Abstract
Mitochondrial function is integral to maintaining cellular homeostasis through the production of ATP, the generation of reactive oxygen species (ROS) for signaling, and the regulation of the apoptotic cascade. A number of small molecules, including nitric oxide (NO), are well characterized regulators of mitochondrial function. Nitrite, an NO metabolite, has recently been described as an endocrine reserve of NO that is reduced to bioavailable NO during hypoxia to mediate physiological responses. Accumulating data suggests that mitochondria may play a role in metabolizing nitrite and that nitrite is a regulator of mitochondrial function. Here what is known about the interactions of nitrite with the mitochondria is reviewed, with a focus on the role of the mitochondrion as a metabolizer and target of nitrite.
Keywords: Nitrite, mitochondria, nitric oxide, cytochrome c oxidase, nitrite reductase
INTRODUCTION
Nitrite (NO2−), once regarded as a physiologically inert metabolite of nitric oxide (NO), is now considered to be an endocrine reserve of NO in the blood and tissues that can be utilized during hypoxia[1; 2]. In conditions of low oxygen and pH, a number of proteins reduce nitrite to generate bioavailable NO to mediate biological responses such as hypoxic vasodilation[3; 4], gene and protein expression[5; 6], angiogenesis[7], and cytoprotection after ischemia/reperfusion (I/R) [8; 9; 10; 11; 12; 13; 14]. While nitrite has been shown to mediate numerous physiological responses, the molecular mechanisms for these responses and subcellular targets for nitrite are still being elucidated. In the last five years, a great deal of interest has emerged in the interactions of nitrite with mitochondria. A recent study showed that the extent of nitrite reductase activity (the ability to convert nitrite to NO) of mammalian tissues correlated directly with the tissue’s capacity for mitochondrial oxygen consumption[15]. These data, along with the central homeostatic role of the organelle in cellular ATP generation, redox signaling and regulation of cell death[16; 17], suggests that mitochondria are likely important either in the reduction of nitrite to NO or as critical targets of the products of nitrite reduction. This review will focus on the known interactions between nitrite and mitochondria. The potential role of the mitochondrion in regulating nitrite concentration and metabolism, as well as the role of nitrite in regulating mitochondrial function will be explored. The physiological implications and emerging therapeutic potential of these interactions will also be discussed.
MITOCHONDRIA AS REGULATORS OF NITRITE CONCENTRATION
Mitochondrial function - beyond the “powerhouse”
While mitochondria have traditionally been called the “powerhouse” of the cell, it is now known that the function of this organelle extends well beyond ATP generation. For ATP synthesis, electrons, generated from the breakdown of substrates, enter the respiratory chain at complex I or complex II and are transferred through complexes III and IV, down an electrochemical gradient. At complex IV, cytochrome c oxidase, oxygen binds and acts as the terminal electron acceptor to be reduced to water. This transfer of electrons from complex I to IV provides the energy needed to pump protons from the mitochondrial matrix to the innermembrane space, which establishes a proton gradient that is then used by complex V to generate ATP. While the majority of electrons make it through the chain, a small proportion of electrons escape the chain at complex I or III to generate superoxide (Figure 1). This mitochondrial generation of ROS is regulated and plays an important role in many cell signaling pathways[18; 19]. In addition, mitochondrial release of the small electron transporter, cytochrome c, leads to the initiation of the apoptotic cascade [17; 20]. Through the production of ATP, redox signaling, and regulation of cell death, mitochondria play an integral homeostatic role in the cell[16; 17]. This section discusses a proposed novel role for the mitochondrion, as a potential nitrite synthase and nitrite reductase.
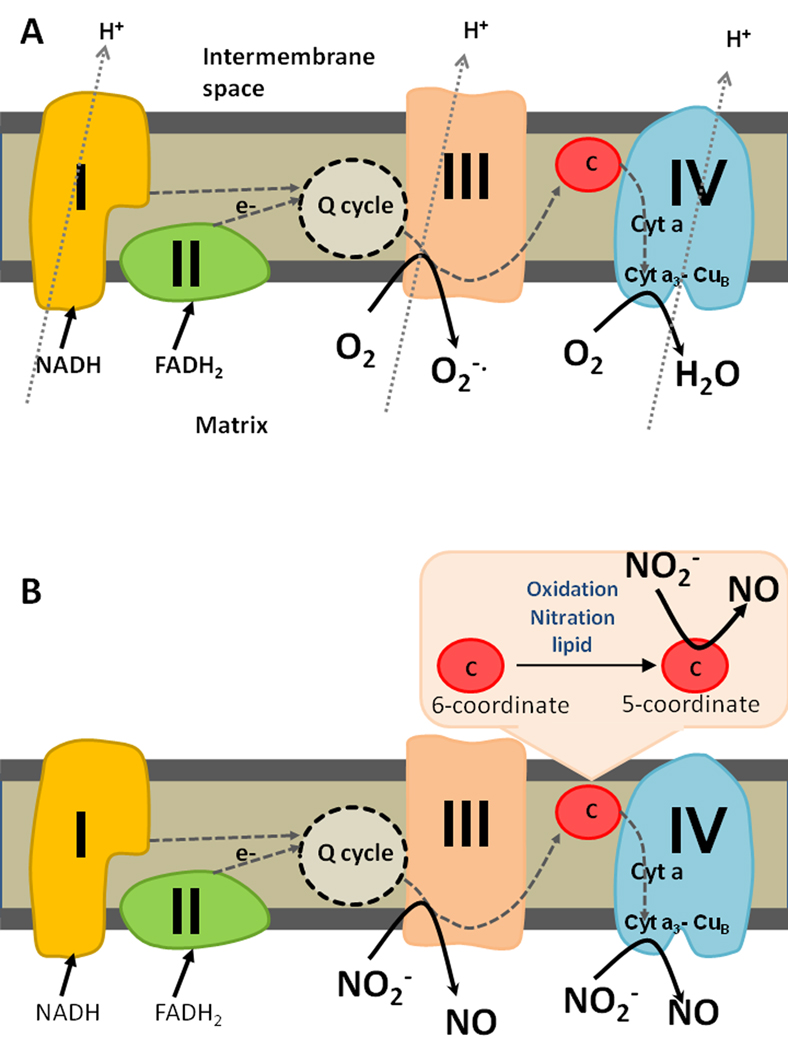
Figure 1. Sites of mitochondrial nitrite reduction.
(A) In normoxia, electrons enter the respiratory chain at complex I or II and are shuttled through the Q cycle to complex III. While most electrons are then shuttled to cytochrome c and then to complex IV, where oxygen binds the binuclear center (cyta3 CuB) and acts as the terminal electron acceptor, some electrons escape at complex III to generate superoxide. Protons are pumped from the matrix to the intermembrane space through the complexes to set up a proton gradient for ATP generation. (B) During hypoxia, nitrite can be reduced at complex III or cytochrome c oxidase (complex IV). If cytochrome c is converted to its pentacoordinate form (through oxidation, nitration or association with anionic lipid), it can act as a site of nitrite reduction.
Nitrite formation in vivo
Nitrite concentrations in vivo are derived from two sources: dietary consumption of nitrite and nitrate[2] and the oxidation of endogenously generated NO[21]. The contribution of dietary nitrate to basal nitrite concentration involves the reduction of nitrate by an entero-salivary pathway (reviewed in [22]). Briefly, once nitrate is consumed, it is absorbed in the upper gastrointestinal tract and enters the circulation. While a large fraction of the nitrate is excreted, a small proportion is actively taken up by the salivary gland and concentrated approximately 20-fold in the saliva. Once in the saliva, commensal bacteria in the oral cavity reduce nitrate to nitrite[22]. Consistent with a central role for commensal bacteria in this pathway, Lundberg and colleagues showed in rats that the increase in plasma nitrite concentration after the ingestion of nitrate was attenuated when antiseptic mouthwash was used to eliminate commensal bacteria.[23].
The second source of nitrite in vivo is the oxidation of NO generated by the NO synthase (NOS) enzymes. Nitrite is generated through the relatively slow reaction of NO with oxygen (k = 2 × 106 M−2s−1) [24]. While this reaction can be accelerated by lipid membranes including the inner mitochondrial membrane [25; 26], in most biological compartments, this reaction is kinetically unfavorable in comparison with the reaction of NO with other targets, particularly heme containing proteins. For example, in the blood, NO reacts rapidly with oxygenated hemoglobin (k = 3.4 × 107 M−1s−1), generating nitrate[27]. Hence, it is unexpected that any nitrite would be formed from the oxidation of NO. However, it has been shown that the acute phase protein, ceruloplasmin, a multi-copper oxidase present in the plasma in micromolar levels, catalyzes the oxidation of NO to nitrite[28]. Consistent with this role, ceruloplasmin knockout mice and humans deficient in ceruloplasmin have significantly lower basal plasma nitrite concentrations than wildtype mice and healthy controls respectively[28].
