Abstract
In this review we want to consider some of the requirements for autoimmune disease to develop and how this may be reproduced in animal models. Besides a genetic predisposition, environmental triggering factors seem to play a central role in the etiology of many autoimmune diseases. In theory, a structural similarity or identity between the host and an invading pathogen might cause the immune system of the host to react not only to the pathogen but also to self-components. However, in order for such a process of molecular mimicry to induce autoimmunity the mechanisms of maintaining tolerance or ignorance to the self-components need to be circumvented. Subsequently, in order to advance autoimmunity to overt autoimmune disease the frequency and avidity of autoaggressive lymphocytes has to be of sufficient magnitude. Intuitively, one would assume that tolerance might be stronger to identical structures than to structures that just share a certain degree of similarity. Self-reactive lymphocytes with high-avidity are more likely to be deleted or functionally silenced by central and/or peripheral tolerance mechanisms. Thus, perfect mimicry between identical structures might fail in inducing autoimmunity because of efficient tolerance mechanisms. In contrast, imperfect mimicry between similar but not identical structures might on one hand circumvent tolerance but on the other hand result in the generation of lymphocytes with only low- to intermediate avidity. Here we examine animal models that use the concept of molecular mimicry as a potential mechanism for inducing or accelerating autoimmunity. We focus on the RIP-LCMV model for type 1 diabetes and the novel cytochrome P450 2D6 (CYP2D6) model for autoimmune hepatitis, which use either identical or similar triggering and target antigens.
Keywords: Cytochrome P450, virus infection, tolerance, liver disease, inflammation
Virus infections and autoimmune diseases
In the past decades multiple associations have been established between infections and autoimmune diseases such as multiple sclerosis, type 1 diabetes, ankylosing spondilitis, myasthenia gravis, systemic lupus erythematosus, autoimmune myocarditis and many others [1-3]. Infection with a pathogen sharing similar structures with autoantigens is one possibility how pathogens might induce or accelerate autoimmunity. Such ‘molecular mimicry’ indeed exists [4] and has been detected between pathogens and autoantigens recognized by antibodies or T cells of patients with a broad variety of autoimmune diseases [5]. However, in most cases no firm proof for pathogens as inducers for autoimmune diseases has been found so far. An exception might be the Guillain Barré syndrome (GBS), where the association with the infection with Campylobacter jejuni, which shares a structural homology of the lipo-oligosaccahride with the peripheral nerve GM1 ganglioside, could be convincingly reproduced in an animal model [6]. In addition, one of the best examples of postinfectious autoimmunity due to molecular mimicry has been established for Streptococcus pyogenes-induced acute rheumatic fever (ARF), where the lysoganglioside of the host shares a structural similarity to N-acetyl-β-D-glucosamine, the dominant epitope of the group A streptococcal carbohydrate [7]. Recently, an animal model for true molecular mimicry has been established in the laboratory of M. Eric Gershwin at UC Davis. Anti-mitochondrial antibodies directed against the E2 subunit of pyruvate dehydrogenase complex (PDC-E2) are the hallmark of primary biliary cirrhosis (PBC) [8]. Interestingly, the Gershwin group found several potential environmental inducers for PBC, including bacteria, such as Novosphingobium aromaticivorans, and chemical xenobiotics, that confer molecular mimicry to the immunodominant structure in PDC-E2 containing the prosthetic group lipoic acid [9, 10]. Among the chemical xenobiotics the cosmetic and food additive 2-octoynoic acid (2-OA) shows a high structural similarity to lipoic acid and injection of 2-OA coupled to bovine serum albumin (BSA) resulted in the generation of PBC like disease in wildtype C57BL/6 mice [11]. Mice treated with 2-OA-BSA manifested autoimmune cholangitis, anti-mitochondrial antibodies and infiltration of the liver by activated CD8 T cells [11].
Several animal models for human autoimmune diseases have been engineered to test the concept of molecular mimicry in that transgenic animals expressing specific target antigens are infected with pathogens bearing identical or similar antigens as triggering factors for the autoimmune process. In order to induce autoimmune disease in these models a sufficient number of autoaggressive lymphocytes has to be generated to destroy the target tissue. In this context, infection with a pathogen carrying a structure with molecular identity to the target antigen has the advantage of a ‘perfect fit’ and therefore can result in the generation of more lymphocytes with higher avidity to the identical transgenic target (auto)antigen. However, even in the absence of a possible thymic expression of the target antigen, mechanisms of peripheral tolerance can result in a certain degree of unresponsiveness to the identical antigen present on the triggering virus that might prevent a sufficiently strong aggressive immune response. Infection with pathogens with similar but not identical structures might circumvent tolerance induction by the host, but then the question is whether autoimmunity initiated by such molecular mimicry generates enough autoaggressive lymphocytes with sufficient avidity to cross the threshold for clinical autoimmune disease.
