Abstract
Background
To determine if radiofrequency ablation (RFA) can safely reduce pain from osseous metastatic disease.
Methods
A single arm prospective trial in patients with a single painful bone metastasis with unremitting pain of at least a score above 50 on a pain scale of 0–100. Percutaneous CT guided RFA of the bone metastasis to temperatures above 60 degrees Celsius was performed.
Endpoints were the toxicity and pain effects of RFA before and at 2 weeks, one and three months after RFA.
Results
55 patients completed RFA. Grade 3 toxicities occurred in 3 of 55 patients (5%). RFA reduced pain at 1- and 3-month for all pain assessment measures. The average increase in pain relief from pre-RFA to 1-month follow-up is 26.27 (95% CI, 17.65 to 34.89, P<0.0001) and the increase from pre-RFA to 3-month follow-up is 16.38 (95% CI, 3.37 to 29.39, P=0.02). The average decrease in pain intensity from pre-RFA to 1-month follow-up was 26.9 (P<0.0001) and 14.2 for 3-month follow-up (P=0.02). The odds of being in lower pain severity at 1-month follow-up is 14.03 (95% CI, 2.33 to 25.73, P<0.0001) times higher than that at pre-RFA, and the odds at 3-month follow-up is 8.00 (95% CI, 0.85 to 15.15, P<0.001) times higher than that at pre-RFA. The average increase in mood from pre-RFA to 1-month follow-up was 19.9 (P<0.0001) and 14.9 for 3-month follow-up (P=0.005).
Conclusion
This cooperative group trial strongly suggests that RFA can safely palliate pain from bone metastases.
Keywords: bone metastases, radiofrequency ablation, pain palliation, tumor ablation
INTRODUCTION
Metastatic cancer is the most common neoplasm involving the skeletal system.1 Pain from bone metastases can be related to mechanical or chemical factors. Pressure effects on the periosteum or adjacent neural structures can cause local or radiating pain.2 Primary treatment has relied upon radiation therapy3 with or without systemic chemotherapy or hormonal therapy. Newer systemic treatments with radionuclides4–5 and bisphosphonates6–7 have also shown some success. Many prospective trials have been performed studying the ability of external beam radiation therapy to palliate pain or control progression of osseous metastatic disease.8–16 Meta-analysis of radiotherapy data have revealed that one month after treatment over 40% of patients can expect a 50% reduction in pain and fewer than 30% can expect complete pain relief.17 Despite the availability of effective treatments many studies have documented the under treatment of pain in cancer patients18 is not always completely effective and many patients live with inadequate analgesia requiring increasing doses of narcotics. Radiofrequency ablation (RFA) is an image-guided minimally invasive treatment for solid tumors. Patients that have not responded to conventional treatment, have a contraindication to initial or repeat radiation or have limited disease may benefit from palliation with RFA. RFA has been used for patients that have persistent pain from a solitary focus of metastatic disease that has been previously treated or in localized disease where a more local ablative therapy can be performed as an alternative to external beam radiotherapy.19–20 The hypothesis is RFA can safely reduce pain as measured by multiple pain scale parameters. This multi-center trial was planned to determine the safety and toxicity of RFA as well as the palliative efficacy in patients with pain from a dominant site of osseous metastatic disease.
Patients
Patients were required to have pathologically-confirmed malignant disease with a bone lesion that has the clinical and imaging features of metastatic disease. Patients with primary musculoskeletal malignancies, lymphoma and leukemia were not eligible. Pain was required to be from a solitary site of metastatic disease to the bone although patients may have had subclinical bone metastases in other areas and the painful site had to be amenable to RFA utilizing a percutaneous CT-guided approach defined as a location in which a radiofrequency electrode could be safely placed without significant harm to normal structures. To avoid damage to contiguous vital structures certain criteria had to be met. Tumors within 3cm of a major neurovascular bundle required continuous motor nerve testing during the ablation to reduce thermal nerve toxicity. The tumor mass could not come in contact with hollow viscera. Patients were not eligible if the tumor involved a weight-bearing long-bone of the lower extremity. The site of the tumor could not have been previously surgically stabilized with metallic hardware. Patients with spinal tumors were eligible if they had an intact cortex between the mass and the spinal canal and exiting nerve roots. Patients were required to have intractable pain that resulted in a return visit to the oncologist. Intractable pain was defined as unremitting pain despite active treatment with narcotics by their medical oncologist. The measurable pain must be above a pain scale of 50 based on a 1–100 scale. The maximum size of the bone metastasis had to be no greater than 8cm. Patients could not have a pacemaker. Patients were required to have a platelet count > 70,000/ul and no uncontrolled coagulopathy or bleeding diatheses. Previous treatment with bisphosphonates, or radiotherapy (radionuclide or external beam) was not an exclusion for this study. Patients could not have had previous radiation within 30 days. Chemotherapy was not allowed within 14 days prior to and within 14 days post- RFA. All patients underwent a physical examination, laboratory assessment and imaging of the bone metastases by CT or MRI within 2 weeks of RFA. Patients had to be cognitively intact. All patients signed informed written consent according to institutional and federal guidelines.
Methods
Aspirin and nonsteroidal anti-inflammatory medications, antiplatelet medications, or warfarin was discontinued prior to the RFA for a time period that was appropriate to the drug half life. Low molecular weight heparin preparations were discontinued 24 hours prior to procedure.