Similar to the scavenging of NO by hemoglobin in blood, myoglobin in cardiac and skeletal muscle or other analogous heme proteins can oxidize NO to nitrate in tissues[29]. However, like in blood, significant levels of nitrite exist in the tissue basally (1–20 µM)[30]. Although ceruloplasmin is expressed at low levels in heart and liver, it is unknown whether this protein plays a role in nitrite synthesis in tissues. It is possible that other oxidases, such as cytochrome c oxidase, play a role in NO oxidation in tissues.
Cytochrome c oxidase, complex IV of the mitochondrial respiratory chain, is the terminal component of the mitochondrial electron transport chain where oxygen binds and is reduced to water. The enzyme contains two heme groups and two copper centers, with one heme group (hemea3) and the second copper (CuB) closely associated to form the binuclear center, the site of oxygen binding. The enzyme, which oxidizes its substrate, cytochrome c, to catalyze the four electron reduction of dioxygen, bears structural and functional resemblance to ceruloplasmin[31]. The binding of NO to the binuclear site of the enzyme is well characterized and two different products of this reaction are formed depending on the oxidation state of the heme-copper center[32; 33]. In the reduced state of the enzyme, NO is thought to nitrosylate the hemea3 and then be released. However, in the fully oxidized enzyme, NO has been shown to be oxidized to nitrite in the active site. While the off rate of the nitrite formed by the purified enzyme has been described to be relatively slow (0.024 min−1), this rate was increased in the presence of reductants[33]. These studies raise the question as to whether mitochondria are involved in the synthesis of nitrite in the cell. Although it appears that purified cytochrome c oxidase is mechanistically capable of oxidizing NO to nitrite, further study is necessary to determine whether this enzyme plays a role in NO oxidation in whole cells and in vivo. As discussed in the next sections, much more is known about the role of the mitochondrion in metabolizing nitrite to NO than in generating nitrite.
Nitrite Reduction
The mitochondrion as a nitrite reductase
The majority of nitrite-dependent cell signaling appears to occur through its reduction to NO during hypoxia. A number of proteins have been described to catalyze this reaction, and much focus has been placed on the mitochondrion as a nitrite reductase. The first report of mitochondrially catalyzed nitrite reduction was published in 1965 by Taylor and colleagues, who observed that porcine mitochondria generate nitrosylated cytochrome c when incubated with nitrite in anoxic conditions[34]. While this observation was thought to be important to the understanding of the mechanisms by which nitrite preserves meat during the meat curing process, the authors did not speculate on a physiological role for this reaction. Reutov and colleagues later proposed that, in a primordial anoxic environment, nitrite was the terminal electron acceptor for mitochondrial oxidative phosphorylation, and that this reduction of nitrite generated NO[35]. While it is still unclear whether mitochondria can utilize nitrite to facilitate ATP generation, recent studies have shown that components of the respiratory chain are capable of reducing nitrite to NO[36; 37; 38]. Initial studies by Nohl and colleagues demonstrated that the isolated mitochondria and submitochondrial particles both possessed nitrite reductase activity that is localized to the respiratory chain and dependent on the presence of respiratory substrates[38]. Furthermore, inhibitor studies demonstrated that NO generation was attenuated by myxathiozol, an inhibitor of the bc1 complex of complex III. In contrast, the Complex I inhibitor, rotenone, had no effect, suggesting that the nitrite reductase activity was localized to complex III[38].
Poyton and colleagues showed that the terminal complex of the electron transport chain, cytochrome c oxidase, is a mediator of mitochondrial nitrite reduction[37]. This complex accepts electrons from cytochrome c and uses these electrons to reduce oxygen, which binds to its hemea3/CuB binuclear center. It has now been demonstrated in yeast cells, isolated liver mitochondria and purified cytochrome c oxidase protein that cytochrome c oxidase can reduce nitrite to NO in the presence of cytochrome c. Characterization of this activity showed that nitrite reduction is inhibited by cyanide and that the rate of NO generation, while minimal at pH 7, increases as pH is decreased[37]. While the exact chemical mechanism of this reduction is unknown, it has previously been shown that when purified bovine cytochrome c oxidase is incubated with excess nitrite and reducing agents, a hemea3-nitrosyl complex is formed at the binuclear site of the enzyme and NO can be released from this complex at a slow rate (0.01 s−1)[39; 40].
NO generation by cytochrome c oxidase is regulated by oxygen on multiple levels. On a biochemical level, cytochrome c oxidase-dependent nitrite reduction is inhibited by oxygen, such that NO generation is observed only in hypoxia, below a dissolved oxygen concentration of 2% [37; 41]. In addition, oxygen also appears to regulate nitrite reduction through the modulation of gene expression. Of the 13 protein subunits that comprise the complete cytochrome c oxidase enzyme, different isoforms of several of the subunits exist and are expressed under varying conditions. In particular, homologous isoforms of mammalian subunit IV (yeast subunit V), a subunit required for enzymatic activity but not part of the catalytic site, are differentially expressed depending on the presence of oxygen. In normoxic conditions the majority of cytochrome c oxidase contains mammalian subunit IV-1 (yeast subunit Va), while hypoxic conditions induce the expression of isozyme IV-2 (yeast subunit Vb), which enhances the rate of enzyme turnover by 3–4 fold. This differential expression allows the assembly of varying forms of the enzyme with different catalytic rates depending on oxygen availability[42; 43; 44]. Interestingly, yeast cells grown in hypoxia and expressing the hypoxic isozyme (subunit Vb) were shown to be more efficient nitrite reductases, with the rate of reduction being 5 times higher than cells expressing subunit Va and NO generation occurring at a higher oxygen tension (80%)[41]. This data demonstrates that nitrite reduction and hypoxia are intimately linked on many levels. Further, it supports the hypothesis that cytochrome c oxidase evolved before the presence of atmospheric oxygen and utilized nitrite as a terminal electron acceptor.
Since cytochrome c oxidase must be in turnover to reduce nitrite to NO, its substrate, cytochrome c is required for complex IV dependent nitrite reduction[37]. However, recently Basu and colleagues demonstrated that under certain conditions, cytochrome c, even in the absence of cytochrome c oxidase, is able to reduce nitrite to NO[36]. This 12 kilodalton protein loosely associated with the mitochondrial inner membrane contains one heme center in which the iron normally exists in a hexacoordinate state, with the heme iron bound by histidine-18 and methionine-80. However, under specific conditions, such as when the protein is oxidized, nitrated, or associated with anionic lipid (such as that present in the inner mitochondrial membrane), weakening and breakage of the iron-methionine bond can occur, shifting cytochrome c to a pentacoordinate state[45; 46; 47]. In this pentacoordinate state, cytochrome c is able to reduce nitrite to NO by a reaction that occurs in anoxic conditions and at acidic pH. In vitro experiments, using purified protein incubated in anoxia with anionic lipid and a range of nitrite concentrations (5 µM- 5mM) at pH 5.4, demonstrated that significant (micromolar) concentrations of NO are generated in one minute. This phenomenon is inhibited in the absence of the anionic lipid or in the presence of oxygen[36].
Respirometry experiments in which submitochondrial particles reconstituted with cytochrome c were incubated with increasing anionic lipid concentrations during hypoxia showed that as lipid concentration was increased in the presence of nitrite, respiratory rate of the submitochondrial particles decreased. This was presumably due to the increased generation of NO, a potent respiratory inhibitor, by the lipid induced increase in the concentration of pentacoordinate cytochrome c[36]. While these data suggest that cytochrome c dependent nitrite reduction may be able to modulate mitochondrial respiration, the physiological role for this reaction is not yet known. The physiological relevance of this reaction has been questioned due to the inability of pentacoordinate cytochrome c to function as an electron carrier. However, it is important to note that significant nitrite reductase activity may exist even if only a small fraction of the total cytochrome c pool is in its pentacoordinate state. Moreover, in addition to acting as an electron shuttle to cytochrome c oxidase, cytochrome c is a signaling molecule whose release from the mitochondria initiates the apoptotic cascade[48]. Data suggesting that the pentacoordination of cytochrome c may influence its release from the mitochondrion have raised the speculation that cytochrome c-dependent nitrite reduction may be important in generating NO, a regulator of the mitochondrial apoptotic pathway, before the initiation of the apoptotic cascade.