The RIP-LCMV model for type 1 diabetes: The avidity of self-reactive lymphocytes determines the course of disease
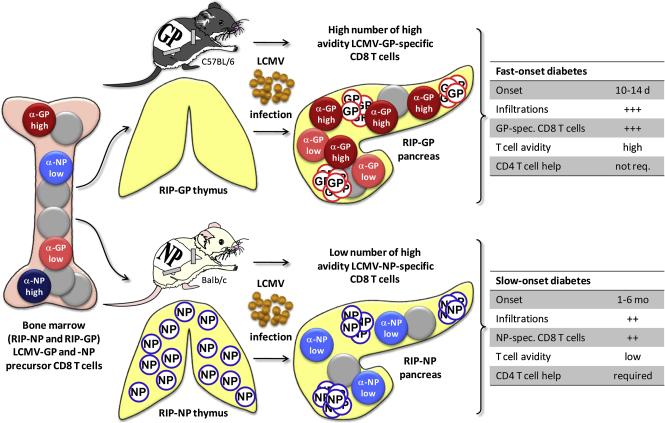
RIP-LCMV mice express the glycoprotein (GP) or the nucleoprotein (NP) of the lymphocytic choriomeningitis virus (LCMV) under the control of the rat insulin promoter (RIP) specifically in the β-cells of the pancreatic islets of Langerhans [12]. Upon infection with LCMV, RIP-LCMV-GP mice (C57BL/6 background) and RIP-LCMV-NP mice (Balb/c background) develop type 1 diabetes (T1D) within 10-14 days and 1-6 months, respectively. The affinity of the immunodominant LCMV-GP peptide GP33 to H-2Db is about equal to the immunodominant LCMV-NP peptide NP118 to H-2Ld [13]. However, the affinity of the TcRs encountered in vivo in RIP-LCMV transgenic mouse strains that are either RIP-LCMV-GP or RIP-LCMV-NP expressers of GP33-specific CD8 T cells to the H-2Db-GP33 complex is about 10-fold higher than of NP118-specific CD8 T cells to the H-2Ld-NP118 complex [14, 15]. This is largely due to the expression of LCMV-NP in the thymus of RIP-LCMV-NP mice, which results in the deletion of high avidity NP118-specific T cells [14, 15]. Thus, even though a similar number of GP33-specific and NP118-specific CD8 T cells are generated upon LCMV-infection of RIP-LCMV-GP and RIP-LCMV-NP mice, respectively, the pathogenesis of T1D is accelerated in RIP-LCMV-GP mice (figure 1). Importantly, the low avidity LCMV-NP-specific CD8 T cells were not sufficient to induce disease in absence of CD4 help, since CD4-depleted RIP-LCMV-NP mice did not develop disease upon LCMV-infection [14, 15]. In contrast, LCMV-infected RIP-LCMV-GP mice kept their fast-onset diabetic profile even after CD4 depletion [14, 15]. These findings indicate that the avidity of autoaggressive lymphocytes to presented antigenic epitopes has a great impact on the course of disease. Tian et al. demonstrated that the affinity of the MHC/peptide complex to the TcR correlated well the functional activity of T cells [16]. They analyzed the interactions between the TcR of P14 mice expressing LCMV-GP33-specific TcRs and a set of altered GP33 peptides and found a correlation of the affinity of the P14 TcR to the MHC/peptide complex with both cytotoxicity and IFNγ-production. In contrast, no correlation was found between MHC/peptide-TcR interaction and other kinetic parameters, such as the dissociation rate [16]. Further, Gronski et al. have shown that a reduction in the affinity of the TcR results in the loss of the ability to induce autoimmune disease even though cytotoxic T cells (CTLs) are being generated [17]. They infected P14 mice with LCMV variants expressing altered GP33 sequences and demonstrated that a fivefold reduction of the affinity of the P14 TcR to an altered GP33 peptide/MHC complex resulted in a 50% reduction of the T1D incidence and a reduction by a factor of 20 completely abrogated disease. Surprisingly, infected mice still generated functional CTLs and developed insulitis [17]. The data indicate that the local infection of the pancreas caused inflammation and attraction of functional CTLs at a high frequency. However, due to their low avidity, infiltrated autoaggressive cells failed to cause sufficient damage for autoimmune disease.
Figure 1. RIP-LCMV model: Thymic expression of the target antigen influences onset of autoimmune disease.
LCMV-GP in exclusively expressed in the pancreas of RIP-LCMV-GP mice (C57BL/6 background). In contrast, LCMV-NP is expressed in the thymus of RIP-LCMV-NP mice (Balb/c background) as well, which results in the deletion of the higher avidity LCMV-NP-specific CD8 T cells. The remaining LCMV-NP118-specific CD8 T cells are of low avidity and cause delayed-onset of T1D after LCMV-infection of RIP-LCMV-NP mice when compared to RIP-LCMV-GP mice. In addition, RIP-LCMV-NP mice require ‘help’ of LCMV-NP-specific CD4 T cells for the development of T1D.
