Abstract
Many phytochemicals function as noxious agents that protect plants against insects and other damaging organisms. However, at subtoxic doses the same phytochemicals may activate adaptive cellular stress response pathways that can protect cells against a variety of adverse conditions. We screened a panel of botanical pesticides using cultured human and rodent neuronal cell models, and identified plumbagin as a novel potent activator of the nuclear factor E2-related factor 2 (Nrf2)/antioxidant response element (ARE) pathway. In vitro, plumbagin increases nuclear localization and transcriptional activity of Nrf2, and induces the expression of the Nrf2/ARE-dependent genes, such as heme oxygenase 1 in human neuroblastoma cells. Plumbagin specifically activates the Nrf2/ARE pathway in primary mixed cultures from ARE-human placental alkaline phosphatase reporter mice. Exposure of neuroblastoma cells and primary cortical neurons to plumbagin provides protection against subsequent oxidative and metabolic insults. The neuroprotective effects of plumbagin are abolished by RNA interference-mediated knockdown of Nrf2 expression. In vivo, administration of plumbagin significantly reduces the amount of brain damage and ameliorates associated neurological deficits in a mouse model of focal ischemic stroke. Our findings establish precedence for the identification and characterization of neuroprotective phytochemicals based upon their ability to activate adaptive cellular stress response pathways.
Keywords: Nrf2/ARE, stroke, neuroprotection, phytochemicals
Introduction
Plants, plant extracts, and compounds purified from plants have long been used in traditional medicine in various civilizations, and many drugs currently used in modern medicine are derived from natural products (De Smet, 1997). While some phytochemicals may act by inhibiting enzymes or via intrinsic antioxidant properties, others may activate specific signal transduction pathways. For example, tetrahydrocannabinol (the psychoactive chemical in marijuana) activates cannabinoid receptors on neurons (Wilson and Nicoll, 2002) and caffeine activates ryanodine receptors to mobilize intracellular calcium and thereby increase heart rate and neuronal excitability (Tarnopolsky, 1994). Interestingly, several widely used dietary components or supplements with medicinal properties have recently been shown to activate adaptive stress response pathways in cells. For example, sulforaphane (present in high amounts in the broccoli sprouts) (Juge et al., 2007), and curcumin (concentrated in the roots of the turmeric plant) each activate the transcription factor Nrf2 which binds to the antioxidant response element (ARE) of genes that encode antioxidant and phase 2 enzymes (Dinkova-Kostova and Talalay, 2008). Other adaptive stress response pathways activated by phytochemicals include the sirtuin – FOXO pathway (resveratrol) and the transient receptor potential - calcium pathway (Calixto et al., 2005). The mechanism of action of such phytochemicals can therefore be considered as a form of hormesis in which exposure to a low amount of a stressor triggers an adaptive response which increases resistance to more severe stress and disease (Calabrese et al., 2007).
Converging evidence from several fields suggests that many phytochemicals, particularly those concentrated in exposed surfaces such as the skins of fruits or roots, function to repel insects and other organisms (Mattson and Cheng, 2006). Indeed, the goal of the botanical pesticide (BP) industry is to identify, isolate and produce large quantities of phytochemicals that dissuade insects from eating plants (Isman, 2006; Wright et al., 2007). Hundreds of such BPs have been isolated and include a range of molecular structures such as flavonoids, terpenoids, alkaloids, indoles, quinones, coumarins, phenylpropanols and cardenolides (Koul and Dhaliwal, 2001). Because they are toxic at high doses, some BPs have been evaluated for their ability to prevent the growth of or kill cancer cells (Sugie et al. 1998). However, bioassays used to identify pesticides include those that measure insect repellant activity and so reveal chemicals that act on sensory neurons to elicit a withdrawal response. It is therefore likely that many botanical pesticides elicit cellular stress responses in neurons that mediate the antifeedant response. However, the possibility that subtoxic doses of certain BPs might activate adaptive stress response pathways in neurons that may protect neurons against more severe stress has not been investigated. In the present study we used neuronal cell-based assays to screen BPs for their ability to activate the Nrf2/ARE pathway. We show that, among the BPs tested, the naphthoquinone plumbagin activates the Nrf2/ARE pathway resulting in the upregulation of target genes and increased resistance of neurons to oxidative and metabolic insults in culture, and to ischemic stroke in vivo.
Materials and Methods
Cell Cultures and Experimental Treatments
Human neuroblastoma SH-SY5Y cells were maintained at 37°C in a humidified 6% CO2 atmosphere in Dulbecco’s Modified Eagle’s Media (DMEM) (GIBCO, Grand Island, NY) supplemented with 2 mM L-glutamine, 10% Hyclone serum and 25 mg/L gentamicin sulfate (Sigma, St. Louis, MO). Dissociated cell cultures of primary cerebral cortical neurons were prepared using methods similar to those described previously (Camandola et al., 2005). Briefly, cerebral cortices were removed from embryonic day 18 Sprague-Dawley rats (Harlan Sprague Dawley, Inc., Indianapolis, IN), or ARE-hPAP mice (Johnson et al., 2002). Cells were dissociated by mild trypsinization and trituration. For neuronal cultures the cell suspension was plated in polyethyleneimine-coated culture dishes containing Eagle’s minimum essential medium supplemented with 26 mM NaHCO3, 40 mM glucose, 20 mM KCl, 1 mM sodium pyruvate, 10% (v/v) fetal bovine serum, and 0.001% gentamycin sulfate. After a 3–5-h incubation period to allow for cell attachment, the medium was replaced with Neurobasal medium with B27 supplements (Invitrogen, Grand Island, NY). Glucose deprivation, and combined oxygen and glucose deprivation, were performed as previously described (Arumugam et al., 2007; Tang et al., 2007).
Quantification of cell survival
Cells seeded in 96-well plates (0.01–0.1 × 106 cells/well) were treated with the experimental agents at the indicated concentrations for 24 hours in medium containing 2% serum. Cytotoxicity was determined using Alamar blue, Celltiter 96® AQUEOUS one solution reagent (Promega, Madison, WI), lactate dehydrogenase activity assay (Roche, Nutley, NJ), and random blinded cell counting of Hoechst 33421 stained cells.
Cell Transfection and Reporter Assay
Human SH-SY5Y neuroblastoma cells were transfected with 3 μg of TF-driven firefly luciferase reporter (TF= ARE, AP-1, Foxo, NF-κB), and 0.5 μg of pRL-TK Vector (Promega) expressing renilla luciferase, using Cell line Nucleofector kit V and Nucleofector I device (Amaxa Biosciences, Gaithersburg, MD) according to the manufacturer’s instructions. 12 hours after nucleofection cells were incubated with the indicated concentration of the phytochemicals for 24 hours, and luciferase activity was quantified using a Dual-Luciferase-Reporter System (Promega). All analyses were performed in triplicate.