While a number of enzymes within the mitochondria are now known to reduce nitrite to NO and mechanisms of regulation of these individual enzymes are being elucidated, little is known about whether all these proteins work simultaneously or whether they reduce nitrite in different situations. Nohl and colleagues have proposed that nitrite concentration dictates the site of mitochondrial reduction, with low concentrations (micromolar) being reduced by complex III and higher concentrations being reduced at cytochrome c oxidase [49; 50]. However, this is countered by data demonstrating that cytochrome c oxidase can reduce 20µM nitrite at pH 6 [37]. It is likely that complex III and IV function as nitrite reductases in different situations; however further in vivo and in vitro work is needed to define the conditions that determine which site is active.
Comparison of mitochondria to other nitrite reductases
A number of proteins, other than cytochrome c oxidase, have been described to catalyze the reduction of nitrite to NO in hypoxic conditions. Perhaps the most widely characterized mammalian nitrite reductase is the family of heme globins, the oxygen binding proteins which include hemoglobin in red blood cells[4; 51; 52], neuroglobin in the brain and retina[53], and myoglobin in cardiac and skeletal muscle[52; 54]. Although these proteins differ in their rates of nitrite reduction, the heme globins generate NO from nitrite by the same reaction (Reaction 1)[52]. The reaction of nitrite and a proton with deoxygenated ferrous heme (myoglobin in this case) generates NO and oxidizes the heme in the process.
| (Reaction 1) |
The necessity for deoxygenated heme and a proton renders this reaction susceptible to modulation by oxygen and pH. For each heme protein, nitrite reduction only occurs below the p50 (oxygen concentration at which the heme is half saturated with oxygen) of the protein[52]. Hence nitrite reduction catalyzed by hemoglobin (p50= 20 mm Hg) occurs at a higher oxygen tension than that catalyzed by myoglobin (p50= 2.2 mm Hg)[52]. In addition, as pH is decreased, the rate of the reaction increases. The efficiency of nitrite reduction by each heme globin varies according to structure, with myoglobin (bimolecular rate =12 M−1s−1)[54]greater than hemoglobin, but less than neuroglobin[51; 52]. Beyond the heme globins, a number of other nitrite reductases have been characterized in tissue [55], including xanthine oxidoreductase[56], cytochrome P450 enzymes[57], and nitric oxide synthase[58]. While all of these proteins function in conditions of low oxygen and become more efficient at low pH, the involvement of each of these proteins physiologically in the reduction of nitrite appears to be tissue specific[15]. For the purposes of this review, focus will be placed on myoglobin-dependent nitrite reduction due to the localization of myoglobin in mitochondria-rich tissue and the ability of myoglobin to regulate mitochondrial function.
Although several in vitro studies have now demonstrated that the mitochondrion is able to function as a nitrite reductase, the relative contribution of the organelle to NO generation in comparison to other tissue nitrite reductases remains unclear. In all published studies to date, mitochondrial proteins are incubated with millimolar concentrations of nitrite (Table 1), well above the physiological nitrite concentration in tissue (1–10µM)[30; 36; 37; 41]. In addition, with the exception of one study ([38]), acidic pH is necessary to observe measurable NO generation even from 1mM of nitrite. In contrast, myoglobin, which is also localized in tissue compartments has been shown to generate measurable concentrations of NO from nitrite concentrations as low as 2–10 µM[12; 54]. Interestingly, while Nohl and colleagues demonstrated mitochondrial dependent NO generation from sub-millimolar concentrations (50–100 µM) of nitrite, these studies utilized the generation of iron-nitrosyl hemoglobin as a measure of NO generation[38]. Hence, these studies are confounded by the presence of deoxyhemoglobin, which has also been shown to reduce micromolar concentrations of nitrite to NO[3; 4; 52; 59].
Table 1.
Rates and conditions of NO generation for mitochondrial nitrite reductases
One argument for the necessity of high nitrite concentrations in the mitochondrial nitrite reductase studies is that nitrite transport into the mitochondrion is inefficient [37]. Castello and colleagues showed that approximately 10% of nitrite added to isolated mitochondria is internalized [37]. Furthermore, it is unknown what proportion of the internalized nitrite reaches the sites of nitrite reduction within the mitochondria since these targets are buried within the hydrophobic inner membrane, which nitrite is not likely to cross by free diffusion. While the mechanism of nitrite uptake by mitochondria or whole cells is unclear, it is interesting to note that measurable myoglobin-dependent NO generation is observed when micromolar concentrations of nitrite are added to intact cardiomyocytes or intact isolated hearts[54; 60]. This suggests that mitochondria may contribute to nitrite reduction only when pathological levels of nitrite are reached. Alternatively, this may suggest a mechanism by which hypoxic NO generation is compartmentalized such that low levels of nitrite already present within the mitochondrion are reduced during hypoxia to regulate mitochondrial function locally.
In addition to the necessity of high concentration, mitochondria-dependent nitrite reduction requires low pH. Although heme globin- and xanthine oxidase- dependent NO generation are both accelerated by decreased pH, significant concentrations of nitrite are reduced at neutral pH[4; 52; 54; 60]. It is interesting to speculate that while other nitrite reductases, such as myoglobin and xanthine oxidoreductase, may play a major role in hypoxic NO generation physiologically, mitochondrial nitrite reduction may contribute more during pathological conditions. Interestingly, a pH gradient exists across the inner mitochondrial membrane such that physiologically the intermembrane space has an estimated pH of 7.2 and the matrix has a more alkaline pH of 7.9[61]. Based on in vitro studies [36; 37], minimal mitochondrial nitrite reduction would occur at this pH. However, in pathology, such as during tissue ischemia, cytosolic pH can drop below pH 6, which would decrease pH both in the intermembrane space and matrix. This in turn could present a more optimal condition for mitochondrial nitrite reduction. Although the biochemical aspects of mitochondrial nitrite reduction have been relatively well characterized in vitro, much more study is needed to determine the contribution of this pathway to hypoxic NO generation in vivo.
MITOCHONDRIA AS TARGETS OF NITRITE
While the physiological role of the mitochondrion as a nitrite reductase is still controversial, accumulating data now suggests that the mitochondrion may be a central physiological target for nitrite during hypoxia and ischemia[6; 13; 15; 54; 60; 62]. Although several potential protein targets exist within the mitochondrion, to date, the nitrite-dependent modification of two sites within the respiratory chain and the consequence of these modifications on mitochondrial function have been explored in depth. The first site of interaction, cytochrome c oxidase, has been described in previous sections as the site of nitrite reduction. In the following sections, the enzyme will be described as a target of NO generated by myoglobin-dependent nitrite reduction. The implications of the nitrosylation of the heme a3 at the binuclear center of the enzyme will be discussed in the context of the regulation of respiration and how it potentially plays a role in regulating tissue oxygen gradients during hypoxia. The second mitochondrial target of nitrite is complex I, which has been shown to be S-nitrosated during pathological ischemia/reperfusion, an effect that is hypothesized to be central to nitrite-mediated cytoprotection after ischemia/reperfusion[13]. The remainder of this review will focus on these two nitrite-dependent modifications of these specific targets and their effect on mitochondrial function. The nitrosylation of cytochrome c oxidase will be reviewed first and the implications of this modification on the regulation of oxygen gradients discussed. The S-nitrosation of complex I will then be discussed in the context of ischemia/reperfusion.
Nitrosylation of cytochrome c oxidase
For decades myoglobin has been considered to be an oxygen storage protein, whose major function is to release oxygen in order to support mitochondrial respiration during hypoxia[63]. However, the close association of myoglobin with mitochondria in cardiac tissue and skeletal muscle has generated questions as to whether additional mechanisms of crosstalk exist between myoglobin and the organelle[29; 64]. Considering the finding that myoglobin is an efficient nitrite reductase, multiple groups have recently hypothesized that the reduction of nitrite by myoglobin during hypoxia generates bioavailable NO that is able to regulate mitochondrial function[54; 60]. The most well-characterized interaction of NO with mitochondria is the binding of NO to the binuclear center of cytochrome c oxidase[65; 66; 67; 68]. As described above, dependent on the redox status of the enzyme, NO can either reversibly bind and be released from this site or be metabolized to nitrite. However, in either case, binding of NO to the binuclear center precludes the binding of oxygen to this site, and leads to the inhibition of cytochrome c oxidase activity and hence mitochondrial respiration[33]. This inhibition is completely reversible and regulated by oxygen, with the inhibition being more potent as oxygen concentration is decreased[26].