In this context, it is important to note that the generation of higher T cell avidity of T cells does not always correlate with higher autoimmune damage. Studies of Pere Santamaria’s research group demonstrated in the NOD mouse that lower avidity T cells are more pathogenic than high avidity T cells. They showed that avidity maturation of NRP-A7/H-2Kd- and islet-specific glucose-6 phosphatase catalytic subunit-related protein (IGRP)-specific T cells resulted in the generation of high avidity T cells that are significantly more diabetogenic than intermediate and low-avidity T cells [18, 19]. However, central and peripheral tolerance mechanisms limited the contribution of these high avidity islet-antigen specific T cells to the overall islet damage and β-cell killing [18, 19]. In addition, they could recently demonstrate that peptide therapy using an IGRP peptide (IGRP206-214) at doses that depleted all but the low-avidity IGRP206-214-specific T cells resulted in the abrogation of disease [20]. These data indicate that a blockade of the islet microenvironment by T cells that albeit islet antigen-specific are non-pathogenic and low-avidity might be the mechanism behind a successful peptide therapy of T1D in the NOD mouse [20].
The overall functional avidity of T cells to APCs is not only governed by the affinity of the TcR to the MHC/peptide complex, but also by adhesion molecules and the internal signal transduction machinery. Slifka and Whitton could demonstrate in an elegant study, that T cells undergo avidity maturation within the first week of virus infection that is independent of TcR affinity and adhesion molecule expression [21]. They rather found an increase in Lck expression suggesting a preassembly of the TcR signal transduction cascade and therefore allowing for an expedited T cell activation [21].
In terms of models for T1D the differences between the two RIP-LCMV mouse lines was useful, since it offered the possibility to study a variety of mechanisms involved in the immunopathogenesis of T1D as well as evaluate therapeutic intervention strategies. On the one hand the use of the fast-onset model, which has a well synchronized pathogenesis in between individual mice, allows for an exact analysis of inflammatory events occurring after virus-infection and of the transition phase between inflammation and autoimmunity [22-24]. On the other hand the slow-onset model more closely reflects a potential situation that might occur in human T1D patients, where the disease becomes overt only after a lag phase of several years following a possible triggering event in individuals at genetic risk to develop T1D. Thus, several ways of disease prevention have been tested in the slow-onset model over the last two decades covering areas such as DNA-vaccination, oral tolerance induction, cytokine or chemokine blockade and even virus infection [23, 25-29]. In addition, the use of the slow- rather than the fast-onset model allows for identification of disease-accelerating factors, such as islet-specific chemokine expression or secondary virus infection (figure 2) [30, 31].
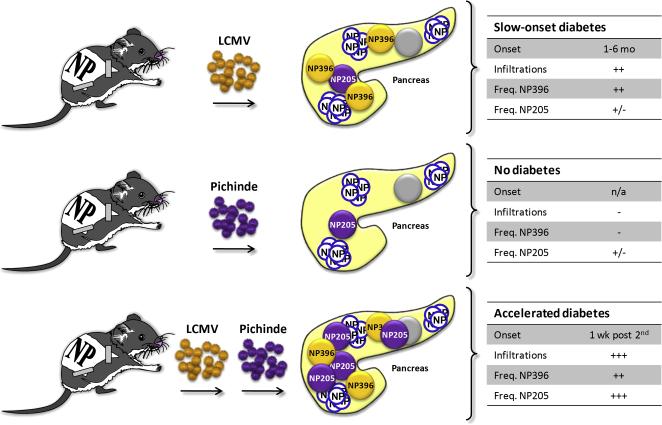
Figure 2. RIP-LCMV model: Molecular mimicry boosts the frequency of autoaggressive CD8 T cells and accelerates autoimmune disease.
Infection of RIP-LCMV-NP mice with Pichinde virus, which confers molecular mimicry to a subdominant LCMV-NP epitope (NP205) fails to induce autoimmune disease. In contrast, heterologous virus infection with LCMV followed by Pichinde virus expands LCMV-NP205-specific CD8 T cells. The presence of high frequencies of both LCMV-NP396- and LCMV-NP205-specific CD T cells rushes the ongoing autoimmune process to overt autoimmune disease.
Heterologous infection in the RIP-LCMV model: The number of self-reactive lymphocytes determines the course of disease
As important as the avidity of autoaggressive CD8 T cells can be, their number is possibly of even greater significance. As demonstrated in the RIP-LCMV model, autoimmune disease only developed when the quantity of functionally active CD8 T cells (CTLs) is exceeding a critical threshold [32]. Infection of RIP-LCMV-NP mice (Balb/c, H-2d background) with several strains of LCMV demonstrated that T1D occurred only in those mice infected with viruses eliciting a frequency of LCMV-specific CTLs of at least 1-2% of all CD8 T cells, such as LCMV-strain Armstrong or E-350. In contrast, infection with LCMV strain Pasteur and Traub, which only induce CTL frequencies of <<1% did not induce disease [32].
