GFP cells. In contrast, increased Fpn protein expression in ponasterone A induced HEK293T-Fpn-GFP cells was associated with a reversal of the Mn-induced (100 and 250 µM) decrease in glutamate uptake. The effect of 500 µM Mn on glutamate uptake remained significant decrease (p<0.001) in ponasterone A induced HEK293T-Fpn-GFP cells compared with WT HEK293T cells. However, glutamate uptake in these cells (ponasterone A induced HEK293T-Fpn-GFP cells) was significantly higher vs. WT HEK293T (p<0.05 or 0.001) and ponasterone A uninduced HEK293T-Fpn-GFP cells (p<0.05 or 0.001) at the same Mn treatments (Fig. 3), corroborating a protective effect of increased Fpn expression on Mn-induced glutamate uptake inhibition.
Figure 3. Fpn protein expression enhanced glutamate uptake in Mn treated HEK cells.
Six hour of Mn exposure (100, 200, or 500 µM) induced decrease of glutamate uptake in a concentration-dependent manner in WT HEK293T and ponasterone A uninduced HEK293T cells; increased Fpn protein expression in ponasterone A induced HEK293T-Fpn-GFP cells was associated with a reversal of the Mn-induced (100 and 250 µΜ) decrease in glutamate uptake. High level (500 µM) of Mn on glutamate uptake remained significantly decreased in induced HEK293T-Fpn-GFP cells compared with WT cells. Glutamate uptake in ponasterone A induced HEK293T-Fpn-GFP cells was significantly higher vs. WT HEK293T and ponasterone A uninduced HEK293T-Fpn-GFP cells at the same Mn treatments. Values are mean ± SEM derived from three independent experiments with three or more samples per experiment. * p<0.05, *** p<0.001 compared to control; Δ p<0.05, ΔΔΔ p<0.001 compared between treatments at the same concentration in the different groups.
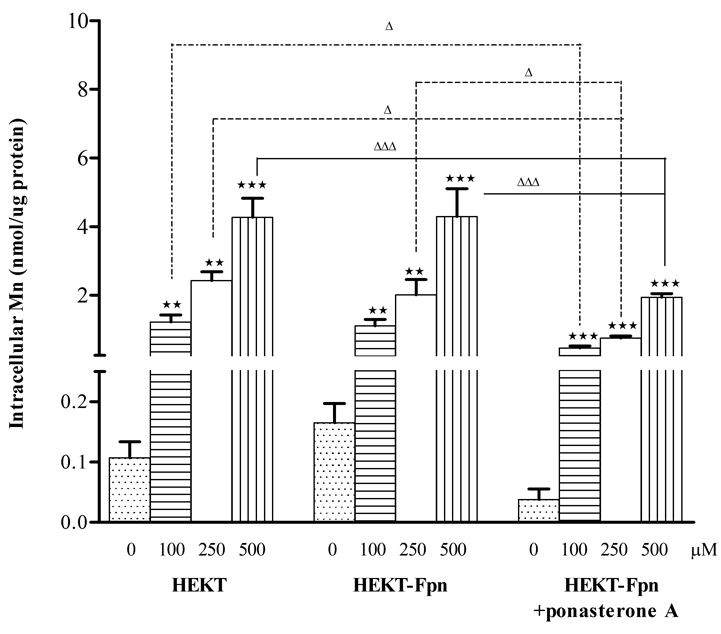
Fpn reduces HEK cell intracellular Mn concentrations
Treatment (6 hours) with Mn (100, 250, or 500 µM) resulted in significant (p<0.01 or 0.001) concentration-dependent increase in intracellular Mn levels in all 3 cell types. However, intracellular Mn concentrations were significantly lower in ponasterone A induced HEK293T-Fpn-GFP cells vs. ponasterone A uninduced HEK293T-Fpn-GFP (p<0.05 or 0.001) and WT HEK293T cells (p<0.05 or 0.001) at the same Mn treatments (Fig. 4).
Figure 4. Fpn protein expression reduced intracellular Mn concentration.
Mn exposure resulted in increase of intracellular Mn level in a concentration-dependent manner in all three WT HEK293T, ponasterone A uninduced and induced HEK293T-Fpn-GFP cells. However, intracellular Mn concentrations were significantly lower in ponasterone A induced HEK293T-Fpn-GFP cells vs. ponasterone A uninduced HEK293T-Fpn-GFP or WT HEK293T cells at the same concentration of Mn treatments. Values are mean ± SEM derived from three independent experiments with three or more samples in each experiment. ** p<0.01, *** p<0.001 compared to control; Δ p<0.05, ΔΔΔ p<0.001 compared between the same concentration treatment in the different groups.