Primary neuronal cultures were transfected with pEF (control vector), and pEF-DN-Nrf2 using Rat neuron Nucleofactor kit and Nucleofactor I device. Glucose deprivation experiments were performed as described 72 hours post-transfection (Tang et al., 2007).
Protein Cell Extraction and Western Blot Analysis
Whole cell lysates and nuclear fractions were prepared as described previously (Camandola et al., 2005). The primary antibodies used in this study were: HO-1 (Santa Cruz Biotechnology, Santa Cruz, CA), Nrf2 (Santa Cruz Biothechnology), NQO1 (Sigma), cleaved caspase 3 (Cell Signaling Technology, Danvers, MA), α-tubulin (Sigma) and β-actin (Sigma).
RNA extraction and real-time PCR
RNA from vehicle (control) or plumbagin treated cells was isolated using Trizol (Invitrogen) and purified with an RNA Micro Kit (Qiagen, Valencia, CA). Following treatment with DNase I, RNA was quantified and equal amounts were retrotranscribed using random hexamers and the SuperScript First Strand Synthesis System (Invitrogen Life Technologies). Real-time PCR analysis was performed with a PTC 200 Pelthier Thermo Cycler and Chromo 4 Fluorescent Detector (BioRad, Hercules, CA), and Sybr® Green PCR Master Mix according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Each reaction included 3 μl of diluted (1:3) cDNA and was performed in triplicate. PCR was performed under the following conditions: 10 min at 95 °C, followed by 40 cycles of 30s at 95 °C, 30s at 60 °C and 1 min at 72°C. The primers used in this study were as follows: human NQO1(NM_000903), 5′-TGC AGC GGC TTT GAA GAA GAA AGG-3′ and 5′-TCG GCA GGA TAC TGA AAG TTC GCA-3′; human GCLM (NM_002061), 5′-CTG CTG TGT GAT GCC ACC AGA TTT-3′ AND 5′-GTG CGC TTG AAT GTC AGG AAT GCT-3′; human TX (NM_182743) 5′-TTG CAA TCC AGG CAG GAA GAT TGC-3′ and 5′-AGC TTT CTC CTC AGA AAG GCC ACA-3′; human HO-1 (NM_002133), 5′-GGGCCA GCA ACA AAG TGC AAG ATT-3′ and 5′-TCG CCA CCA GAA AGC TGA GTG TAA-3′; human GSTpi (NM_000852), 5′-AGG ACC TCC GCT GCA AAT ACA TCT-3′ and 5′-TCT CCC ACA ATG AAG GTC TTG CCT-3′; human GAPDH (NM_002046), 5′-TCG ACA GTC AGC CGC ATC TTC TTT-3′ and 5′-ACC AAA TCC GTT GAC TCC GAC CTT-3′.
Immunocytochemistry
Cells were cultured in Lab-Tek chamber slides (Nalgene Nunc, Rochester, NY). Following experimental treatments cells were fixed in 4% paraformaldehyde in PBS, permeabilized for 5 min in 0.2% Triton X-100 (Sigma) in PBS, incubated for 1 h in blocking solution and then incubated overnight at 4°C with HO-1 or hPAP antibodies. The primary antibody was detected using Alexa Flour 488-conjugated secondary antibody. Nonspecific labeling was determined by omission of the primary antibody. The cell nuclei were counterstained with Hoechst 33421 dye. Confocal images were acquired using a Zeiss 510 LSM microscope. All images were acquired using the same laser intensity and photodetector gain to allow quantitative comparisons of relative levels of immunoreactivity among cells. The average pixel intensity per cell body was determined using the software provided by the manufacturer (Zeiss, Thornwood, NY).
Assessment of oxidative stress and cerebral levels of plumbagin
Levels of oxidative stress were determined by measuring intracellular reactive oxygen species (ROS) generation, as well as levels of the lipid peroxidation end product 4-hydroxy-2,3-nonenal (4HNE). ROS production was determined using 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) (Sigma), and a HTS 7000 Plus BioAssay Reader (Perkin Elmer). 4HNE levels were measured by mass spectrometry as previously described (Cutler et al., 2002). Cerebral concentration of plumbagin was measured by high performance liquid chromatography (HLPC) tandem mass spectrometry 1–2 hrs post injection. Following extraction the lipid fraction was analyzed by HPLC using a Hewlett Packard 1100 series degasser pump (isocratic model phase consisting of 80% methanol with 10 mM ammonium formate, 20% water with 0.6% formic acid, at a flow rate of 200μl/minute), and a Hypersil-Keystone Aquasil C18 150 × 2.1 mm, 5 micron column by Thermo (Bellefonte, PA). Eluates were detected by injection into a electro-spray ionization tandem mass spectrometry (ESI/MS/MS) Sciex 3000 (Thornhill, ON, Canada) using positive polarity, neutral loss scan of 188.18 amu, face plate temperature of 400°C, while scanning for 35 minutes. Plumbagin (Sigma, St. Louis, MO) standards were used to develop the method, and determine the linear detection range and limits.
Focal cerebral ischemia/reperfusion injury, neurological evaluation and quantification of cerebral infarct
Three month old C57BL/6 mice were subjected to transient middle cerebral artery occlusion as described previously (Arumugam et al., 2007; Tang et al., 2007). The functional outcome of the stroke injury was determined blinded to the treatment history of the mice using a 5 grade neurological deficit score (0, no deficit; 1, failure to extend right paw; 2, circling to the right; 3, falling to the right; 4, lacking spontaneous locomotion). Cerebral infarct size was quantified in mice euthanized 72 h post stroke as described previously (Arumugam et al., 2007). For cerebral blood flow measurements, vehicle and plumbagin treated animals were anesthetized and prepared for middle cerebral artery occlusion. The craniotomy to access the left middle cerebral artery was extended to allow positioning of a 0.5-mm Doppler probe (Moor LAB, Moor Instruments, Devon, UK) over the underlying parietal cortex, approximately 1 mm posterior to bregma and 1 mm lateral to the midline. Laser-Doppler recordings are expressed as percentages of the pre-ischemic baseline and averaged over 30-min periods during 1 h of ischemia, and 0, 30, 90, 120 and 180 and min of post-ischemic reperfusion. Mean arterial pressure and heart rate were monitored using LabChart Pro® analysis software (AdInstruments, NSW Australia) in vehicle or drug treated sham and ischemia/reperfusion animals before (−15) and after the induction of ischemia.
Statistical Analyses
Data are presented as means and S.D. (or S.E.M, where indicated). One-way analysis of variance combined with Fisher’s protected least significant difference post hoc test was used for pairwise comparisons.