As shown by a series of experiments and publications by Ray Welsh and Lisa Selin the history of infections influences the overall immune repertoire and the frequency of T cells specific for individual viral components [33, 34]. Thus, heterologous infections with viruses sharing similar epitopes (molecular mimicry) might have a significant impact on the severity and/or kinetics of autoimmune disease. We have been investigating the impact of such sequential infections with viruses conferring molecular mimicry in the RIP-LCMV-NP mouse (C57BL/6, H-2b background) (figure 2). LCMV and Pichinde virus (PV) share a structural similarity in a region of the NP, which constitutes an H-2Kb-restricted subdominant epitope in wildtype C57BL/6 mice after single infection with LCMV or PV. LCMV-NP205-212 (YTVKYPNL) shares 6 out of 8 aminoacids with PV-NP205-212 (YTVKFPNM). Single infection of mice with either LCMV or PV results in the generation of ~1% LCMV-NP205- or PV-NP205-specific CD8 T cells [30, 33, 34]. In contrast, the frequencies CD8 T cells recognizing the individual immunodominant epitopes LCMV-GP33, LCMV-NP396 and PV-NP38 are much higher, ranging from 10%-20% [15, 30, 33, 34]. Infection of RIP-LCMV-NP mice with LCMV containing the immunodominant LCMV-NP396 as well as the subdominant LCMV-NP205 epitopes results in a slow-onset T1D [15, 30]. In contrast, infection of RIP-LCMV-NP mice with PV, which contains only the structural similar PV-NP205 epitopes does not induce T1D [30]. Thus, the frequency of ~1% of LCMV-NP-specific CD8 T cells induced by molecular mimicry of a subdominant epitope is not sufficient to induce autoimmune disease in the RIP-LCMV-NP mouse (C57BL/6, H-2b background). Similar to the infection of RIP-LCMV-NP mice (Balb/c, H-2d background) with the LCMV strains Pasteur and Traub [32], autoimmunity develops and cross-reactive CTL are generated but their frequency is too low to induce clinical disease. Here, the ‘glass of molecular mimicry’ is half empty.
However, heterologous infections with LCMV followed by PV have demonstrated that molecular mimicry can accelerate the ongoing autoimmune destruction (figure 2) [30]. The reason for this acceleration lies in the expansion of LCMV-NP205-specific memory CD8 T cells after secondary infection of LCMV-immune RIP-LCMV-NP mice with PV. The frequency of LCMV-NP205-specific CD8 T cells increased from ~1% (single infection with LCMV or PV) to over 10% of all CD8 T cells, which is approximately the same frequency as CD8 T cells recognizing the immunodominant LCMV-NP396 epitope [30]. This increase in frequency of LCMV-specific CD8 T cells (LCMV-NP396 plus LCMV-NP205) resulted in a dramatic acceleration of T1D in mice that received heterologous infections with LCMV and PV. As early as one week after secondary infection with PV, most of the LCMV-immune RIP-LCMV-NP mice turned diabetic, whereas mice that did not receive a secondary infection remained non-diabetic for several weeks [30]. These data confirm that the frequency of autoaggressive T cells is of central importance for the transition from autoimmunity to autoimmune disease. Infection with a heterologous virus resulted in a boost of the frequency of autoaggressive T cells above a critical threshold and therefore dramatically accelerated the course of disease. Thus, it might be important to consider multiple rather than just one single triggering event to be involved in the etiology of autoimmune diseases.
In this context it might be important to realize that the memory CD8 T cell compartment is rather flexible and is able to grow in size with immunological experience [35]. Vezys et al could show that challenge of the memory compartment with several heterologous virus infections did not result in a displacement of previously generated memory CD8 T cells. The memory compartment has rather a certain plasticity and can grow in size with additional immunological experience [35]. This indicates that T cell memory seems not to forget triggering events that may have occurred at any time in the past and resulted in a low frequency of autoaggressive T cells insufficient to induce autoimmune disease. Thus, multiple triggering events occurring over an extended period of time may nevertheless result in an accumulation of autoaggressive T cells over a critical threshold.
The CYP2D6 mouse model for autoimmune hepatitis: Is mimicry better than molecular identity?