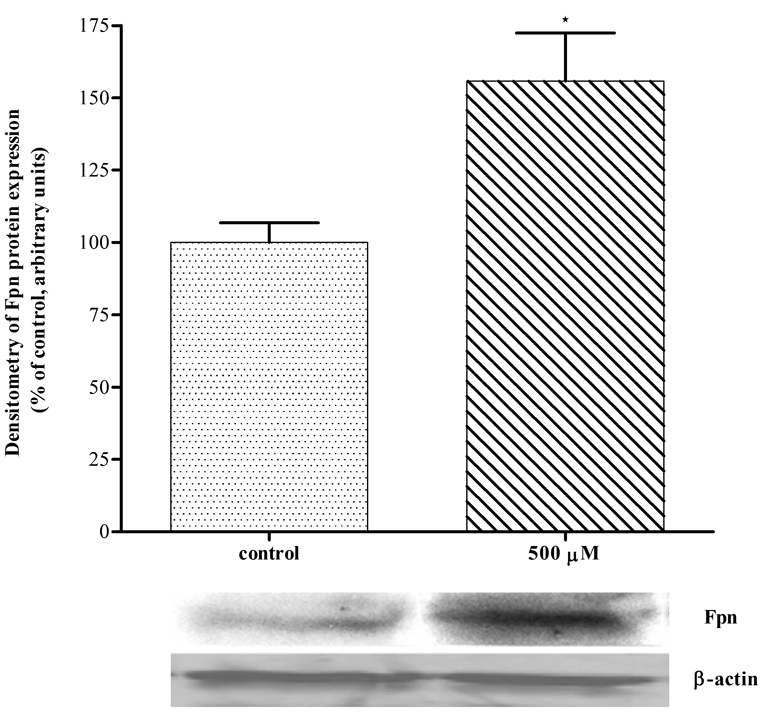
Mn increases Fpn protein expression in HEK293T cells
As shown in Figure 5, treatment with 500 µM for 6 hours significantly increased (p<0.05) Fpn protein expression in WT HEK293T cells vs. non-Mn exposed cells.
Figure 5. Mn increased Fpn protein expression in HEK293T cells.
Mn treatment (500 µM) for 6 hour resulted in significant increase of Fpn protein expression in WT HEK293T cells. Values are mean ± SEM derived from three independent experiments each carried out in triplicates. * p<0.05 compared to non-Mn treated WT HEK293T cells
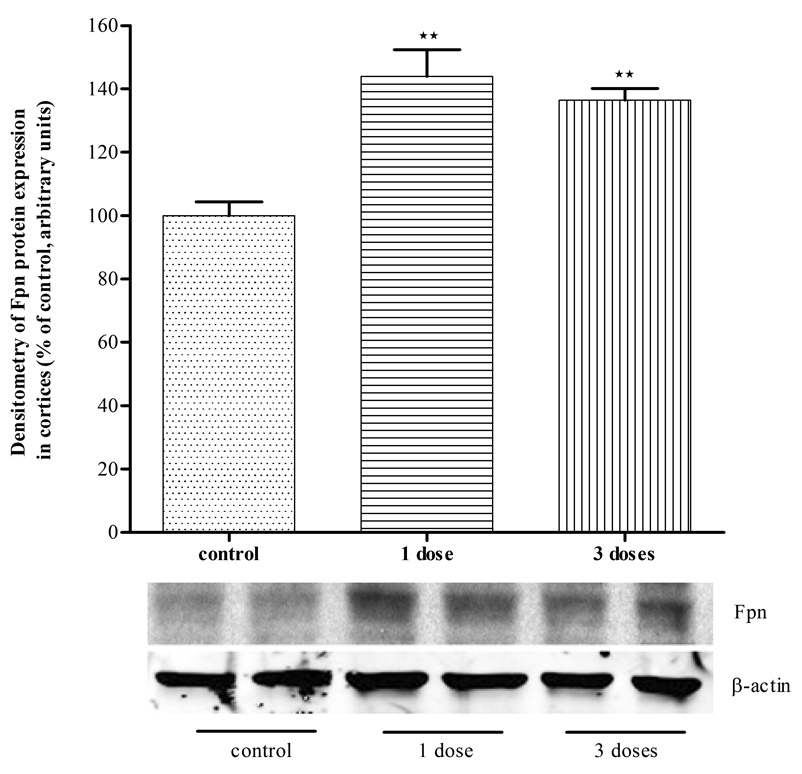
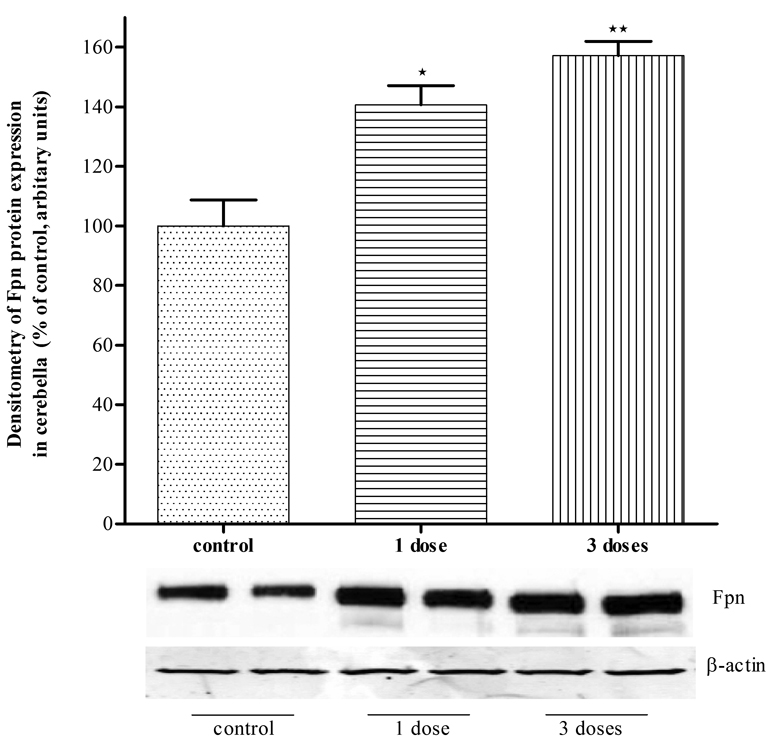
Mn increases Fpn protein expression in mice cerebella and cortices
To corroborate that Mn can increase Fpn protein expression in vivo mice were injected with Mn (s.c., 1–3 doses of Mn at 100 mg/kg body weight). As shown in Fig. 6, twenty-four hours post Mn injection, levels of Fpn protein expression significantly increased (p<0.01) both in the cortices (Fig 6A) and cerebella (Fig 6B) of Mn treated mice compared with untreated controls.
Figure 6. Mn injection increased Fpn expression in mice brains.
Mn injection (100 mg/kg body weight) 1 dose or three doses increased Fpn expression significantly in mice brain cortices (A) or cerebella (B). Values are mean ± SEM of 4–6 animals in each group. * p<0.05, ** p<0.01 compared to control.
Discussion