Results
Screening of BPs identifies plumbagin as a selective activator of the ARE pathway
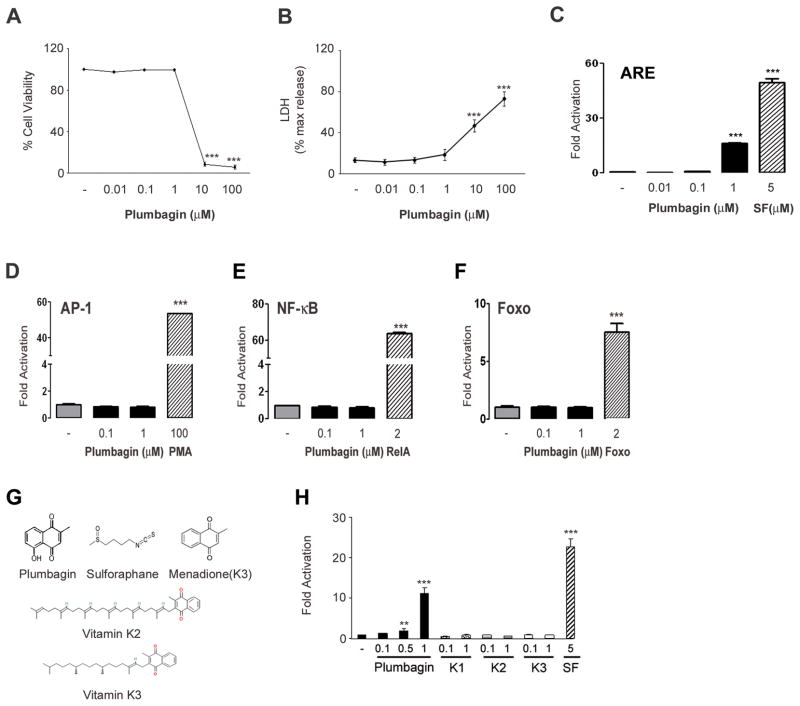
In order to test the hypothesis that subtoxic levels of BPs exert a hormetic effect on neurons through the activation of adaptive stress response pathways, we selected 30 BPs comprising a range of structures including alkaloids, terpenoids, flavonoids, phenols, amides, aldehydes, alcohols, a glucoside and a peptide (Supplemental table 1). We first performed dose-response assays to determine the cytotoxicity of each BP across a broad concentration range (0.01 – 100 μM) using human neuroblastoma cells and Alamar blue and LDH assays (Fig. 1A, B). Based on the toxicity studies, we selected subtoxic concentrations of each BP to test its ability to transactivate an ARE-driven reporter gene in human neuroblastoma cells. Among the 30 BPs tested, only plumbagin (5-hydroxy-2-methyl-1,4-naphtoquinone) significantly increased ARE-driven luciferase activity, being effective at concentrations of 0.1 and 1.0 μM (Fig. 1C and Supplemental table 1). As a positive control we treated reporter cells with sulforaphane, a phytochemical previously shown to activate the Nrf2/ARE pathway (Kraft et al., 2004).
Figure 1.
Plumbagin activates ARE- driven transcription at subtoxic concentrations. SH-SY5Y cells were cultured on 96 well plates and incubated with the indicated concentrations of plumbagin for 24 hours. Viability was assessed by alamar blue (A) and lactate dehydrogenase activity (LDH) (B) assays. Data are presented as mean and S.D. (n=3). ***, p< 0.001 compared with DMSO (−) treated cells. SH-SY5Y cells were transfected with ARE- (C), AP-1- (D), NF-κB- (E) or Foxo- (F) firefly luciferase reporter and pRL-TK control vector, and treated with the indicated concentrations of plumbagin or, respectively, 5 μM sulforaphane (SF), 100 nM phorbol-12-myristate-13-acetate (PMA), 2 μg pRelA (RelA), or 2 μg pECE-Foxo3A (Foxo) as positive controls for 24 hours. The relative light intensities were normalized, and signal averages were determined. Data are mean and S.D. (n=3–6).***, p< 0.001 compared with DMSO (−) treated cells. (G) Structure of sulforaphane, plumbagin and its analogs. (H) Effect of plumbagin and its analogs on ARE-driven luciferase activity. SH-SY5Y were transfected with ARE-luciferase reporter and treated with the indicated concentrations of phytochemicals. Luciferase activity was assessed after 24 hours. Data are normalized values compared to the activity of DMSO treated cells set equal to 1. Data are mean and S.D. of triplicate samples from 2–3 separate experiments. **, p< 0.05 compared with control group; ***, p< 0.001 compared with control group.
Plumbagin is a naturally occurring pigment in the genus Plumbago of the family Plumbaginaceae of flowering plants that possesses antimicrobial activity (de Paiva et al., 2003). Menadione (vitamin K3), which differs from plumbagin by only the absence of a hydroxyl group, did not activate ARE-mediated transcription, and neither did vitamins K1 and K2 (Fig. 1G, H). In addition to natural plumbagin analogs we tested a series of synthetic derivatives. We found that the only compound retaining the ability to induce ARE-dependent transcription maintain both the 2′-methyl and 5′-hydroxyl groups. We thus believe that such groups are indispensable for the activation of the ARE/Nrf2 pathway (Son et al, in preparation). In order to further evaluate the selectivity of plumbagin for the ARE/Nrf2 pathway, we measured its influence on three other oxidative stress-sensitive transcription factors, AP-1, NF-κB and Foxo. At the same concentration range shown to activate ARE, plumbagin failed to induce AP-1-, NF-κB-, and Foxo-driven luciferase activities (Fig. 1D, E, F).
Plumbagin induces ARE-regulated genes
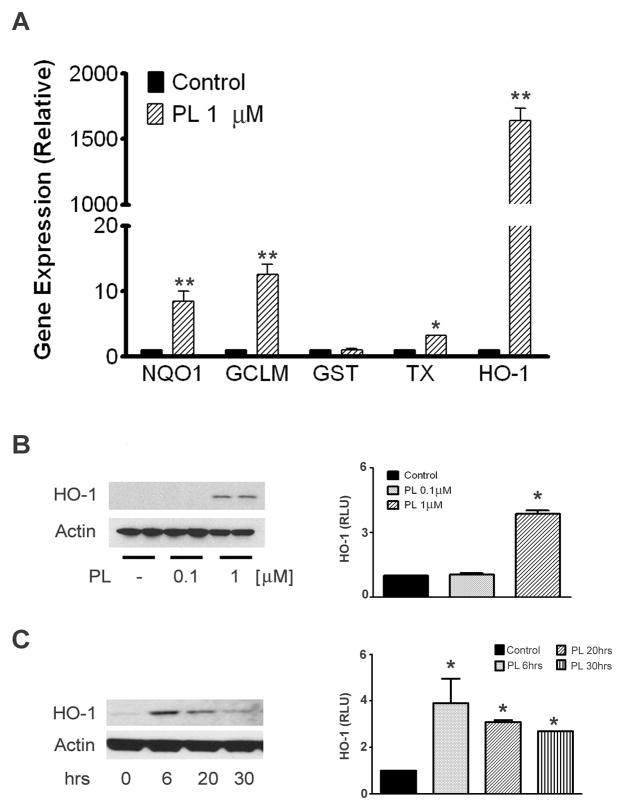
We next determined whether plumbagin induces the expression of endogenous ARE-regulated genes. qRT-PCR was used to determine transcript levels of the ARE-target genes, HO-1, thioredoxin reductase 1 (TX), NAD(P)H quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase modifier subunit (GCLM), and glutathione-S-transferase 1 (GST) in neuroblastoma cells treated with 1 μM plumbagin for 6 hours. With the exception of GST, at the chosen time point plumbagin significantly increased the transcription of all ARE target genes examined (Fig. 2A). We confirmed the effect on endogenous gene expression by western blot analysis of HO-1 levels. Neuroblastoma cells were either treated for 20 hours with increasing concentrations of plumbagin, or treated with 1 μM plumbagin and collected at different time points. Plumbagin treatment increased the protein levels of HO-1 in a concentration-dependant manner (Fig. 2B). The up-regulation of HO-1 was time-dependent, with a peak increase at 6 hours and a gradual return to basal levels by 30 hours post-treatment (Fig. 2C). In addition, plumbagin also induced the activation of the Nrf2/ARE pathway in cultured primary murine cortical neurons and astrocytes (Supplementary Figure 1A). Together these results show that plumbagin rapidly induces the expression of endogenous Nrf2-regulated genes.