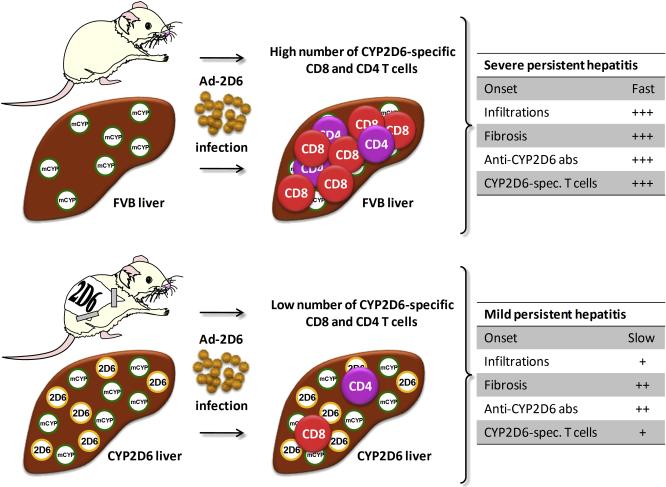
As discussed previously, the traditional RIP-LCMV model for T1D uses identical molecules as trigger and target antigens. Experiments with a heterologous virus infection demonstrated that molecular mimicry of a subdominant LCMV-epitope accelerated an already ongoing autoimmune process but was not sufficient for a de novo induction of autoimmune disease. Thus, in the RIP-LCMV model molecular identity between the triggering virus and the target antigen seems to be required to induce T1D. However, in other animal models for human autoimmune diseases the presence of a structural similarity rather than identity has proven to be more efficient in breaking self-tolerance to the target antigen. We have recently generated a novel mouse model for human autoimmune hepatitis [36]. As a trigger for the autoimmune liver damage we used an Adenovirus (Ad-2D6) expressing the human cytochrome P450 2D6 (CYP2D6), which is the major natural autoantigen in autoimmune hepatitis (AIH) type 2 [37, 38]. As targets we used either wildtype FVB mice, which express mouse cytochrome P450 isoenzymes with a structural and sequential similarity to human CYP2D6 (molecular mimicry), or transgenic CYP2D6 mice [39], with express in addition the identical human CYP2D6 (molecular identity). Infection of FVB mice with Ad-2D6 resulted in the rapid development (onset 2-4 week post-infection) of a persistent form autoimmune hepatitis, which was characterized by widespread infiltration of the liver by mononuclear cells, massive fibrosis, and generation of anti-CYP2D6 antibodies and CYP2D6-specific T cells [36]. In contrast, liver damage and fibrosis progressed significantly slower (onset 4-8 weeks post-infection) in transgenic CYP2D6 mice, which exhibited less infiltration and reduced anti-CYP2D6 antibody titers compared to Ad-2D6-infected FVB mice [36]. The most striking difference in the immune response to Ad-2D6 challenge between the ‘molecular mimicry’ vs. the ‘molecular identity’ model was the frequency of CYP2D6-specific T cells, while the quality of the CYP2D6 T cell response appeared similar in FVB and CYP2D6 mice, which both recognize several CD8 T cell epitopes and one single CD4 T cell epitope. In contrast, the frequency of CYP2D6-specific CD8 T cells in the liver and the spleen of CYP2D6 mice, where autoimmune hepatitis is triggered by molecular identity, was 10-20 times lower than in wildtype FVB mice, where cross-reactivity between mouse and human 2D6 elicits autoimmunity (molecular mimicry) [36, 40]. Thus, it is most likely the difference in the frequency of CYP2D6-specific CD4 and CD8 T cells, rather than anti-CYP2D6 antibody titer or overall inflammation of the liver that accounts for the reduced severity and delayed onset of disease in humanized CYP2D6 mice compared with FVB mice [40]. It still remains to be investigated how possible differences in the affinities of the wildtype vs. transgenic TcRs to the MHC/human CYP2D6 peptide and MHC/mouse CYP2D6 homologues influence the course of disease. From these studies we can conclude that molecular mimicry might, in some cases, be more efficient than molecular identity to trigger autoimmunity, because tolerance induction to ‘almost-self’ antigens can be less efficient than tolerance induction to true autoantigens. In the CYP2D6 mouse model for autoimmune hepatitis, the ‘glass of molecular mimicry’ is half full’.
Summary
The number self-reactive lymphocytes appears to be of paramount importance for the pathogenesis of autoimmune diseases. Animal models demonstrate that overt disease only develops, when a critical threshold of autoaggressive lymphocytes has been generated. Depending on the individual model and the antigens used, a sufficiently high frequency of autoaggressive lymphocytes is induced by infection with a pathogen bearing structures which are identical or similar to the target antigen (Table 1). RIP-LCMV mice developed T1D only after infection with LCMV strains that elicit an adequate CTL frequency of >1% [32]. Further, heterologous virus infection experiments demonstrated that in the RIP-LCMV model infection with a virus conferring true molecular mimicry of a subdominant epitope (LCMV/PV-NP205) was insufficient to induce T1D. However, by increasing the total number of LCMV-NP-specific autoaggressive CD T cells (LCMV-NP396 and LCMV/PV-NP205) secondary infection with a virus sharing structural similarities with the primary infecting virus and the host an already ongoing autoimmune process can be rapidly accelerated to result in overt autoimmune disease (figure 2). Thus, at least in the RIP-LCMV model a ‘perfect fit’ of the trigger and the target is required for the de novo induction of autoimmune disease.
Table 1.