In the present study, we used WT HEK293T, ponasterone A uninduced HEK293T-Fpn-GFP and ponasterone A induced HEK293T-Fpn-GFP cells to investigate the role of Fpn in Mn efflux, and to ascertain whether increased Fpn protein expression attenuates the net intracellular Mn concentrations, and its effects on glutamate uptake and LDH release. Results presented in this study demonstrate, for the first time, that Mn exposure enhances Fpn protein expression in vitro in WT HEK293T cells and that in vivo s.c. Mn injections promote Fpn protein expression in mice cortices and cerebella. In addition, increased Fpn protein expression in HEK293T cells is associated with decreased net intracellular Mn accumulation and attenuated Mn toxicity, exemplified by reversal of Mn-induced glutamate uptake and diminished cellular LDH release.
Fpn is the only known cytoplasmic exporter of Fe in mammalian cells, regulating Fe absorption and recycling (Abbound and Haile, 2000; Knutson and Wessling-Resnick 2003; Donovan et al., 2005). Fpn is densely expressed on the surface of cells with high capacity for Fe export, such as macrophages and enterocytes (Abboud and Haile, 2000; Delaby et al., 2005), but it is present in almost all cells, including neurons and oligodendrocytes (Moos and Rosenbren Neilsen, 2006; Wu et al., 2004; Rouault and Cooperman, 2006). Mutations in the Fpn gene in humans (Pietrangelo, 2004) or deletion of the gene in animal models have established the importance of Fpn protein in Fe homeostasis (Donovan et al., 2005). Patients with Fpn mutation exhibit early Fe overload in the reticuloendocytic macrophages (Montosi et al., 2001) and deletion of the Fpn gene in the intestinal epithelium of postnatal (a period in which the intestine is the only route for Fe entry) mice is incompatible with development (Donovan et al., 2005).