Fig. 2.
Effect of plumbagin on Nrf2 endogenous genes. (A) SH-SY5Y cells were treated with 1 μM plumbagin for 6 hrs. Total RNA was extracted, retrotranscribed, and the transcript levels of the indicated Nrf2-dependent genes were analyzed by semi quantitative RT-PCR. Data are mean and S.D. from three independent experiments. *, p<0.01 compared with vehicle treated cells; **, p<0.001 compared with vehicle treated cells. Protein steady state levels of HO-1 were measured in cell extracts from SH-SY5Y treated either for 20 hrs with increasing concentrations of plumbagin (B), or with 1 μM plumbagin for the indicated time points (C). Autoradiograms are representative of two-three independent experiments. Right panels show densitometric quantitation as mean and S.D. of 3–4 samples.*p<0.05 compared control set equal to 1.
Plumbagin protects cells against oxidative stress-induced death
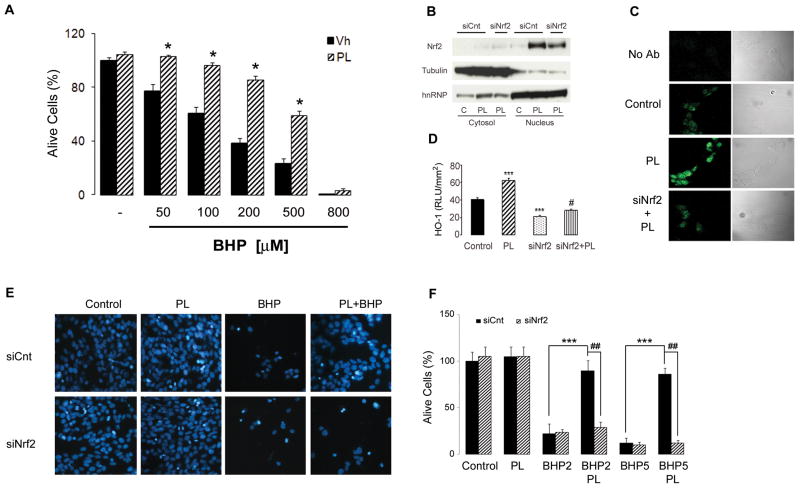
Activation of the Nrf2/ARE pathway is known to confer resistance of cells to oxidative stress (Itoh et al., 2004; Hanneken et al., 2006). We assessed the ability of PL to prevent stress induced cell death by challenging SH-SY5Y with the radical generator tert-butyl-hydroperoxide (BHP). As shown in Figure 3A BHP-induced cells death was completely abolished, at low concentration, or significantly attenuated, at higher doses, by PL pretreatment. To establish that Nrf2 is a major transcription factor activated by plumbagin, and necessary for its protective effect, we treated neuroblastoma cells with either control (siCnt) or Nrf2-specific (siNrf2) small interfering RNAs for 48 hours, and then exposed the cells to DMSO or 1 μM plumbagin and then evaluated Nrf2 nuclear translocation, and HO-1 levels. The siNrf2 treatment significantly decreased the levels of Nrf2 in nuclear extracts from cells treated with plumbagin (Fig. 3B), and completely prevented the up-regulation of its target gene, HO-1 (Fig. 3C–D). We subsequently exposed siCnt- or siNrf2-treated cells to 1 μM plumbagin and then challenged them with BHP 200–500 μM. Plumbagin protected siCnt-treated cells against BHP-induced cell death, but was significantly less effective in protecting siNrf2-treated cells from being killed by BHP (Fig. 3E–F).
Fig. 3.
Plumbagin affords cell protection trough the Nrf2/ARE pathway. (A) SH-SY5Y were treated with either vehicle (Vh) or 1 μM plumbagin (PL) for 6 hrs, and subsequently exposed to the indicated concentrations of tert-butyl-hydroperoxide (BHP). Cell survival was assessed by blinded random counting of Hoescht stained cells. Data are mean and S.E.M. of three independent experiments. *, p<0.0001 compared to the corresponding BHP concentration in Vh treated cells. SH-SY5Y were treated for 48 hrs with control (siCnt) or Nrf2 siRNA (siNrf2), than subjected to plumbagin (PL) for 6 hrs. (B) Nuclear extracts were assessed for Nrf2 levels, using tubulin and hnRNP as internal controls for subcellular fractionation purity. HO-1 representative immunostaining (C) and quantification (D) following PL treatment in control and Nrf2 siRNA treated cells. Data are mean and S.D. of three independent experiments (90–100 cells per treatment). ***, p<0.001 compared to control; #, p<0.001 compared to PL. (E) Representative images and (F) survival quantification following challenge with BHP 200 μM (BHP2) and 500 μM (BHP5) in cells pretreated with control (siCnt) or Nrf2 siRNA (siNrf2) for 48 hrs, then vehicle or 1 μM PL for 6 hrs.; ***, p<0.0001 compared to control siRNA BHP2 or BHP5; ##, p<0.0001 compared to control siRNA PL BHP2 or control siRNA PL BHP5.