Molecular mimicry between trigger and target
| Mouse | Target | Trigger | Mimicry | T cell frequency | T cell avidity | Antibody titer | Autoimmune Disease |
|---|---|---|---|---|---|---|---|
| RIP-LCMV | LCMV-GP | LCMV | identical | high | high | n.d | +++ |
| RIP-LCMV | LCMV-NP | LCMV | identical | high | low | n.d | + |
| RIP-LCMV | LCMV-NP | PV | similar | low | n.d. | n.d | - |
| RIP-LCMV | LCMV-NP | LCMV + PV |
identical + similar |
very high | n.d | n.d | +++ |
| FVB | mouse CYP | hCYP2D6 (Ad-2D6) |
similar | high | n.d | very high | +++ |
| hCYP2D6 | mouse CYP + hCYP2D6 |
hCYP2D6 (Ad-2D6) |
similar + identical |
very low | n.d | high | + |
n.d.: not determined
In contrast, in the CYP2D6 model for autoimmune hepatitis, mice with structural similarity to the invading pathogen seem to be more susceptible to autoimmune liver disease than mice that share identical structures (figure 3). The reason for this might be a stronger peripheral tolerance to the triggering factor, when the degree of similarity reaches identity. The occurrence of molecular mimicry between trigger and target seems to circumvent peripheral tolerance to the target antigen resulting in the generation of a high frequency of target antigen-specific T cells, which surpasses a critical threshold for induction or acceleration of autoimmune disease. The discrepancy between the mouse models for autoimmune diseases affecting the pancreas or the liver might be explained by the presence of several peripheral tolerance mechanisms, which protect the liver from excessive autoimmune damage. Both organs the liver as well as the pancreas are target of potential environmental triggering factors for autoimmune diseases, such as Hepatitis-, Coxsackie- and Herpes simplex viruses and many more. However, because of its function as a detoxifying and drug metabolizing organ the liver is prone to cellular destruction and neo-antigen formation (i.e. protein-adduct formation by reactive metabolites [41, 42]). It has further been suggested that the liver may act as a sink for activated lymphocytes [43, 44]. Several mechanisms for the maintenance of a tight hepatic tolerance by the liver microenvironment have been suggested in the past, including T-cell inactivation by antigenic priming in the liver [45], tolerance induction via cross-presentation by liver sinusoidal endothelial cells (LSEC) [46], induction of regulatory T-cells [47, 48] and hepatic stellate cell (HSC) induced T-cell apoptosis [49].
Figure 3. CYP2D6 model: Molecular mimicry induces a higher frequency of autoaggressive lymphocytes than molecular identity.
The CYP2D6 model for autoimmune hepatitis uses an Adenovirus expressing the natural human autoantigen CYP2D6 as a trigger. Infection wildtype FVB mice expressing the mouse homologues of CYP2D6 only develop massive form of chronic autoimmune hepatitis. In contrast, transgenic CYP2D6 mice, which in addition to the similar mouse CYP2D6 homologues express the identical human CYP2D6 in the liver, develop a significantly milder form of hepatitis with a delayed kinetics. Most strikingly, CYP2D6 mice generate a 10-20 times lower frequencies of CYP2D6-specific CD8 and CD4 T cells.
The avidity of the T cell to the target cell is another factor to be considered. The two RIP-LCMV models for fast-(RIP-LCMV-GP, C57BL/6 background) and slow-onset (RIP-LCMV-NP, Balb/c background) T1D demonstrate that even though similar frequencies of autoaggressive CD8 T cells are generated after LCMV-infection, only the presence of high-avidity T cells results in the rapid destruction of the majority of β-cell in the pancreas. In contrast, thymic expression of LCMV-NP prevents the release of high-avidity LCMV-NP-specific T cells from the thymus into the periphery resulting in a massively delayed kinetic of T1D in RIP-LCMV-NP mice (figure 1). Studies in the NOD mouse showed that high avidity T cells are indeed more pathogenic per se, but are often deleted or functionally silenced by central and/or peripheral tolerance mechanisms before they can cause damage [18, 19].
It is important to note that besides genetic predisposition and environmental factors, local inflammation might play a key role in the transition from autoimmunity to autoimmune disease. Both the RIP-LCMV as well as the CYP2D6 model require the direct infection of the target organ by the triggering virus, which results in a massive local inflammation. Blockade of critical cytokines or chemokines, results in a reduced frequency of autoaggressive T cells within the target organ, inhibits the destructive process and abrogates disease [23, 24, 27]. In addition, just like the balance between pro- and anti-inflammatory chemokines and cytokines, which determine the local inflammatory milieu or the proportion of apoptotic and anti-apoptotic signals influencing the fate of a cell, the ratio between aggressive and regulatory T cells (Treg) will be as important as the absolute number of autoaggressive lymphocytes. Thus, current therapeutic intervention protocols that aim to generate antigen-specific Treg need to consider both the avidity and the frequency of the Treg. Although a change of the avidity of already generated autoaggressive T cell population seems to be a very difficult endeavor, recent developments in peptide therapy of T1D demonstrated that administration of autoantigenic peptides at the correct dose modulates the overall avidity maturation and results in a depletion of high-affinity islet-antigen-specific T cells [20]. Interestingly, the remaining low avidity T cells seem to clog the islet microenvironment preventing additional damage for an extended period of time. In addition, a combinatory treatment, which on the one hand reduces the frequency of autoaggressive T cells (i.e. by depletion using anti-CD3 antibodies) and on the other hand induces Tregs, seems to be a reasonable goal as well. Indeed, such an approach has been proven to work in two models for T1D [25] and is currently evaluated for clinical trials in T1D patients.