Consistent with the shared chemical and physical characteristics of Mn and Fe, animal studies demonstrated that ID enhances Mn absorption independent of body Mn stores (Chandra and Shukla, 1976; Shukla et al., 1976) and leads to a significant increase in Mn concentrations throughout the rat brain (Erikson et al., 2002; Erikson et al., 2004). The inverse association between body Fe stores and Mn absorption has also been demonstrated in humans (Finley, 1999). A G185R mutation in the Belgrade (b/b) rat is associated with complete disruption of DMT-1 transport of Mn across the small-intestine, which is absent in heterozygous +/b rats or +/+ Wistar rats (Knopfel et al., 2005). Consistent with shared transporters for Fe and Mn, nasal absorption of Mn was significantly attenuated in b/b rats and the protein level of olfactory DMT-1 was significantly elevated in ID b/b rats (Thompson et al., 2007).
The present study demonstrates that net intracellular Mn concentration increased in a concentration-dependent manner in all three HEK293T cells (Fig. 4). However, in ponasterone A induced HEK293T-Fpn-GFP cells, intracellular Mn levels were significantly lower compared with WT HEK293T cells and ponasterone A uninduced HEK293T cells treated with the same Mn concentrations (Fig. 4). Though it is possible that Fpn overexpression caused down-regulation of a Mn importer, such as TfR and DMT1, the most likely explanation for these observations is that Mn increased Fpn expression (Fig. 5 and 6), promoting the efflux of Mn. Notably, our results also establish that in vivo basal levels of Fpn expression significantly differ amongst various mouse brain regions (e.g., cerebella vs. cortices ; Fig. 6 A and B). Whether such differences, and by inference, relatively low Fpn expression levels account for the propensity of striatal tissue to accumulate large amounts of Mn (e.g., 6.5 fold increase in striatal Mn levels relative to vehicle mice per method by Dodd et al., 2005) has yet to be established. Further studies could be profitably directed at establishing the distribution of Fpn expression in various brain regions to determine whether Fpn expression levels correlate with Mn accumulation.
Consistent with the reduced net Mn concentrations in ponasterone A induced HEK293T-Fpn-GFP cells, Fpn expression also decreased LDH leakage (Fig. 2) and restored glutamate uptake (Fig. 3) in these cells. Notably, an established mechanism of Mn-induced neurotoxicity is associated with attenuated glutamate uptake (Choi 1988; Brouillet et al., 1993; Westergaard et al., 1995; Aschner et al., 2007), resulting in increased extracellular glutamate concentration and activation of neuronal N-methyl D-aspartate (NMDA) receptors (Rosenberg et al., 1992). Notably, increased Fpn protein expression reversed the Mn-induced inhibition of glutamate uptake (Fig. 3) inherent to the lower Mn treatments (100 and 250 µM) in ponasterone A induced HEK293T-Fpn-GFP cells to levels that were indistinguishable from controls. This, as well as the Fpn associated reversal of the Mn-induced effects on LDH leakage (Fig. 2) was consistent with the data corroborating indistinguishable intracellular Mn concentrations in WT HEK293T and ponasterone A induced HEK293T-Fpn-GFP cells. These results indicate that increased Fpn protein expression reduces Mn toxicity via stimulation of Mn efflux and concomitant decrease in net intracellular concentrations of this metal.
In summary, we report the discovery that Mn exposure increases Fpn protein expression in HEK293T cells and mouse brain. Increased Fpn protein expression in HEK293T cells reduces cellular membrane leakage. Increased Fpn protein expression also reverses the inhibitory effect of Mn on glutamate uptake. Furthermore, increased Fpn protein levels reduce intracellular Mn concentrations following exposure, strongly suggesting that Fpn can actively transport Mn from these cells to decrease Mn cytotoxicity. These results suggest a shared mechanism for Fe and Mn efflux, paving the way for novel interventions to modulate intracellular levels of these metals.