Evidence for the involvement of the PI3 kinase – AKT kinase pathway in the neuroprotective effect of plumbagin
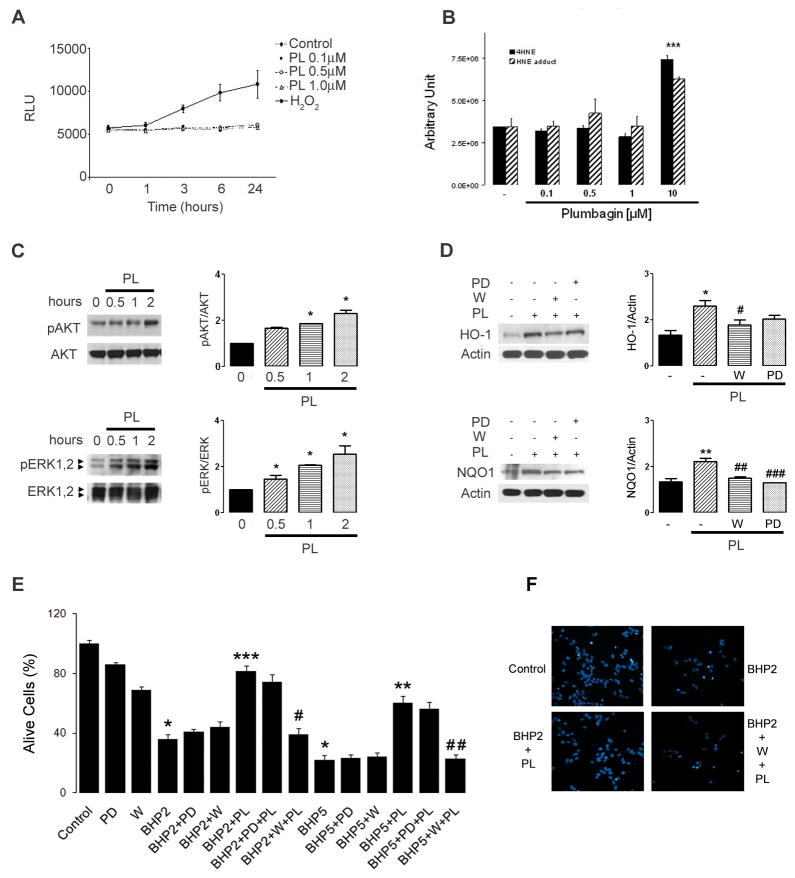
The Nrf2/ARE pathway can be activated by both ROS and electrophilic compounds (Alam et al., 1999). Because plumbagin has been shown to generate ROS in some cells (Inbaraj and Chignell, 2004), we tested the possibility that plumbagin activates Nrf2 via radical generation. Neuroblastoma cells were pre-loaded with the ROS dye indicator dichlorofluorescein diacetate (DCFDA) and were then treated with subtoxic concentrations of plumbagin, or with hydrogen peroxide as a positive control. While hydrogen peroxide treated cells showed a significant increase of fluorescence as result of ROS production, no changes were observed in plumbagin treated cells (Fig. 4A). In addition to the direct ROS production measurement, we also assessed the accumulation of the lipid peroxidation end product 4-hydroxy-2,3-nonenal (4HNE) and its adducts in cells treated for 24 hrs with both subtoxic and toxic concentrations of plumbagin. In agreement with the results obtained with DCFDA, mass spectrometry showed that only the cytotoxic concentration of plumbagin induced ROS generation thus a significant accumulation of 4HNE and its adducts (Fig. 4B). These results together with the lack of activation of other redox sensitive transcription factors (i.e. AP-1, NF-κB, Foxo) strongly suggest that the induction of the Nrf2/ARE pathway observed with low concentrations of plumbagin is not mediated by ROS.
Fig. 4.
Plumbagin activates the PI3K/AKT and ERK pathways. (A) SH-SY5Y cells were loaded with 25 μM DCFDA for 45 min at 37 °C, washed with PBS and exposed to 400 μM hydrogen peroxide (H2O2) or the indicated concentrations of plumbagin (PL). The relative levels of DCF fluorescence were quantified at the indicated time points. (B) Levels of the lipid peroxidation end product 4-hydroxy-2,3-nonenal (4HNE) were assessed in SH-SY5Y cells treated for 24 hrs with increasing concentrations of plumbagin. Total lipids extracts were analyzed by mass spectrometry. Data are mean and S.E.M of triplicate samples. ***, p<0.001 versus DMSO (−) treated cells. (C) SH-SY5Y cells were treated with 1 μM plumbagin for the indicated time points, and cell lysates were analyzed by western blotting using anti- pAKT, AKT, pERK and ERK antibodies. Right panels show densitometric quantitation as mean and S.D. (n=3).*p<0.05 compared to time zero. (D) Cells were pretreated with 1 μM wortmannin (W) (PI3K inhibitor), or 10 μM PD98059 (PD) (MEK1 inhibitor) for 1 h, were washed with PBS and treated with 1 μM plumbagin for 6 hrs. Cell lysates were blotted with anti-HO-1 and NQO1 antibodies. Right panels show quantification as mean and S.D. (n=3).*, p=0.026 compared to control (−); #, p=0.024 compared to PL; **, p=0.0007 compared to control; ##, p=0.0023 compared to PL; ###. P=0.0005 compared to PL. (E) Cell survival quantification and (F) representative images of cells pretreated as above mentioned than subjected to either 200 μM tert-butyl hydroperoxide (BHP2) or 500 μM (BHP5) for 20 hrs. Values are the mean ± S.E.M. of three experiments. *, p<0.0001 compared to control; ***, p<0.0001 compared to BHP2; **, p<0.0001 compared to BHP5; #, p<0.0001 compared to BHP2+PL; ##, p<0.0001 compared to BHP5+PL.
In addition to ROS and electrophiles, Nrf2 activation can be achieved through induction of protein kinases such as mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3 kinase (PI3K) (Lee et al., 2001; Nakaso et al., 2003; Na et al., 2008). Treatment of neuroblastoma cells with plumbagin resulted in time-dependent increases in phosphorylation (activation) of AKT and ERK1,2 (p42/p44 MAP) kinases (Fig. 4C), without affecting JNK or p38 (data not shown). Moreover, the plumbagin-induced up-regulation of HO-1 and NQO1 protein levels was significantly attenuated by a 1 hour pretreatment with the PI3K inhibitor wortmannin, or the MEK1 inhibitor PD98059 (Fig. 4D). These results indicate that plumbagin-induced Nrf2 activation is mediated by the PI3K and MAPK signaling pathways. We next pretreated cells for 1 hour with either wortmannin (PI3K inhibitor) or PD98059 (MEK1 inhibitor) and then exposed the cells to 1 μM plumbagin for 6 hours before challenging them with BHP. Preventing the activation of the PI3K/AKT pathway completely abolished the ability of plumbagin to prevent BHP-induced cell death (Fig. 4E–F). On the other hand, blockage of the MEK1/MAPK pathway marginally affected plumbagin cytoprotection (Fig. 4E). These findings suggest that plumbagin activates both PI3K/AKT and MAPK pathways, and that PI3K/AKT mediates the activation of Nrf2/ARE-dependent gene expression necessary for the cytoprotective action of plumbagin.