Acknowledgments
U.C. is supported by the American Liver Foundation (Liver Fellow), a NIH grant R21 DK071577 and a grant of the German Research Foundation. M.v.H. is supported by NIAID Po1 grant.
Abbreviations
- GBS
Guillain Barré syndrome
- PDC
pyruvate dehydrogenase complex
- PBC
primary biliary cirrhosis
- 1-OA
2-octoynoic acid
- AIH
autoimmune hepatitis
- T1D
type 1 diabetes
- LCMV
lymphocytic choriomeningitis virus
- GP
glycoprotein
- NP
nucleoprotein
- RIP
rat insulin promoter
- NOD
non-obese diabetic
- IGRP
islet-specific glucose-6 phosphatase catalytic subunit-related protein
- PV
Pichinde virus
- CYP2D6
cytochrome P450 2D6
- Ad-2D6
Adenovirus expressing CYP2D6
- LSEC
liver sinusoidal endothelial cells
- HSC
hepatic stellate cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no competing financial interests.
References
- 1.Christen U, Herrath MG. Initiation of autoimmunity. Curr Opin Immunol. 2004;16:759–67. doi: 10.1016/j.coi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Christen U, von Herrath MG. Infections and autoimmunity--good or bad? J Immunol. 2005;174:7481–6. doi: 10.4049/jimmunol.174.12.7481. [DOI] [PubMed] [Google Scholar]
- 3.Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155:1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srinivasappa J, Saegusa J, Prabhakar BS, Gentry MK, Buchmeier MJ, Wiktor TJ, Koprowski H, Oldstone MB, Notkins AL. Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol. 1986;57:397–401. doi: 10.1128/jvi.57.1.397-401.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christen U, von Herrath MG. Induction, acceleration or prevention of autoimmunity by molecular mimicry. Mol Immunol. 2004;40:1113–20. doi: 10.1016/j.molimm.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Ang CW, Jacobs BC, Laman JD. The Guillain-Barre syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25:61–6. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–20. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- 8.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–45. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 9.Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH, et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–7. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 10.Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, Kurth MJ, Nantz MH, Ansari AA, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–83. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, Yoshida K, Yang GX, Hibi T, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–40. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldstone MBA, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: Role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 13.van der Most RG, Murali-Krishna K, Whitton JL, Oseroff C, Alexander J, Southwood S, Sidney J, Chesnut RW, Sette A, Ahmed R. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology. 1998;240:158–67. doi: 10.1006/viro.1997.8934. [DOI] [PubMed] [Google Scholar]
- 14.von Herrath MG, Dockter J, Nerenberg M, Gairin JE, Oldstone MB. Thymic selection and adaptability of cytotoxic T lymphocyte responses in transgenic mice expressing a viral protein in the thymus. J Exp Med. 1994;180:1901–10. doi: 10.1084/jem.180.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Herrath MG, Dockter J, Oldstone MBA. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 16.Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179:2952–60. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 17.Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, et al. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–9. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 18.Amrani A, Verdaguer J, Serra P, Tafuro S, Tan R, Santamaria P. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406:739–42. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 19.Han B, Serra P, Yamanouchi J, Amrani A, Elliott JF, Dickie P, Dilorenzo TP, Santamaria P. Developmental control of CD8 T cell-avidity maturation in autoimmune diabetes. J Clin Invest. 2005;115:1879–87. doi: 10.1172/JCI24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han B, Serra P, Amrani A, Yamanouchi J, Maree AF, Edelstein-Keshet L, Santamaria P. Prevention of diabetes by manipulation of anti-IGRP autoimmunity: high efficiency of a low-affinity peptide. Nat Med. 2005;11:645–52. doi: 10.1038/nm1250. [DOI] [PubMed] [Google Scholar]
- 21.Slifka MK, Whitton JL. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–7. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 22.von Herrath M, Holz A. Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM. J Autoimmun. 1997;10:231–8. doi: 10.1006/jaut.1997.0131. [DOI] [PubMed] [Google Scholar]
- 23.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol. 2003;171:6838–45. doi: 10.4049/jimmunol.171.12.6838. [DOI] [PubMed] [Google Scholar]
- 24.Seewaldt S, Thomas H, Ejrnaes M, Christen U, Wolfe T, Rodrigo E, Coon B, Michelsen B, Kay T, von Herrath MG. Virus-induced autoimune diabetes: Most b-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) CTL. Diabetes. 2000;49:1801–1809. doi: 10.2337/diabetes.49.11.1801. [DOI] [PubMed] [Google Scholar]