In initial experiments to determine whether myoglobin- dependent nitrite reduction could generate NO that subsequently inhibits mitochondrial respiration, isolated rat liver mitochondria and purified deoxygenated myoglobin were co-incubated with physiological concentrations of nitrite (5–20 µM) and mitochondrial oxygen consumption was monitored during hypoxia[54]. In these experiments, respiration was inhibited in the presence of both nitrite and deoxygenated myoglobin, but no effect was observed in the presence of nitrite or myoglobin alone. Moreover, this phenomenon was absent in experiments in which the myoglobin was oxidized or in which the NO scavenger PTIO (2-Phenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl) was present, confirming that the inhibition of respiration under these conditions was indeed due to an interaction of nitrite and myoglobin that generates NO (Reaction 1)[54]. These data have now been recapitulated in more physiological systems, including intact cardiomyocytes, which endogenously contain physiological levels of mitochondria and myoglobin[54]. Furthermore, physiological levels of nitrite (2–20 µM) concentration dependently inhibit hypoxic respiration in heart homogenates from wildtype mice, while this effect is blunted in homogenates made from myoglobin knockout mice[12]. In addition, perfusion of isolated intact hearts with nitrite (10 µM) during hypoxia, showed a nitrite dependent increase in NO generation and NO modified proteins (iron nitrosyl and S-nitrosothiols) in the wildtype, but not the myoglobin deficient hearts. This increase in NO generation was associated with a decrease in myocardial oxygen consumption in the wildtype hearts, which was also absent in hearts from wildtype animals[60]. In the myocardium, this mechanism of nitrite-dependent downregulation of oxygen consumption may be central to a phenomenon known as “short term hibernation” by which the myocardium decreases its contractile activity in response to decreased perfusion[60].
Cumulatively, these data demonstrate that nitrite, through its reduction by myoglobin, is a regulator of cytochrome c oxidase. In addition, these studies establish a mechanism of crosstalk between the mitochondria and myoglobin, beyond simple oxygen delivery, in which nitrite is essential. Physiologically, this novel mechanism of nitrite and myoglobin dependent modulation of mitochondrial function may play a role in regulating tissue oxygen gradients as discussed below.
Extension of tissue oxygen gradients and modulation of exercise capacity
Previous studies have proposed that the partial inhibition of respiration can regulate tissue oxygen gradients and conserve oxygen, particularly in conditions of physiological hypoxia[69; 70]. Partial inhibition of the most actively respiring mitochondria and those closest to the oxygen source would allow oxygen to diffuse beyond these mitochondria and further into the tissue to those sections of the tissue that are more distant from the oxygen source (Figure 2). Additionally, as described by Thomas et al., this extension of the oxygen gradient deeper into the tissue would also extend the NO gradient in the tissue, thereby increasing the apparent bioavailability of both oxygen and NO [70]. In normoxic and intermediate oxygen tensions, mitochondrial inhibition is most probably mediated by NOS dependent NO. However, in hypoxic conditions when NOS lacks oxygen, a required substrate for enzymatic NO generation, reduction of the existing tissue nitrite pool may be the primary source of NO. The proximity of myoglobin to the mitochondrion within cardiac and skeletal muscle provides an optimal metabolome for the reduction of nitrite and subsequent inhibition of mitochondrial respiration[2].
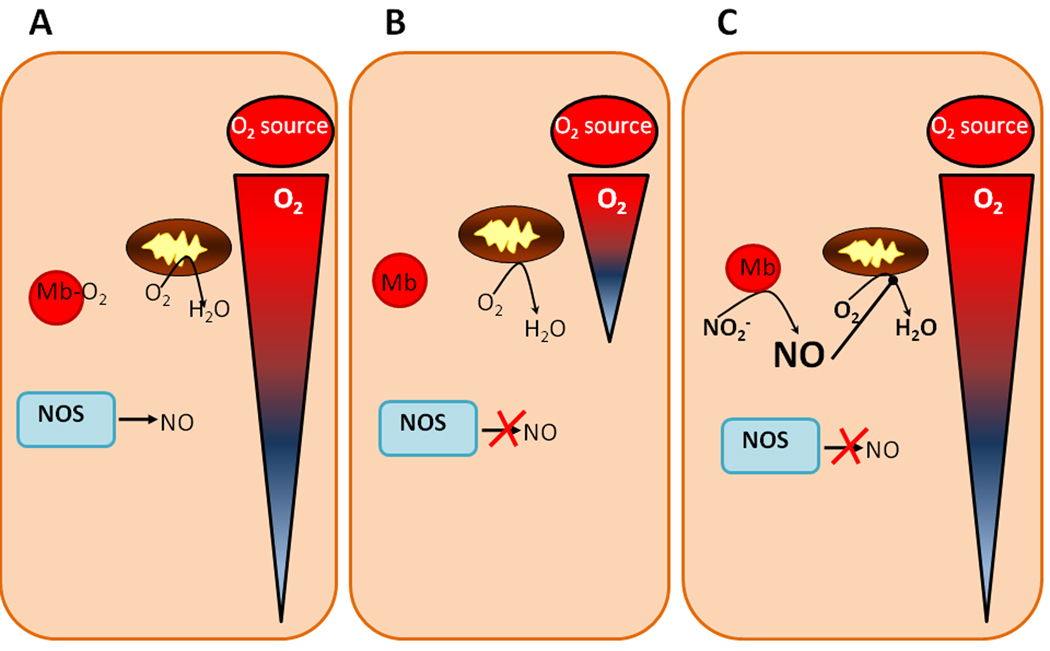
Figure 2. Nitrite-dependent extension of oxygen gradients.
(A) During normoxia, NOS is functional, myoglobin is oxygenated, and sufficient oxygen is available to diffuse from the source of oxygen through the tissue. (B) In hypoxic conditions, NOS is substrate limited and cannot make NO and myoglobin becomes deoxygenated. The majority of oxygen present is consumed by mitochondria close to the oxygen source, leading to a shortened oxygen gradient. (C) If nitrite is present during hypoxic conditions, it can be reduced by deoxygenated myoglobin. The NO generated can then partially inhibit mitochondrial oxygen consumption, allowing more oxygen to diffuse past these mitochondria and further into the tissue (elongation of oxygen gradient).
As tissue oxygen concentration decreases below the Km of NOS for oxygen binding (reported at values between 5 and 100µM), NOS activity becomes inhibited[71]. As this occurs, the p50 of myoglobin is reached (3 µM)[72] and as the protein begins to deoxygenate, it reduces available tissue nitrite. The NO generated from this reaction then inhibits mitochondrial cytochrome c oxidase (Km for oxygen binding < 1 µM)[73], leading to oxygen diffusion beyond these mitochondria and deeper into the tissue. Once oxygen concentrations increase in the tissue, inhibition is reversed and respiration is restored.
It is seemingly paradoxical that the inhibition of respiration, which would ultimately inhibit ATP generation, could be beneficial in hypoxia. However, it is important to note that inhibition of respiration does not always result in a decreased rate of ATP production[74]. Studies of respiratory control demonstrate that a large “reserve capacity” of cytochrome c oxidase activity exists in the mitochondria, such that slight inhibition of the enzyme (and hence inhibition of oxygen consumption) does not significantly impact the rate of ATP generation[74; 75]. Hence, it is possible that nitrite (through its reduction to NO) can reversibly and dynamically modulate oxygen consumption without negatively impacting ATP generation.
A recent study by Larsen and colleagues showed that whole body oxygen consumption during submaximal exercise was decreased in healthy volunteers when their diets were supplemented with nitrate[76]. This effect was associated with an increase in plasma nitrite concentration, and while oxygen consumption was decreased by an average of 0.16 L/min, there was no difference in maximal attainable work rate[76]. Although the mechanism of this increased muscular efficiency is not yet clear, it is interesting to speculate that in the hypoxic environment of an exercising muscle, deoxygenated myoglobin may reduce nitrite to NO, leading to the partial inhibition of respiration. This slight decrease in oxygen consumption, while large enough to improve efficiency of the muscle, may not be enough to inhibit ATP generation. While further study is needed to determine whether the nitrosylation of cytochrome c oxidase specifically is involved in the nitrite-dependent improvement of exercise efficiency, it is likely that the mitochondrion is involved in this phenomenon in some regard.
Nitrite, mitochondria and ischemia/reperfusion
Ischemia/reperfusion injury is a major component of many disease processes including myocardial infarction, solid organ transplant, cardiac arrest and stroke. While the pathology of I/R injury is complicated and involves a number of processes, mitochondrial dysfunction plays a central role[77; 78; 79]. During ischemia, mitochondrial respiration is inhibited due to the lack of oxygen and ATP stores are depleted leading to a bioenergetic deficit[80; 81; 82]. While the re-introduction of oxygen (reperfusion) to the tissue is necessary to restore cellular energetics, reperfusion itself exacerbates mitochondrial damage and tissue injury. Specifically, the rapid entrance of electrons accumulated during ischemia into the respiratory chain leads to the production of a burst of reactive oxygen species upon reperfusion, which oxidizes key proteins and lipids within the organelle[80; 83]. This oxidative stress along with a large influx of calcium into the mitochondria at the moment of reperfusion, triggers opening of the mitochondrial permeability transition pore and ultimately ends in the release of cytochrome c to initiate the apoptotic cascade[84; 85; 86; 87].