Acknowledgements
This study was supported by grants RO1ES010563 (MA) and RO1ES016931 (ABB) from the National Institutes of Health and National Institute of Environmental Health Sciences; and grant W81XWH-05-0239 from the Department of Defense (MA).
Abbreviations
- DMT1
divalent metal transporter 1
- Fpn
ferroportin
- ID
iron deficiency
- Mn
manganese
- TfR
transferrin receptor
References
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- Allen JW, El-Oqayli H, Aschner M, Syversen T, Sonnewald U. Methylmercury has a selective effect on mitochondria in cultured astrocytes in the presence of [U-13C]glutamate. Brain Res. 2001;908:149–154. doi: 10.1016/s0006-8993(01)02628-2. [DOI] [PubMed] [Google Scholar]
- Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol. Appl. Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology. 2008;29:569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M, Dietz MC, Ihrig A, Triebig G. Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int. Arch. Occup. Environ. Health. 1999;72:521–527. doi: 10.1007/s004200050410. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Koller W, Schulz PE. Parkinsonism due to manganism in a welder: neurological and neuropsychological sequelae. Neurotoxicology. 2006;27:327–332. doi: 10.1016/j.neuro.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Brouillet EP, Shinobu L, McGarvey U, Hochberg F, Beal MF. Manganese injection into the rat striatum produces excitotoxic lesions by impairing energy metabolism. Exp. Neurol. 1993;120:89–94. doi: 10.1006/exnr.1993.1042. [DOI] [PubMed] [Google Scholar]
- Burdo JR, Antonetti DA, Wolpert EB, Connor JR. Mechanisms and regulation of transferrin and iron transport in a model blood-brain barrier system. Neuroscience. 2003;121:883–890. doi: 10.1016/s0306-4522(03)00590-6. [DOI] [PubMed] [Google Scholar]
- Chandra SV, Shukla GS. Role of iron deficiency in inducing susceptibility to manganese toxicity. Arch. Toxicol. 1976;35:319–323. doi: 10.1007/BF00570272. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Chua AC, Morgan EH. Manganese metabolism is impaired in the Belgrade laboratory rat. J. Comp. Physiol. B. 1997;167:361–369. doi: 10.1007/s003600050085. [DOI] [PubMed] [Google Scholar]
- Cotzias GC, Horiuchi K, Fuenzalida S, Mena I. Chronic manganese poisoning. Clearance of tissue manganese concentrations with persistence of the neurological picture. Neurology. 1968;18:376–382. doi: 10.1212/wnl.18.4.376. [DOI] [PubMed] [Google Scholar]
- Darshan D, Anderson GJ. Liver-gut axis in the regulation of iron homeostasis. World J. Gastroenterol. 2007;13:4737–4745. doi: 10.3748/wjg.v13.i35.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaby C, Pilard N, Hetet G, Driss F, Grandchamp B, Beaumont C, Canonne-Hergaux F. A physiological model to study iron recycling in macrophages. Exp. Cell Res. 2005;310:43–53. doi: 10.1016/j.yexcr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Dodd CA, Ward DL, Klein BG. Basal ganglia accumulation and motor assessment following manganese chloride exposure in the C57Cl/6 mouse. Int. J. Toxicol. 2005;24:389–397. doi: 10.1080/10915810500366500. [DOI] [PubMed] [Google Scholar]
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A, Barut B, Zapata A, Law TC, Brugnara C, Lux SE, Pinkus GS, Pinkus JL, Kingsley PD, Palis J, Fleming MD, Andrews NC, Zon LI. Postional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403:776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Konstantinov R, Bast-Pettersen R, Merkurjeva L, Chashchin M, Thomassen Y, Chashchin V. A neurobehavioral study of current and former welders exposed to manganese. Neurotoxicology. 2008;29:48–59. doi: 10.1016/j.neuro.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol. Trace Elem. Res. 2002;87:143–156. doi: 10.1385/BTER:87:1-3:143. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem. Int. 2003;43:475–480. doi: 10.1016/s0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, Aschner M. Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J. Nutr. Biochem. 2004;15:335–341. doi: 10.1016/j.jnutbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Increased manganese uptake by primary astrocyte cultures with altered iron status is mediated primarily by divalent metal transporter. Neurotoxicology. 2006;27:125–130. doi: 10.1016/j.neuro.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Finley JW. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr. 1999;70:37–43. doi: 10.1093/ajcn/70.1.37. [DOI] [PubMed] [Google Scholar]