- 25.Bresson D, Togher L, Rodrigo E, Chen Y, Bluestone JA, Herold KC, von Herrath M. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J Clin Invest. 2006;116:1371–81. doi: 10.1172/JCI27191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christen U, Benke D, Wolfe T, Rodrigo E, Rhode A, Hughes AC, Oldstone MB, Von Herrath MG. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113:74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christen U, Wolfe T, Mohrle U, Hughes AC, Rodrigo E, Green EA, Flavell RA, von Herrath MG. A dual role for TNF-alpha in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J Immunol. 2001;166:7023–32. doi: 10.4049/jimmunol.166.12.7023. [DOI] [PubMed] [Google Scholar]
- 28.Coon B, An L-L, Whitton JL, von Herrath MG. DNA immunization to prevent autoimmune diabetes. J Clin Invest. 1999;104:189–194. doi: 10.1172/JCI7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Homann D, Jahreis A, Wolfe T, Hughes A, Coon B, van Stipdonk MJ, Prilliman KR, Schoenberger SP, von Herrath MG. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity. 2002;16:403–15. doi: 10.1016/s1074-7613(02)00290-x. [DOI] [PubMed] [Google Scholar]
- 30.Christen U, Edelmann KH, McGavern DB, Wolfe T, Coon B, Teague MK, Miller SD, Oldstone MB, von Herrath MG. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest. 2004;114:1290–1298. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhode A, Pauza ME, Barral AM, Rodrigo E, Oldstone MB, von Herrath MG, Christen U. Islet-specific expression of CXCL10 causes spontaneous islet infiltration and accelerates diabetes development. J Immunol. 2005;175:3516–24. doi: 10.4049/jimmunol.175.6.3516. [DOI] [PubMed] [Google Scholar]
- 32.Sevilla N, Homann D, von Herrath M, Rodriguez F, Harkins S, Whitton JL, Oldstone MB. Virus-induced diabetes in a transgenic model: role of cross-reacting viruses and quantitation of effector T cells needed to cause disease. J Virol. 2000;74:3284–92. doi: 10.1128/jvi.74.7.3284-3292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–34. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 34.Selin LK, Lin MY, Kraemer KA, Pardoll DM, Schneck JP, Varga SM, Santolucito PA, Pinto AK, Welsh RM. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–42. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 35.Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–9. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- 36.Holdener M, Hintermann E, Bayer M, Rhode A, Rodrigo E, Hintereder G, Johnson EF, Gonzalez FJ, Pfeilschifter J, Manns MP, et al. Breaking tolerance to the natural human liver autoantigen cytochrome P450 2D6 by virus infection. J Exp Med. 2008;205:1409–22. doi: 10.1084/jem.20071859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manns MP, Johnson EF, Griffin KJ, Tan EM, Sullivan KF. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450db1. J Clin Invest. 1989;83:1066–72. doi: 10.1172/JCI113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zanger UM, Hauri HP, Loeper J, Homberg JC, Meyer UA. Antibodies against human cytochrome P-450db1 in autoimmune hepatitis type II. Proc Natl Acad Sci U S A. 1988;85:8256–60. doi: 10.1073/pnas.85.21.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR, Gonzalez FJ. The CYP2D6 Humanized Mouse: Effect of the Human CYP2D6 Transgene and HNF4alpha on the Disposition of Debrisoquine in the Mouse. Mol Pharmacol. 2001;60:1260–1267. doi: 10.1124/mol.60.6.1260. [DOI] [PubMed] [Google Scholar]
- 40.Holdener M, Bogdanos DP, Christen S, Bayer M, Pfeilschifter J, Hintermann E, Christen U. The CYP2D6 model for autoimmune hepatitis: Characterization of the CD4 and CD8 T cells response. 2009 manuscript submitted. [Google Scholar]
- 41.Nelson SD, Pearson PG. Covalent and noncovalent interactions in acute lethal cell injury caused by chemicals. Annu Rev Pharmacol Toxicol. 1990;30:169–95. doi: 10.1146/annurev.pa.30.040190.001125. [DOI] [PubMed] [Google Scholar]
- 42.Park BK, Kitteringham NR. Drug-protein conjugation and its immunological consequences. Drug Metab Rev. 1990;22:87–144. doi: 10.3109/03602539008991445. [DOI] [PubMed] [Google Scholar]
- 43.Mehal WZ, Juedes AE, Crispe IN. Selective retention of activated CD8+ T cells by the normal liver. J Immunol. 1999;163:3202–10. [PubMed] [Google Scholar]
- 44.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 45.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–12. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–54. doi: 10.1038/82161. [DOI] [PubMed] [Google Scholar]
- 47.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 48.Luth S, Huber S, Schramm C, Buch T, Zander S, Stadelmann C, Bruck W, Wraith DC, Herkel J, Lohse AW. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118:3403–10. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CH, Kuo LM, Chang Y, Wu W, Goldbach C, Ross MA, Stolz DB, Chen L, Fung JJ, Lu L, et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]