Plumbagin protects neurons against ischemic injury
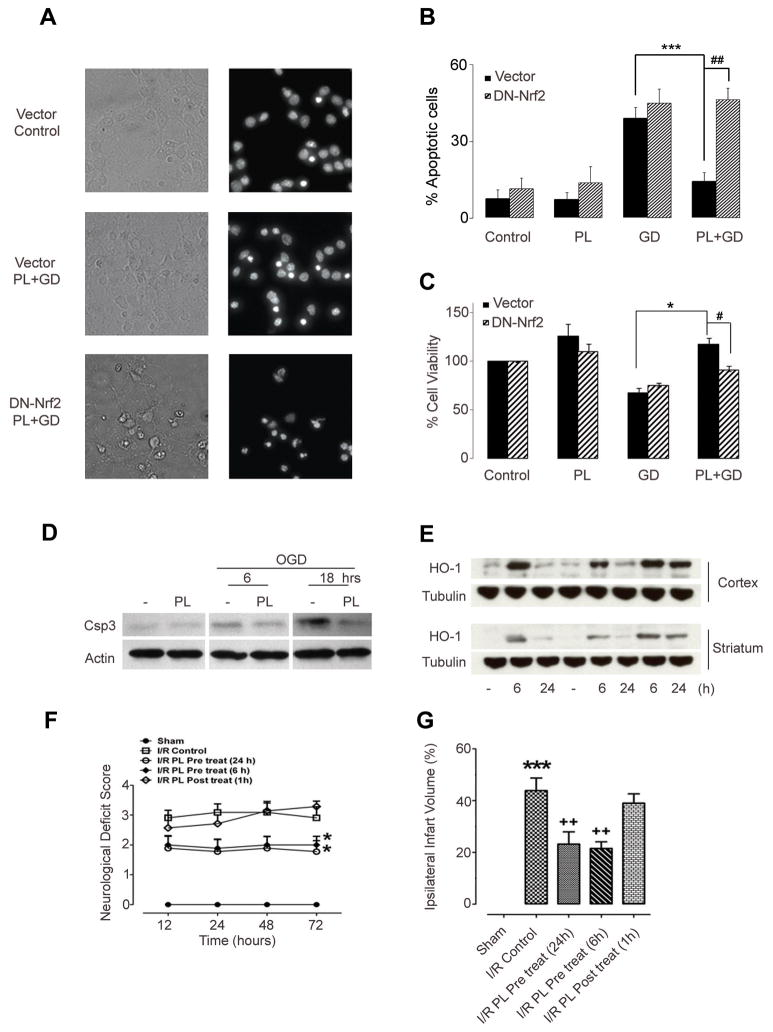
To determine whether the cytoprotective/hormetic effect of exposure to subtoxic levels of plumbagin also applies to neurons, we used a primary rat cortical neuron culture model. Cultures were exposed to increasing concentrations of plumbagin and neuron viability was quantified after 24 and 48 hours. In the concentration range 0.1–0.8 μM, plumbagin did not cause cell death (Supplemental Fig 1A), and induced ARE/Nrf2-dependent transcription in both neurons and astrocytes from ARE reporter mice (Supplemental Fig. 1B). We next pretreated cortical neurons with 0.5 μM of plumbagin and then subjected them to glucose deprivation (GD) or combined oxygen and glucose deprivation (OGD). Plumbagin protected neurons from being killed by GD (Fig. 5A–C and Supplemental Fig 1C), and attenuated the cleavage/activation of caspase-3 induced by OGD (Fig. 5D). To confirm that the protection afforded by plumbagin requires Nrf2 activation, we inhibited endogenous Nrf2 activity by transfecting primary neurons with a dominant negative Nrf2 (DN-Nrf2). The dominant-negative Nrf2 lacks the N-terminal trans-activating domain, but retain the C-terminal DNA binding and heterodimerization domains (Alam et al., 1999). Inhibition of Nrf2 activity by the DN is achieved through competition for binding partners and/or DNA binding sites (Alam et al.,1999; Kraft et al., 2004). Prevention of Nrf2/ARE activation in primary neurons significantly attenuated plumbagin protection against GD (Fig. 5A–C).
Fig. 5.
Plumbagin protects cultured primary neurons against OGD, reduces brain damage and improves functional outcome in a mouse stroke model.
Neuronal cultures transfected with pEF (Vector) or dominant negative Nrf2(DN-Nrf2) were treated with either vehicle (Control) or 0.5 μM plumbagin (PL) then subjected to glucose deprivation (GD) for 24 hrs. Cell survival was assessed by random blinded cell counting and MTT assays. Representative images (A) and quantification of apoptotic neurons (B). Data are mean and S.D. of three independent experiments (~300–600 neurons per experiment). ***, p<0.0001 compared to GD; ##, p<0.0001 compared to vector transfected cells. (C) MTT data are mean and S.E.M. (n=3). *, p=0.003 compared to GD; #, p=0.02 compared to vector transfected cells. (D) Immunoblot showing levels of the pro-apoptotic marker cleaved caspase 3 (csp3) in neurons pretreated with vehicle or plumbagin (PL) and subjected to oxygen and glucose deprivation (OGD) for the indicated time points. (E) Levels of the Nrf-2-target gene HO-1 in cortex and striatum from mice injected with either vehicle or 3 mg/kg of plumbagin for the indicated time points. (F) Neurological scores of mice sham operated or subjected to 1 hr ischemia followed by reperfusion (I/R) for the indicated time points. Mice were intravenously injected with either vehicle (control) (n=11) or 3 mg/kg of plumbagin (PL) 6 hrs (n=9) and 24 hrs (n=9) before surgery, or 1 hr post ischemia (n=7). *p<0.001 compared to control mice. (G) Ischemic infarct size 72 hrs post ischemia/reperfusion in vehicle-treated (n=9), 6 hrs PL-pretreated (n=10), 24 hrs PL-pretreated (n=8), or 1 hr PL post treated (n=9) mice. ***p,0.001 compared to sham operated mice. ++ p<0.001 compared to vehicle- treated mice.
Because plumbagin protected cultured neurons against insults relevant to ischemic stroke, we next tested the in vivo neuroprotective potential of plumbagin in a mouse focal ischemia model stroke. In preliminary experiments we tested several routes and doses for systemic administration of plumbagin for their ability to up-regulate HO-1 in the cerebral cortex and striatum. We found that intravenous administration of 3 mg/kg of plumbagin had no adverse effects on the animals, resulted in detectable levels of PL in the brain tissue (Supplemental Fig 2A), and increased the expression of the Nrf2 target gene HO-1 in the cortex and striatum (Fig. 5E). Plumbagin-induced HO-1 expression peaked at 6 hours, and then declined by approximately 50% by 24 hours (Fig. 5E). When mice were pretreated with 3 mg/kg of plumbagin (i.v.) either 24 or 6 hours prior to middle cerebral artery occlusion/reperfusion, their neurological deficits were significantly less than vehicle-treated mice at 12, 24, 48 and 72 hours after the experimental stroke (Fig. 5F). The amount of brain damage (infarct volume) assessed 72 hours post-stroke was significantly less in mice pretreated for 24 or 6 hours compared to vehicle-treated mice (Fig. 5F). In contrast, when plumbagin was administered 1 hour after the onset of reperfusion, neurological deficits and cerebral infarct volumes were not significantly different than vehicle-treated mice (Fig. 5F, G). No differences in mean arterial pressure (Supplemental Fig. 2B), and heart rate (Supplemental Fig. 2C) were observed between vehicle and plumbagin treated animals at any time point before or after middle cerebral artery occlusion. The cerebral blood flow was similar between the groups before, during or after middle cerebral artery occlusion, although plumbagin significantly improved brain blood flow 3 hrs post ischemia (Supplemental Table 2). These data strongly suggest that the neuroprotection afforded by plumbagin is unlikely the result of an effect on cerebral vasculature.