Nitrite is cytoprotective after ischemia/reperfusion
In the last 5 years, studies by a number of labs and in a number of animal models have demonstrated that nitrite is a potent cytoprotective agent during focal ischemia/reperfusion of the heart[8; 9; 10; 11; 12; 13; 14], liver[10; 13; 88], brain[89] and kidney[90] and in a global ischemic model of cardiopulmonary arrest[91] and resuscitation. These individual studies have previously been reviewed [92; 93] and will not be discussed in depth here. Suffice to say that nitrite is a versatile cytoprotective agent that is effective both in in vitro (Langendorff perfused heart[8; 14], isolated mitochondria anoxia/reoxygenation[13]) and in vivo animal models[9; 10; 11; 89; 90] of I/R. Dose response studies demonstrate that nitrite works within a large range of concentrations, with doses between a range of 0.1 and 100 µM/kg providing significant cytoprotection. Remarkably, even small elevations in plasma nitrite appear to mediate significant cytoprotection. For example, in murine models of hepatic I/R and myocardial infarction, an increase in plasma nitrite of 200nM decreased liver injury by approximately 40% and infarct size by 50% respectively[10].
The cytoprotective effects of nitrite are also evident over a large temporal window. In initial studies nitrite was administered at the midpoint of the ischemic episode, but it is now apparent that nitrite administration immediately before[14; 92], during[10; 11], or at the end[11] of the ischemic period is cytoprotective. Consistent with this, Gonzalez and colleagues demonstrated that in a canine model of myocardial infarction in which an ischemic period of 2 hours was followed by 6 hours of reperfusion, infarct size was significantly decreased whether nitrite was administered one hour or five minutes before the initiation of reperfusion[11]. The cytoprotective effect of nitrite is present even when nitrite is administered 24 hours before the onset of ischemia[92] or supplemented in the diet prior to ischemia[9; 94; 95]. In addition, Kumar and colleagues demonstrated that nitrite treatment increases angiogenesis in a hindlimb model of chronic ischemia, suggesting that chronic nitrite treatment can also be cytoprotective[7]. While it is established that nitrite is a potent and versatile cytoprotective agent during I/R, the mechanism of its protection are not clear.
S-nitrosation of complex I
Given that the mitochondrion is a known target of nitrite, and mitochondrial dysfunction is central to I/R injury, the role of nitrite in the regulation of mitochondrial function during I/R has emerged as an active area of research. While broad gene expression and proteomic studies have shown that nitrite mediates the differential expression of a number of mitochondrial genes and proteins after I/R, the functional consequence of these alterations have not yet been elucidated[6; 94]. This section will focus instead on the nitrite-dependent post-translational modification of mitochondrial complex I, which has been shown to modulate mitochondrial function after I/R.
Studies from our lab have demonstrated that nitrite prevents the three major manifestations of I/R-induced mitochondrial damage, including decreased ATP generation, increased ROS production at reperfusion, and cytochrome c release[92]. Initial studies examining mitochondrial function in the liver of mice subjected to hepatic ischemia/reperfusion demonstrated that the cytoprotective effects of nitrite were associated with the inhibition of complex I dependent respiration at reperfusion. This finding led to the further characterization of nitrite on mitochondrial function in an in vitro model in which isolated mitochondria were subjected to anoxia/reoxygenation in order to mimic tissue I/R in the absence of other cellular components. Studies in this model demonstrated that nitrite did indeed inhibit complex I after anoxia/reoxygenation in a concentration dependent manner[92]. While this inhibition of respiration initially seems paradoxical with tissue protection, this data is consistent with previous literature demonstrating that inhibitors of complex I, such as rotenone, amobarbitol, nitric oxide, and S-nitrosothiols are cytoprotective[96; 97; 98; 99; 100; 101].
Complex I (NADH ubiquinone oxidoreductase), a large protein consisting of 46 subunits, is an entry point of electrons in the respiratory chain and a key site for ROS generation in the mitochondrion. Mechanistically, inhibition of complex I is thought to contribute to cytoprotection by blocking entry of electrons into the respiratory chain and thus attenuating the burst of ROS generation associated with reperfusion. Consistent with this mechanism, when hydrogen peroxide generation was measured in isolated mitochondria which were subjected to in vitro anoxia/reoxygenation, mitochondria treated with nitrite during anoxia generated significantly less hydrogen peroxide than those that were untreated[13] (Figure 3). This decrease in ROS generation was corroborated by data demonstrating that nitrite administration during ischemia in vivo prevented an I/R induced decrease in the activity of aconitase, an iron-sulfur protein which loses its activity upon oxidative modification. Nitrite treatment also protected complex II- dependent respiration and ATP generation from an I/R induced decrease that was observed in untreated animals, suggesting that nitrite-dependent attenuation of ROS prevents the oxidative damage of complex II[13]. In addition to preventing oxidation of key mitochondrial proteins, nitrite treatment prevented the calcium-induced permeability pore opening and release of cytochrome c from mitochondria after anoxia/reoxygenation, suggesting a mechanism for the prevention of apoptosis[13]. The specific mechanism of nitrite-dependent inhibition of complex I appears to involve the S-nitrosation of critical thiol residues on the complex. S-nitrosation of complex I by a number of agents including high concentrations of NO, peroxynitrite, and even ischemic preconditioning, has been linked to the inhibition of the catalytic activity of the complex[102; 103; 104]. While it is unknown whether S-nitrosation of a specific target cysteine within the complex leads to inhibition or whether a group of thiols must be modified, several subunits of the complex have been implicated as putative targets of S-nitrosation[104; 105]. In the case of nitrite, although the exact site of modification has not yet been elucidated, it is clear that nitrite treatment of mitochondria during anoxia concentration-dependently S-nitrosate the mitochondrion, and this increased S-nitrosation is associated with a concomitant decrease in complex I activity[13].
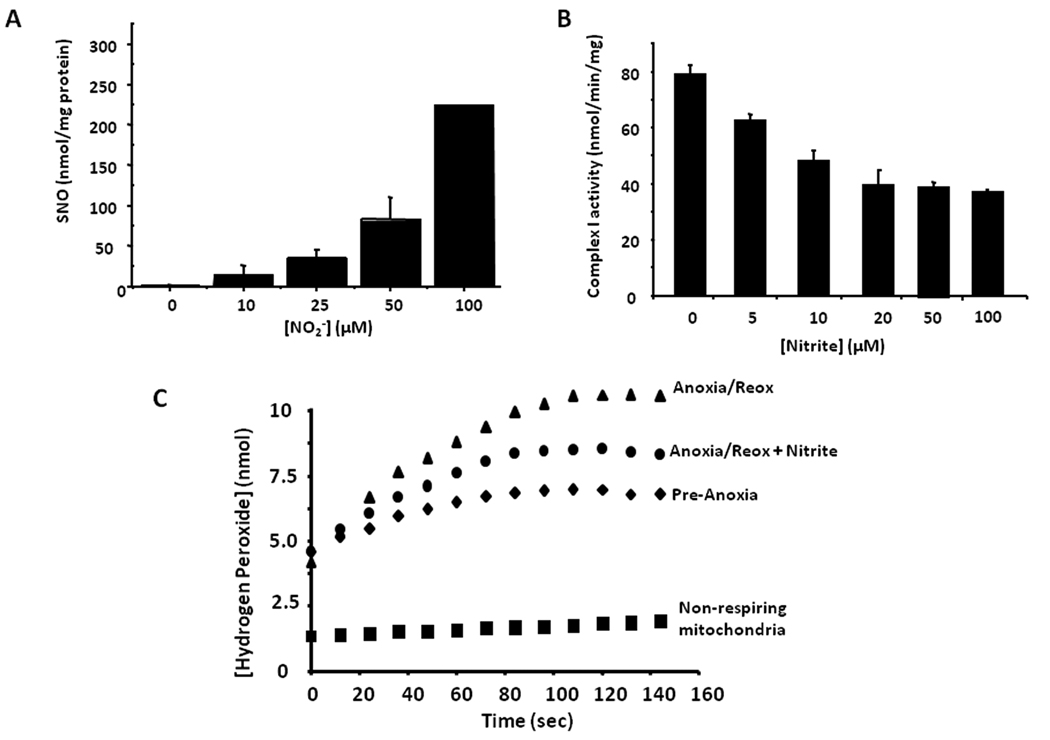
Figure 3. S-nitrosation of mitochondria and decreased ROS generation after anoxia/reoxygenation.