- Fleming RE, Migas MC, Zhou X, Jiang J, Britton RS, Brunt EM, Tomatsu S, Waheed A, Bacon BR, Sly WS. Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1. Proc. Natl. Acad. Sci. USA. 1999;96:3143–3148. doi: 10.1073/pnas.96.6.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T, Nemeth E. Iron imports, IV: hepcidin and regulation of iron metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G199–G203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Gellein K, Syversen T, Aschner M. Iron deficient and manganese supplanted diets alter metals and transporters in the developing rat brain. Toxicol. Sci. 2007;95:516–525. doi: 10.1093/toxsci/kfl139. [DOI] [PubMed] [Google Scholar]
- Garcia Avila M, Penalver Ballina R. Manganese poisoning in the mines of Cuba. Ind. Med. Surg. 1953;22:220–201. [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Tran TT, Beard JL, Crinella FM, Lonnerdal B. Neurobehavioral evaluation of rhesus monkey infants fed cow's milk formula, sow formula or soy formula with added manganese. Neurotoxicol. Teratol. 2005;27:615–627. doi: 10.1016/j.ntt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muchenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- Jensen JH, Szulc K, Hu C, Ramani A, Lu H, Xuan L, Falangola MF, Chandra R, Knopp EA, Schenck J, Zimmerman EA, Helpern JA. Magnetic field correlation as a measure of iron-generated magnetic field inhomogeneities in the brain. Magn. Reson. Med. 2009;61:481–485. doi: 10.1002/mrm.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, Palis J, Abkowitz JL. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park JK, Choi Y, Yoo CI, Lee CR, Lee H, Lee JH, Kim SR, Jeong TH, Yoon CS, Park JH. Blood manganese concentration is elevated in iron deficiency anemia patients, whereas globus pallidus signal intensity is minimally affected. Neurotoxicology. 2005;26:107–111. doi: 10.1016/j.neuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Knopfel M, Zhao L, Garrick MD. Transport of divalent transition-metal ions is lost in small-intestinal tissue of b/b Belgrade rats. Biochemistry. 2005;44:3454–3465. doi: 10.1021/bi048768+. [DOI] [PubMed] [Google Scholar]
- Knutson MD, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit. Rev. Biochem. Mol. Biol. 2003;38:61–88. doi: 10.1080/713609210. [DOI] [PubMed] [Google Scholar]
- Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after rythrophagocytosis is up-regulated by ferroportin 1 overexpression and downregulated by hepcidin. Proc. Natl. Acad. Sci. USA. 2005;102:1324–1328. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu. Rev. Pharmacol. Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- Law NA, Caudle MT, Pecoraro VL. Manganese Redox Enzymes and Model Systems: Properties, Structures, and Reactivity. Advances in inorganic Chemistry. 1998;46:305–440. [Google Scholar]
- Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu. Rev. Pathol. 2009;4:489–515. doi: 10.1146/annurev.pathol.4.110807.092205. [DOI] [PubMed] [Google Scholar]
- Liu XB, Hill P, Haile DJ. Role of the ferroportin iron-responsive element in iron and nitric oxide dependent gene regulation. Blood Cells Mol. Dis. 2002;29:315–326. doi: 10.1006/bcmd.2002.0572. [DOI] [PubMed] [Google Scholar]
- Lymboussaki A, Pignatti E, Montosi G, Garuti C, Haile DJ, Pietrangelo A. The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J. Hepatol. 2003;39:710–715. doi: 10.1016/s0168-8278(03)00408-2. [DOI] [PubMed] [Google Scholar]
- McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- Milatovic D, Yin Z, Gupta RC, Sidoryk M, Albrecht J, Aschner JL, Aschner M. Manganese induces oxidative impairment in cultured rat astrocytes. Toxicol. Sci. 2007;98:198–205. doi: 10.1093/toxsci/kfm095. [DOI] [PubMed] [Google Scholar]
- Mims MP, Prchal JT. Divalent metal transporter 1. Hematology. 2005;10:339–345. doi: 10.1080/10245330500093419. [DOI] [PubMed] [Google Scholar]