Discussion
Several observations led us to test the hypothesis that some BPs can, at subtoxic concentrations, activate an adaptive stress response pathway and thereby protect neurons against oxidative and metabolic insults. First, BPs were developed commercially based upon their ability to induce stress in insects (Koul, 2005; Isman, 2006). Second, evolutionary considerations and the fact that BPs are concentrated in exposed regions of plants suggests that neurons may be particularly sensitive to BPs. Indeed, some BPs have been shown to elicit excitatory responses in neurons (Shafeek et al., 2004). Third, although few BPs have been evaluated for their effects on neurons, several BPs have been shown to induce adaptive stress responses in other cell types and whole organisms. For example, farnesol activates NF-κB in lung carcinoma cells (Joo and Jetten, 2008), juglone induces stress resistance in C. elegans (de Castro et al., 2004), and piperine exerts cytoprotective effects on splenocytes (Pathak and Khandelwal, 2007). Fourth, numerous studies have documented the potential for activation of adaptive stress response pathways by lifestyle factors (exercise, dietary energy restriction, cognitive stimulation) and some drugs and phytochemicals (antidepressants and curcumin) to protect neurons against dysfunction and death in animal models of neurodegenerative disorders (Lim et al., 2001; Nelson et al., 2007; Mattson, 2008).
Among possible stress response pathways, we focused on Nrf2/ARE, knowing that its activation can protect neurons against oxidative, metabolic and excitotoxic insults relevant to stroke, and Alzheimer’s, Parkinson’s and Huntington’s diseases (de Paiva et al., 2003; Lee et al., 2003; Satoh et al., 2006,2008; Wruck et al., 2008). Among 30 BPs evaluated, only plumbagin activated the Nrf2/ARE pathway at subtoxic concentrations. Menadione, a structurally similar quinone was ineffective, as were vitamins K1 and K2. Several Nrf2 target genes that encode antioxidant enzymes were upregulated by plumbagin including HO-1, NQO1, thioredoxin reductase and GCLM. Previous studies have shown that upregulation of HO-1 (Chen et al., 2000; Satoh et al., 2003), NQO1 (Lim et al., 2008), thioredoxin reductase (Tanito et al., 2007) and GCLM (Giordano et al., 2007) in neurons can protect them against oxidative and excitotoxic insults. We found that cells treated with plumbagin exhibit increased activation of AKT kinase and p42/p44 MAP kinases. A PI3 kinase inhibitor blocked the ability of plumbagin to upregulate HO-1 and protect neurons against oxidative stress, whereas an inhibitor of the p42/p44 MAP kinase pathway was relatively ineffective. Collectively, our findings suggest that plumbagin activates the Nrf2/ARE pathway by a PI3 kinase-mediated mechanism, thereby inducing the expression of cytoprotective ARE target genes. Such a mechanism is consistent with previous studies showing that PI3 kinase – AKT (Gines et al., 2003; Nakazawa et al., 2003) and Nrf2/ARE (Kraft et al., 2006)can protect cultured neurons against metabolic, excitotoxic and oxidative stress, and that Nrf2 activation can reduce ischemic brain injury in vivo (Shih et al., 2005; Zhao et al., 2006; Leonard, 2006; Danilov et al., 2009). We found that plumbagin increases ARE target gene (HO-1) levels in the cerebral cortex and striatum when administered intravenously and, when administered prior to focal ischemia/reperfusion, reduces brain cell damage and improves functional outcome in a mouse stroke model. Consistent with a hormetic/preconditioning mechanism of action, post-stroke treatment with plumbagin did not reduce brain damage.
Biphasic dose responses with low dose stimulatory/beneficial effects and high dose toxic effects are common in biological systems (Calabrese et al., 2007). A few natural toxins are currently used in the clinic, with botulinum toxin being a prominent example (Kostrzewa and Segura-Aguillar, 2007). In the present study we demonstrated that neuroprotective agents can be identified by screening known botanical “toxins” for their ability to activate an adaptive cellular stress response at subtoxic doses. We also provide the first evidence that the naftoquinone plumbagin is a activator of the Nrf2/ARE pathway, and affords neuroprotection against ischemic stroke.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Aging Intramural Research Program of the National Institutes of Health (NIH), and by grants from the National Institute of Environmental Sciences (NIEHS ES 10042 and ES08089) to J.A. Johnson. The authors declare no conflict of interest.
Abbreviations
- ARE
antioxidant response element
- BHP
tert-butyl-hydroperoxide
- BP
botanical pesticide
- GCLM
glutamate-cysteine ligase modifier subunit
- GST
glutathione-S-transferase 1
- HO-1
heme oxygenase-1
- 4HNE
4-hydroxy-2,3-nonenal
- hPAP
human placental alkaline phosphatase
- NQO1
NAD(P)H quinone oxidoreductase 1
- Nrf2
nuclear factor E2-related factor 2
- ROS
reactive oxygen species
- TX
thioredoxin reductase 1
Footnotes
Authors contributions: TGS, SC, designed and performed research, analyzed data, and wrote paper; TVA, RGC, RST, MRM, TAM performed research and analyzed data; Q-SY, DAJ, JAJ and NG contributed reagents; MPM designed research and wrote paper.