Isolated rat liver mitochondria treated with nitrite (2.5–100µM) during anoxia show a (A) concentration dependent increase in S-nitrosation and (B) a concomitant inhibition of complex I activity. (C) Nitrite treatment (10 µM) during anoxia prevents hydrogen peroxide generation at reoxygenation.
The chemical mechanism by which nitrite mediates S-nitrosation of complex I is not entirely clear, however several potential pathways exist by which this modification may occur. Nitrous acid, formed by the protonation of nitrite, is a nitrosating agent and may be readily formed in the acidic conditions of tissue ischemia. In addition to simple protonation, evidence now exists for the heme globin conversion of nitrite to dinitrogen trioxide (N2O3), a potent nitrosating species. In the case of hemoglobin, a unique reductive anhydrase pathway has been described for the formation of N2O3 from nitrite[106]. Briefly, one molecule of nitrite reacts with deoxygenated hemoglobin to generate NO and methemoglobin through classical nitrite reductase chemistry (Reaction 1 above). Another molecule of nitrite then binds to methemoglobin, and the resulting nitrite-methemoglobin has an electron configuration which appears to possess Fe 2+- NO2• character (Reaction 2).
| (Reaction 2) |
The NO2• species can react rapidly with a molecule of NO to form N2O3 [106]
| (Reaction 3) |
By this reaction, hemoglobin essentially catalyzes the removal of a molecule of water from two molecules of nitrite to generate dinitrogen trioxide (N2O3) [106]. Once generated, the hydrophobic inner mitochondrial membrane could stabilize N2O3 or NO2• in close proximity to complex I, allowing for the reaction of these species with the protein. It is unknown whether either or both of these mechanisms play a role in nitrite-dependent complex I S-nitrosation. However, it has been shown that in animal models of I/R, as well as in isolated mitochondria, the protective effect of nitrite is inhibited by the NO scavenger PTIO[10; 13; 14; 89; 107]. In addition, in the heart, nitrite-dependent reduction of infarct size after I/R is dependent on the presence of myoglobin[12], suggesting a role for heme catalyzed S-nitrosation.
Although the chemical mechanism of S-nitrosation is unclear, accumulating data in animal models show that nitrite-dependent S-nitrosation of complex I and concomitant inhibition of this complex is associated with cytoprotection after I/R. In a murine model of hepatic I/R in which the diets of mice were supplemented with, or depleted of, nitrite and nitrate for one week before the ischemic episode, mice on a high nitrite/nitrate diet were protected from hepatic injury after I/R[94]. Furthermore, the extent of hepatic protection correlated inversely with complex I activity, supporting the idea that nitrite-dependent complex I inhibition is important in protection from I/R injury. Nitrite dependent S-nitrosation and concomitant inhibition of complex I in the heart has been associated with improved cardiac function and increased survival in a global ischemia/reperfusion model of cardiac arrest and resuscitation[91]. In addition, rats administered one dose of nitrite (480 nmoles) 24 hours before undergoing ischemia showed increased levels of mitochondrial S-nitrosation compared to mitochondria from untreated animals[13].
Consistent with the hypothesis that nitrite decreases ROS generation and inhibition of cytochrome c release, a number of studies have now shown that nitrite decreases oxidative stress during ischemia/reperfusion. For example, Jung and colleagues showed that in a rat model of stroke, in which nitrite protects neurological function and decreases cerebral necrosis, nitrite treatment is associated with a decrease in nitrotyrosine formation in the brain compared to untreated controls[89]. After cardiac arrest and cardiopulmonary resuscitation (CPR), cardiac mitochondria from nitrite treated animals demonstrated decreased hydrogen peroxide from 5 minutes to one hour after CPR[91]. A number of studies also show a decrease in markers of apoptosis, such as TUNEL staining, with nitrite treatment, consistent with the nitrite-dependent prevention of permeability pore opening and cytochrome c release[10; 11; 91].
While it is unclear how complex I inhibition is protective given that prolonged inhibition of electron transfer could lead to the inhibition of ATP synthesis, it is important to note that S-nitrosation is a reversible modification and treatment of mitochondria with metals, thiols, or light has been shown to completely reverse this modification as well as the inhibition of complex I[108]. Thus, the inhibition of complex I may be protective immediately at reperfusion and thereafter the S-nitrosation may be removed and complex I activity restored. It has been hypothesized that the gradual reversal of complex I inhibition over time would allow electrons to enter the electron transport chain incrementally, preventing the burst of ROS formation at reperfusion, a concept termed “gradual wake-up”[78; 83]. Indeed, in a murine model of cardiac arrest and CPR, cardiac complex I activity is inhibited by approximately 40% in nitrite treated animals 5 minutes after reperfusion, but is restored to the levels of sham operated animals by 1 hour into reperfusion[91].
Nitrite is a unique complex I inhibitor
While a number of complex I inhibitors have been shown to mediate cytoprotection in animal models of I/R, few successfully make the transition from bench to the clinic. However, several characteristics make nitrite unique among complex I inhibitors and suggest that it could be used as a therapeutic. First, nitrite is targeted to ischemic tissue. While nitrite treatment has no significant effect on mitochondrial function during normoxia, nitrite-dependent inhibition of complex I is observed after the ischemic episode. This suggests that nitrite selectively modulates mitochondria in ischemic tissues, while allowing normal electron transfer in non-ischemic mitochondria. Second, nitrite is a reversible inhibitor of complex I. This is important not only because prolonged inhibition of respiration can lead to ATP depletion, but also because the chronic presence of complex I inhibitors, such as rotenone, have been shown to lead to Parkinson’s Disease[109]. Finally, unlike complex I inhibitors such as rotenone amobarbitol, nitrite is naturally occurring in the diet. This opens the possibility for dietary modulation of the cytoprotective response. Although much more research is needed to determine the effects of chronic nitrite treatment on mitochondrial function as well as whether mitochondria build tolerance to nitrite, studies to date suggest that nitrite may be a promising pharmacologic regulator of mitochondrial function and mediator of cytoprotection.
CONCLUSIONS
Nitrite, historically thought to be an inert byproduct of NO metabolism, is now regarded as an endocrine store of NO and a signaling molecule in its own right. Although the physiological effects of nitrite and the mechanisms by which these effects are regulated are just beginning to be elucidated, it is clear that the mitochondria are involved in nitrite signaling. In vitro evidence exists for mitochondrial involvement in both the generation and reduction of nitrite. Studies also demonstrate that nitrite is a regulator of mitochondrial function by at least two distinct mechanisms – the nitrosylation of cytochrome c oxidase and the S-nitrosation of complex I (Figure 4). Through these modifications, nitrite may be instrumental in regulating physiological processes such as the extension of oxygen gradients during hypoxia and modulation of exercise efficiency, as well as mediating cytoprotection from I/R injury. While a great deal has been uncovered about the intertwined signaling axes of the mitochondrion and nitrite in the last decade, future studies will no doubt reveal new mitochondrial targets for nitrite and describe new physiological roles for these interactions.
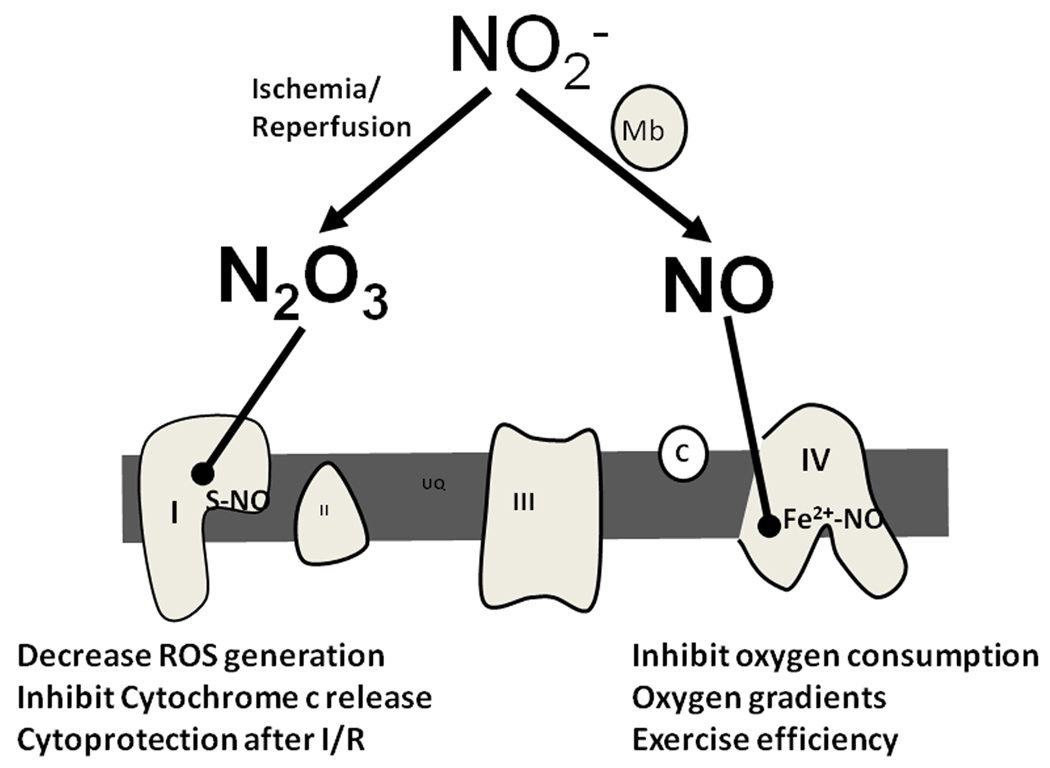
Figure 4. Nitrite regulates mitochondrial function.