- Montosi G, Donovan A, Totaro A, Garuti C, Pignatti E, Cassanelli S, Trenor CC, Gasparini P, Andrews NC, Pietrangelo A. Autosomal-dominant hemochromatosis is associated with a mutation in the ferroportin (SLC11A3) gene. J. Clin. Invest. 2001;108:619–623. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos T, Rosengren Nielsen T. Ferroportin in the postnatal rat brain: implications for axonal transport and neuronal export of iron. Semin. Pediatr. Neurol. 2006;13:149–157. doi: 10.1016/j.spen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Moos T, Rosengren Nielsen T, Skjørringe T, Morgan EH. Iron trafficking inside the brain. J. Neurochem. 2007;103:1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x. [DOI] [PubMed] [Google Scholar]
- Myers JE, Thompson ML, Ramushu S, Young T, Jeebhay MF, London L, Esswein E, Renton K, Spies A, Boulle A, Naik I, Iregren A, Rees DJ. The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology. 2003a;24:885–894. doi: 10.1016/S0161-813X(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Myers JE, teWaterNaude J, Fourie M, Zogoe HB, Naik I, Theodorou P, Tassel H, Daya A, Thompson ML. Nervous system effects of occupational manganese exposure on South African manganese mineworkers. Neurotoxicology. 2003b;24:649–656. doi: 10.1016/S0161-813X(03)00035-4. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward MD, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to Ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu. Rev. Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- Olanow CW. Manganese-induced parkinsonism and Parkinson’s disease. Ann. NY Acad. Sci. 2004;1012:209–223. doi: 10.1196/annals.1306.018. [DOI] [PubMed] [Google Scholar]
- Park JD, Chung YH, Kim CY, Ha CS, Yang SO, Khang HS, Yu IK, Cheong HK, Lee JS, Song CW, Kwon IH, Han JH, Sung JH, Heo JD, Choi BS, Im R, Jeong J, Yu IJ. Comparison of high MRI T1 signals with manganese concentration in brains of cynomolgus monkeys after 8 months of stainless steel welding-fume exposure. Inhal. Toxicol. 2007;19:965–971. doi: 10.1080/08958370701516108. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A. The Ferroportin disease. Blood Cells Mol. Dis. 2004;32:131–138. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Rosenberg PA, Amin S, Leitner M. Glutamate uptake disguises neurotoxic potency of glutamate agonists in cerebral cortex in dissociated cell culture. J. Neurosci. 1992;12:56–61. doi: 10.1523/JNEUROSCI.12-01-00056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA. How Mammals Acquire and Distribute Iron Needed for Oxygen-Based Metabolism. PLos Biology. 2003;1:326–328. doi: 10.1371/journal.pbio.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA, Cooperman S. Brain iron metabolism. Semin. Pediatr. Neurol. 2006;13:142–148. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Shukla GS, Chandra SV, Seth PK. Effect of manganese on the levels of DNA, RNA, DNase and RNase in cerebrum, cerebellum and rest of brain regions of rat. Acta Pharmacol. Toxicol. (Copenh) 1976;39:562–569. doi: 10.1111/j.1600-0773.1976.tb03206.x. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ, Stankowski JN, Aschner M, McLaughlin B. Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J. Neurochem. 2009;110:378–389. doi: 10.1111/j.1471-4159.2009.06145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. Olfactory uptake of manganese requires DMT1 and is enhanced by anemia. FASEB J. 2007;21:223–230. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate glutamine cycle revisited. Dev. Neurosci. 1995;17:203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- Wright DM, Andrews NC. Iron homeostasis and erythropoiesis. Curr. Top Dev. Biol. 2008;82:141–167. doi: 10.1016/S0070-2153(07)00006-3. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Leenders AG, Cooperman S, Meyron-Holtz E, Smith S, Land W, Tsai RY, Berger UV, Sheng ZH, Rouault TA. Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res. 2004;1001:108–117. doi: 10.1016/j.brainres.2003.10.066. [DOI] [PubMed] [Google Scholar]
- Yang F, Liu XB, Quinones M, Melby PC, Ghio A, Haile DJ. Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J. Biol. Chem. 2002;277:39786–39791. doi: 10.1074/jbc.M201485200. [DOI] [PubMed] [Google Scholar]
- Zoller H, Koch RO, Theurl I, Obrist P, Pietrangelo A, Montosi G, Haile DJ, Vogel W, Weiss G. Expression of the duodenal iron transporters divalent-metal transporter 1 and ferroportin 1 in iron deficiency and iron overload. Gastroenterology. 2001;120:1412–1419. doi: 10.1053/gast.2001.24033. [DOI] [PubMed] [Google Scholar]