References
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a cap ‘n’ Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Tang SC, Lathia JD, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci USA. 2007;104:14101–14109. doi: 10.1073/pnas.0700506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Calixto JB, Kassuya CA, Andre E, Ferreira J. Contribution of natural products to the discovery of the transient receptor potential (TRP) channels family and their functions. Pharmacol Ther. 2005;106:179–208. doi: 10.1016/j.pharmthera.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Camandola S, Cutler RG, Gary DS, Milhavet O, Mattson MP. Suppression of calcium release from IP3-sensitive stores mediates the anti-apoptostic function of NF-κB. J Biol Chem. 2005;280:22287–22296. doi: 10.1074/jbc.M410923200. [DOI] [PubMed] [Google Scholar]
- Chen K, Gunter K, Maines MD. Neurons overexpressing heme oxygenase-1 resist oxidative stress-mediated cell death. J Neurochem. 2000;75:304–313. doi: 10.1046/j.1471-4159.2000.0750304.x. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- Danilov CA, Chandrasekaran K, Racz J, Soane L, Zielke C, Fiskum G. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia. 2009;57:645–656. doi: 10.1002/glia.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro E, Hegi de Castro S, Johnson TE. Isolation of long-lived mutants in Caenorhabditis elegans using selection for resistance to juglone. Free Radic Biol Med. 2004;37:139–145. doi: 10.1016/j.freeradbiomed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- de Paiva SR, Figueiredo MR, Aragao TV, Kaplan MA. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Mem Inst Oswaldo Cruz. 2003;98:959–961. doi: 10.1590/s0074-02762003000700017. [DOI] [PubMed] [Google Scholar]
- De Smet PA. The role of plant-derived drugs and herbal medicines in healthcare. Drugs. 1997;54:801–840. doi: 10.2165/00003495-199754060-00003. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52:S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- Gines S, Ivanova E, Seong IS, Saura CA, MacDonald ME. Enhanced Akt signaling is an early pro-survival response that reflects N-methyl-D-aspartate receptor activation in Huntington’s disease knock-in striatal cells. J Biol Chem. 2003;278:50514–50522. doi: 10.1074/jbc.M309348200. [DOI] [PubMed] [Google Scholar]
- Giordano G, White CC, Mohar I, Kavanagh TJ, Costa LG. Glutathione levels modulate domoic acid induced apoptosis in mouse cerebellar granule cells. Toxicol Sci. 2007;100:433–444. doi: 10.1093/toxsci/kfm236. [DOI] [PubMed] [Google Scholar]
- Hanneken A, Lin FF, Johnson J, Maher P. Flavonoids protect human retinal pigment epithelial cells from oxidative-stress-induced death. Invest Ophthalmol Vis Sci. 2006;47:3164–3177. doi: 10.1167/iovs.04-1369. [DOI] [PubMed] [Google Scholar]
- Inbaraj JJ, Chignell CF. Cytotoxic action of juglone and plumbagin: a mechanistic study using HaCaT keratinocytes. Chem Res Toxicol. 2004;17:55–62. doi: 10.1021/tx034132s. [DOI] [PubMed] [Google Scholar]
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Andrews GK, Xu W, Johnson JA. Activation of the antioxidant response element in primary cortical neuronal cultures derived from transgenic reporter mice. J Neurochem. 2002;81:1233–1241. doi: 10.1046/j.1471-4159.2002.00913.x. [DOI] [PubMed] [Google Scholar]
- Joo JH, Jetten AM. NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway. J Biol Chem. 2008;283:16391–16399. doi: 10.1074/jbc.M800945200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrzewa RM, Segura-Aguilar J. Botulinum neurotoxin: evolution from poison, to research tool onto medicinal therapeutic and future pharmaceutical panacea. Neurotox Res. 2007;12:275–290. doi: 10.1007/BF03033911. [DOI] [PubMed] [Google Scholar]
- Koul O. Insect Antifeedants. CRC Press LLC; Boca Raton: 2005. pp. 1–1005. [Google Scholar]
- Koul O, Dhaliwal GS. Phytochemical biopesticides. Harwood Academic Publishers; Amsterdam, Netherlands: 2001. p. 223. [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AD, Lee JM, Johnson DA, Kan YW, Johnson JA. Neuronal sensitivity to kainic acid is dependent on the Nrf2-mediated actions of the antioxidant response element. J Neurochem. 2006;98:1852–1865. doi: 10.1111/j.1471-4159.2006.04019.x. [DOI] [PubMed] [Google Scholar]
- Lee JM, Hanson JM, Chu WA, Johnson JA. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem. 2001;276:20011–20016. doi: 10.1074/jbc.M100734200. [DOI] [PubMed] [Google Scholar]
- Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- Leonard MO. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20:2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Kim KM, Kim SW, Hwang O, Choi HJ. Bromocriptine activates NQO1 via Nrf2-PI3K/Akt signaling: novel cytoprotective mechanism against oxidative damage. Pharmacol Res. 2008;57:325–331. doi: 10.1016/j.phrs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Awareness of hormesis will enhance future research in basic and applied neuroscience. Crit Rev Toxicol. 2008;38:633–639. doi: 10.1080/10408440802026406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Na HK, Kim EH, Jung JH, Lee HH, Hyun JW, Surh YJ. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch Biochem Biophys. 2008;476:171–177. doi: 10.1016/j.abb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Shimura M, Tomita H, Akiyama H, Yoshioka Y, Kudou H, Tamai M. Intrinsic activation of PI3K/Akt signaling pathway and its neuroprotective effect against retinal injury. Curr Eye Res. 2003;26:55–63. doi: 10.1076/ceyr.26.1.55.14254. [DOI] [PubMed] [Google Scholar]
- Nelson RL, Guo Z, Halagappa VM, et al. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3xTgAD mice. Exp Neurol. 2007;205:166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak N, Khandelwal S. Cytoprotective and immunomodulating properties of piperine on murine splenocytes: an in vitro study. Eur J Pharmacol. 2007;576:160–170. doi: 10.1016/j.ejphar.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Satoh T, Baba M, Nakatsuka D, et al. Role of heme oxygenase-1 protein in the neuroprotective effects of cyclopentenone prostaglandin derivatives under oxidative stress. Eur J Neurosci. 2003;17:2249–2255. doi: 10.1046/j.1460-9568.2003.02688.x. [DOI] [PubMed] [Google Scholar]
- Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci USA. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Kosaka K, Itoh K, et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J Neurochem. 2008;104:1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafeek A, Jaya Prasanthi RP, Reddy GH, Chetty CS, Reddy GR. Exposure to azadirachtin produced an excitatory effect on spontaneous electrical activity as well as cercal sensory-mediated giant-fiber responses in the cockroach. Ecotoxicol Environ Saf. 2004;59:205–208. doi: 10.1016/j.ecoenv.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugie S, Okamoto K, Rahman KM, Tanaka T, Kawai K, Yamahara J, Mori H. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998;127:177–183. doi: 10.1016/s0304-3835(98)00035-4. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Cutler RG, et al. Neuroprotective actions of a histidine analogus in models of ischemic stroke. J Neurochem. 2007;101:729–736. doi: 10.1111/j.1471-4159.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky MA. Caffeine and endurance performance. Sports Med. 1994;18:109–125. doi: 10.2165/00007256-199418020-00004. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Wright DA, Dawson R, Cutler SJ, Cutler HG, Orano-Dawson CE. Screening of natural product biocides for control of non-indigenous species. Environ Technol. 2007;28:309–319. doi: 10.1080/09593332808618790. [DOI] [PubMed] [Google Scholar]
- Wruck CJ, Gotz ME, Herdegen T, Varoga D, Brandenburg LO, Pufe T. Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Mol Pharmacol. 2008;73:1785–1795. doi: 10.1124/mol.107.042499. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.