During hypoxia, nitrite is reduced to NO by deoxygenated myoglobin and nitrosylates the binuclear center of complex IV. This results in the inhibition of oxygen consumption which may contribute to the regulation of oxygen gradients and the modulation of exercise efficiency. During ischemia/reperfusion, nitrite is converted to a nitrosating species (possibly N2O3 through its reductive anhydrase reaction with heme) and S-nitrosates complex I at reperfusion. This leads to decreased ROS generation and inhibition of cytochrome c release, which contribute to cytoprotection after I/R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, 3rd, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 3.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 4.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol. 2005;1:290–297. doi: 10.1038/nchembio734. [DOI] [PubMed] [Google Scholar]
- 6.Perlman DH, Bauer SM, Ashrafian H, Bryan NS, Garcia-Saura MF, Lim CC, Fernandez BO, Infusini G, McComb ME, Costello CE, Feelisch M. Mechanistic insights into nitrite-induced cardioprotection using an integrated metabolomic/proteomic approach. Circ Res. 2009;104:796–804. doi: 10.1161/CIRCRESAHA.108.187005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH, Jr, Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A. 2008;105:7540–7545. doi: 10.1073/pnas.0711480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JE, Su J, Fu X, Hsu A, Gross GJ, Tweddell JS, Hogg N. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and K(ATP) channels. J Mol Cell Cardiol. 2007;43:437–444. doi: 10.1016/j.yjmcc.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2007;104:19144–19149. doi: 10.1073/pnas.0706579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, 3rd, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci U S A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura MF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J, Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem. 2008;283:33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandel NS, Schumacker PT. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett. 1999;454:173–176. doi: 10.1016/s0014-5793(99)00783-8. [DOI] [PubMed] [Google Scholar]
- 17.Ow YP, Green DR, Hao Z, Mak TW. Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 18.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 21.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 22.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 23.Petersson J, Carlstrom M, Schreiber O, Phillipson M, Christoffersson G, Jagare A, Roos S, Jansson EA, Persson AE, Lundberg JO, Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Ford PC, Wink DA, Stanbury DM. Autoxidation kinetics of aqueous nitric oxide. FEBS Lett. 1993;326:1–3. doi: 10.1016/0014-5793(93)81748-o. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR., Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiva S, Brookes PS, Patel RP, Anderson PG, Darley-Usmar VM. Nitric oxide partitioning into mitochondrial membranes and the control of respiration at cytochrome c oxidase. Proc Natl Acad Sci U S A. 2001;98:7212–7217. doi: 10.1073/pnas.131128898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 28.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 29.Brunori M. Nitric oxide moves myoglobin centre stage. Trends Biochem Sci. 2001;26:209–210. doi: 10.1016/s0968-0004(01)01824-2. [DOI] [PubMed] [Google Scholar]
- 30.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci U S A. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torres J, Wilson MT. The reactions of copper proteins with nitric oxide. Biochim Biophys Acta. 1999;1411:310–322. doi: 10.1016/s0005-2728(99)00022-5. [DOI] [PubMed] [Google Scholar]
- 32.Giuffre A, Barone MC, Mastronicola D, D'Itri E, Sarti P, Brunori M. Reaction of nitric oxide with the turnover intermediates of cytochrome c oxidase: reaction pathway and functional effects. Biochemistry. 2000;39:15446–15453. doi: 10.1021/bi000447k. [DOI] [PubMed] [Google Scholar]
- 33.Torres J, Sharpe MA, Rosquist A, Cooper CE, Wilson MT. Cytochrome c oxidase rapidly metabolises nitric oxide to nitrite. FEBS Lett. 2000;475:263–266. doi: 10.1016/s0014-5793(00)01682-3. [DOI] [PubMed] [Google Scholar]
- 34.Walters CL, Taylor AM. The Reduction of Nitrite by Skeletal-Muscle Mitochondria. Biochim Biophys Acta. 1965;96:522–524. doi: 10.1016/0005-2787(65)90570-8. [DOI] [PubMed] [Google Scholar]
- 35.Reutov VP, Sorokina EG. NO-synthase and nitrite-reductase components of nitric oxide cycle. Biochemistry (Mosc) 1998;63:874–884. [PubMed] [Google Scholar]
- 36.Basu S, Azarova NA, Font MD, King SB, Hogg N, Gladwin MT, Shiva S, Kim-Shapiro DB. Nitrite reductase activity of cytochrome c. J Biol Chem. 2008;283:32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Nohl H, Staniek K, Sobhian B, Bahrami S, Redl H, Kozlov AV. Mitochondria recycle nitrite back to the bioregulator nitric monoxide. Acta Biochim Pol. 2000;47:913–921. [PubMed] [Google Scholar]
- 39.Brudvig GW, Bocian DF, Gamble RC, Chan SI. Evidence for the absence of photoreduction of the metal centers of cytochrome C oxidase by X-irradiation. Biochim Biophys Acta. 1980;624:78–89. doi: 10.1016/0005-2795(80)90227-5. [DOI] [PubMed] [Google Scholar]
- 40.Sarti P, Giuffre A, Forte E, Mastronicola D, Barone MC, Brunori M. Nitric oxide and cytochrome c oxidase: mechanisms of inhibition and NO degradation. Biochem Biophys Res Commun. 2000;274:183–187. doi: 10.1006/bbrc.2000.3117. [DOI] [PubMed] [Google Scholar]
- 41.Castello PR, Woo DK, Ball K, Wojcik J, Liu L, Poyton RO. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proc Natl Acad Sci U S A. 2008;105:8203–8208. doi: 10.1073/pnas.0709461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke PV, Poyton RO. Structure/function of oxygen-regulated isoforms in cytochrome c oxidase. J Exp Biol. 1998;201:1163–1175. doi: 10.1242/jeb.201.8.1163. [DOI] [PubMed] [Google Scholar]
- 43.Trueblood CE, Wright RM, Poyton RO. Differential regulation of the two genes encoding Saccharomyces cerevisiae cytochrome c oxidase subunit V by heme and the HAP2 and REO1 genes. Mol Cell Biol. 1988;8:4537–4540. doi: 10.1128/mcb.8.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groot GS, Poyton RO. Oxygen control of cytochrome c oxidase synthesis in isolated mitochondria from Saccharomyces cerevisiae. Nature. 1975;255:238–240. doi: 10.1038/255238a0. [DOI] [PubMed] [Google Scholar]
- 45.Castro L, Eiserich JP, Sweeney S, Radi R, Freeman BA. Cytochrome c: a catalyst and target of nitritehydrogen peroxide-dependent protein nitration. Arch Biochem Biophys. 2004;421:99–107. doi: 10.1016/j.abb.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 46.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 47.Vlasova II, Tyurin VA, Kapralov AA, Kurnikov IV, Osipov AN, Potapovich MV, Stoyanovsky DA, Kagan VE. Nitric oxide inhibits peroxidase activity of cytochrome c.cardiolipin complex and blocks cardiolipin oxidation. J Biol Chem. 2006;281:14554–14562. doi: 10.1074/jbc.M509507200. [DOI] [PubMed] [Google Scholar]
- 48.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 49.Kozlov AV, Costantino G, Sobhian B, Szalay L, Umar F, Nohl H, Bahrami S, Redl H. Mechanisms of vasodilatation induced by nitrite instillation in intestinal lumen: possible role of hemoglobin. Antioxid Redox Signal. 2005;7:515–521. doi: 10.1089/ars.2005.7.515. [DOI] [PubMed] [Google Scholar]
- 50.Nohl H, Staniek K, Kozlov AV. The existence and significance of a mitochondrial nitrite reductase. Redox Rep. 2005;10:281–286. doi: 10.1179/135100005X83707. [DOI] [PubMed] [Google Scholar]
- 51.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The reaction between nitrite and deoxyhemoglobin. Reassessment of reaction kinetics and stoichiometry. J Biol Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 52.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petersen MG, Dewilde S, Fago A. Reactions of ferrous neuroglobin and cytoglobin with nitrite under anaerobic conditions. J Inorg Biochem. 2008;102:1777–1782. doi: 10.1016/j.jinorgbio.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 55.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Rev. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia Y, Zweier JL. Substrate control of free radical generation from xanthine oxidase in the postischemic heart. J Biol Chem. 1995;270:18797–18803. doi: 10.1074/jbc.270.32.18797. [DOI] [PubMed] [Google Scholar]
- 57.Kozlov AV, Dietrich B, Nohl H. Various intracellular compartments cooperate in the release of nitric oxide from glycerol trinitrate in liver. Br J Pharmacol. 2003;139:989–997. doi: 10.1038/sj.bjp.0705323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minneci PC, Deans KJ, Shiva S, Zhi H, Banks SM, Kern S, Natanson C, Solomon SB, Gladwin MT. Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis. Am J Physiol Heart Circ Physiol. 2008;295:H743–H754. doi: 10.1152/ajpheart.00151.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res. 2007;100:1749–1754. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 61.Logan DC. The mitochondrial compartment. J Exp Bot. 2007;58:1225–1243. [PubMed] [Google Scholar]
- 62.Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- 63.Brunori M. Nitric oxide, cytochrome-c oxidase and myoglobin. Trends Biochem Sci. 2001;26:21–23. doi: 10.1016/s0968-0004(00)01698-4. [DOI] [PubMed] [Google Scholar]
- 64.Doeller JE, Wittenberg BA. Myoglobin function and energy metabolism of isolated cardiac myocytes: effect of sodium nitrite. Am J Physiol. 1991;261:H53–H62. doi: 10.1152/ajpheart.1991.261.1.H53. [DOI] [PubMed] [Google Scholar]
- 65.Brookes P, Darley-Usmar VM. Hypothesis: the mitochondrial NO(*) signaling pathway, and the transduction of nitrosative to oxidative cell signals: an alternative function for cytochrome C oxidase. Free Radic Biol Med. 2002;32:370–374. doi: 10.1016/s0891-5849(01)00805-x. [DOI] [PubMed] [Google Scholar]
- 66.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 67.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 68.Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic Biol Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 69.Poderoso JJ, Carreras MC, Lisdero C, Riobo N, Schopfer F, Boveris A. Nitric oxide inhibits electron transfer and increases superoxide radical production in rat heart mitochondria and submitochondrial particles. Arch Biochem Biophys. 1996;328:85–92. doi: 10.1006/abbi.1996.0146. [DOI] [PubMed] [Google Scholar]
- 70.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci U S A. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dweik RA, Laskowski D, Abu-Soud HM, Kaneko F, Hutte R, Stuehr DJ, Erzurum SC. Nitric oxide synthesis in the lung. Regulation by oxygen through a kinetic mechanism. J Clin Invest. 1998;101:660–666. doi: 10.1172/JCI1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol. 2003;206:2011–2020. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- 73.Gnaiger E, Kuznetsov AV. Mitochondrial respiration at low levels of oxygen and cytochrome c. Biochem Soc Trans. 2002;30:252–258. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 74.Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol. 1998;201:1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- 75.Brand MD, Murphy MP. Control of electron flux through the respiratory chain in mitochondria and cells. Biol Rev Camb Philos Soc. 1987;62:141–193. doi: 10.1111/j.1469-185x.1987.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 76.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 77.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 78.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wakeup. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 80.Di Lisa F, Menabo R, Canton M, Petronilli V. The role of mitochondria in the salvage and the injury of the ischemic myocardium. Biochim Biophys Acta. 1998;1366:69–78. doi: 10.1016/s0005-2728(98)00121-2. [DOI] [PubMed] [Google Scholar]
- 81.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 82.Lesnefsky EJ, Tandler B, Ye J, Slabe TJ, Turkaly J, Hoppel CL. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am J Physiol. 1997;273:H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 83.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 84.Borutaite V, Mildaziene V, Brown GC, Brand MD. Control and kinetic analysis of ischemia-damaged heart mitochondria: which parts of the oxidative phosphorylation system are affected by ischemia? Biochim Biophys Acta. 1995;1272:154–158. doi: 10.1016/0925-4439(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 85.Brookes PS, Darley-Usmar VM. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am J Physiol Heart Circ Physiol. 2004;286:H39–H46. doi: 10.1152/ajpheart.00742.2003. [DOI] [PubMed] [Google Scholar]
- 86.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stone D, Darley-Usmar V, Smith DR, O'Leary V. Hypoxia-reoxygenation induced increase in cellular Ca2+ in myocytes and perfused hearts: the role of mitochondria. J Mol Cell Cardiol. 1989;21:963–973. doi: 10.1016/0022-2828(89)90795-5. [DOI] [PubMed] [Google Scholar]
- 88.Lang JD, Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 90.Tripatara P, Patel NS, Webb A, Rathod K, Lecomte FM, Mazzon E, Cuzzocrea S, Yaqoob MM, Ahluwalia A, Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J Am Soc Nephrol. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 91.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser D, Munasinghe J, T.L CFC, Vanden Hoek L, Gladwin M. Nitrite Therapy After Cardiac Arrest Reduces Reactive Oxygen Species Generation, Improves Cardiac and Neurological Function, and Enhances Survival via Reversible Inhibition of Mitochondrial Complex I. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.853267. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shiva S, Gladwin MT. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Res Cardiol. 2009;104:113–119. doi: 10.1007/s00395-009-0009-3. [DOI] [PubMed] [Google Scholar]
- 93.Sinha SS, Shiva S, Gladwin MT. Myocardial protection by nitrite: evidence that this reperfusion therapeutic will not be lost in translation. Trends Cardiovasc Med. 2008;18:163–172. doi: 10.1016/j.tcm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 94.Raat H, Cochard A, Raghavachari N, Shiva S, Gladwin MT. Dietary Nitrite Modulates the response to ischemia. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.05.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–415. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 97.Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- 98.Stewart S, Lesnefsky EJ, Chen Q. Reversible blockade of electron transport with amobarbital at the onset of reperfusion attenuates cardiac injury. Transl Res. 2009;153:224–231. doi: 10.1016/j.trsl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 99.Konorev EA, Tarpey MM, Joseph J, Baker JE, Kalyanaraman B. S-nitrosoglutathione improves functional recovery in the isolated rat heart after cardioplegic ischemic arrest-evidence for a cardioprotective effect of nitric oxide. J Pharmacol Exp Ther. 1995;274:200–206. [PubMed] [Google Scholar]
- 100.Nadtochiy SM, Burwell LS, Ingraham CA, Spencer CM, Friedman AE, Pinkert CA, Brookes PS. In vivo cardioprotection by S-nitroso-2-mercaptopropionyl glycine. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Prime TA, Blaikie FH, Evans C, Nadtochiy SM, James AM, Dahm CC, Vitturi DA, Patel RP, Hiley CR, Abakumova I, Requejo R, Chouchani ET, Hurd TR, Garvey JF, Taylor CT, Brookes PS, Smith RA, Murphy MP. A mitochondria-targeted S-nitrosothiol modulates respiration, nitrosates thiols, and protects against ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2009;106:10764–10769. doi: 10.1073/pnas.0903250106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim Biophys Acta. 2004;1658:44–49. doi: 10.1016/j.bbabio.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 103.Dahm CC, Moore K, Murphy MP. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J Biol Chem. 2006;281:10056–10065. doi: 10.1074/jbc.M512203200. [DOI] [PubMed] [Google Scholar]
- 104.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Galkin A, Moncada S. S-nitrosation of mitochondrial complex I depends on its structural conformation. J Biol Chem. 2007;282:37448–37453. doi: 10.1074/jbc.M707543200. [DOI] [PubMed] [Google Scholar]
- 106.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 107.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia-reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–338. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Borutaite V, Budriunaite A, Brown GC. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim Biophys Acta. 2000;1459:405–412. doi: 10.1016/s0005-2728(00)00178-x. [DOI] [PubMed] [Google Scholar]
- 109.Hoglinger GU, Oertel WH, Hirsch EC. The rotenone model of parkinsonism--the five years inspection. J Neural Transm Suppl. 2006:269–272. doi: 10.1007/978-3-211-45295-0_41. [DOI] [PubMed] [Google Scholar]