Radiofrequency ablation was performed utilizing a Radionics CC-1 (Valley Lab, Boulder, CO) radiofrequency generator and single 17-gauge or cluster Cool-tip electrode. RFA was performed under conscious sedation (midazolam (Abbott Laboratories; North Chicago, IL) and fentanyl citrate (Abbott Laboratories; North Chicago, IL). with monitoring by continuous pulse oximetry with blood pressure performed every 5 minutes. General endotracheal anesthesia or monitored anesthesia care was allowed in cases where tumors were not within 3cm of a major neurovascular bundle when clinically appropriate as deemed by the site investigator. Computerized tomography was used to localize the metastasis. Local anesthesia involved 1% lidocaine both intradermally and around the periosteum. A 14-gauge coaxial bone biopsy needle (Ackerman, Cook, Bloomington, IN) was placed into the lesion if the cortical bone was intact. After the core was removed, the inner trephine needle was removed, and the RF electrode was placed through the outer cannula into the metastasis. For tumors that destroyed the bone cortex, the RF electrode was placed directly into the metastasis (Figure 2). Tumors over 4cm in size were treated with a cluster RF electrode consisting of three 17-gauge needles spaced 5mm apart. A cluster electrode creates a spheroid ablation with diameters of thermocoagulation that range from 3–7cm in diameter and 3.5cm in length depending upon tumor vascularity and tissue dielectrical properties. Tumors smaller than 4cm were treated with single RF electrodes (1, 2 or 3cm active tips).; tumors smaller than 2cm were treated with a 1cm active tip; tumors between 2 and 3cm were treated with a 2cm active tip; and tumors 3–4cm were treated with a 3cm active tip length. Single electrodes create elliptical ablations that range in diameter from 2–3cm with lengths approximately 1cm greater than the active tip length. The initial ablation was performed for no longer than 4 minutes utilizing the maximum allowable current given the impedance of the system (typical range 1100–2000mA). For larger lesions over 4cm the goal of the ablation is to focus closer to the margins on the tumor-bone interface. For smaller lesions less peripheral placement can achieve the therapeutic goal of treating the bone/tumor interface. Intratumoral temperature measurements after each ablation ensure adequate thermocoagulation of the metasastasis.
Figure 2.
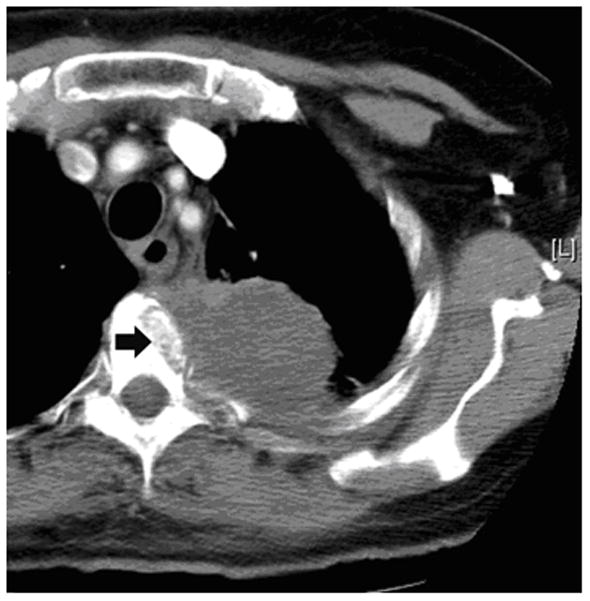
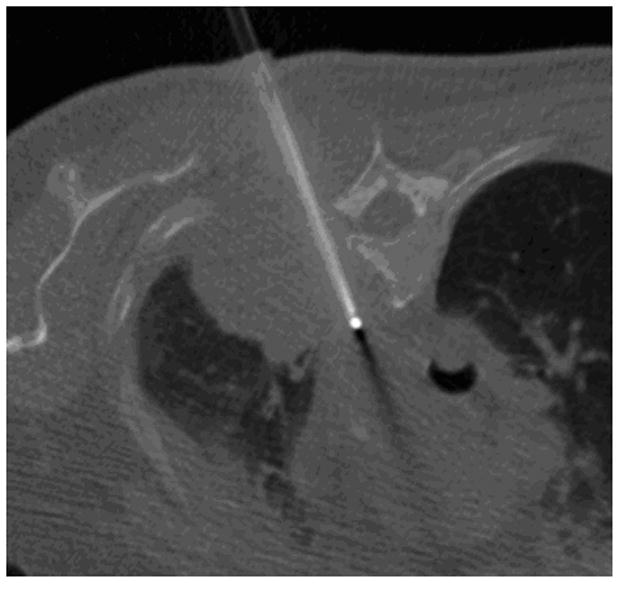
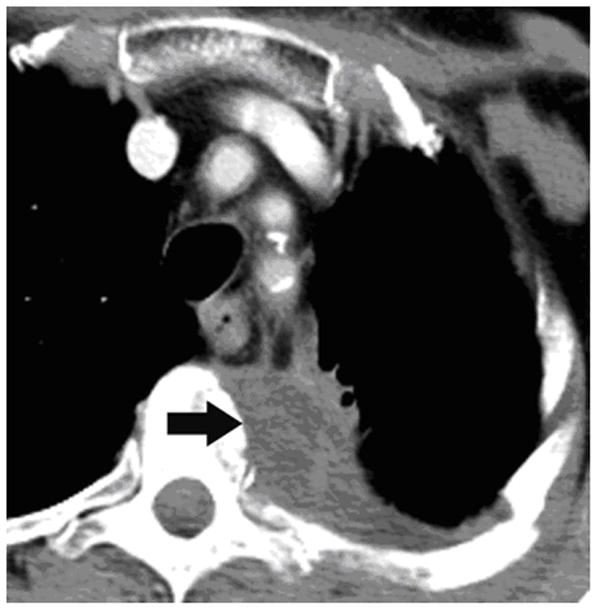
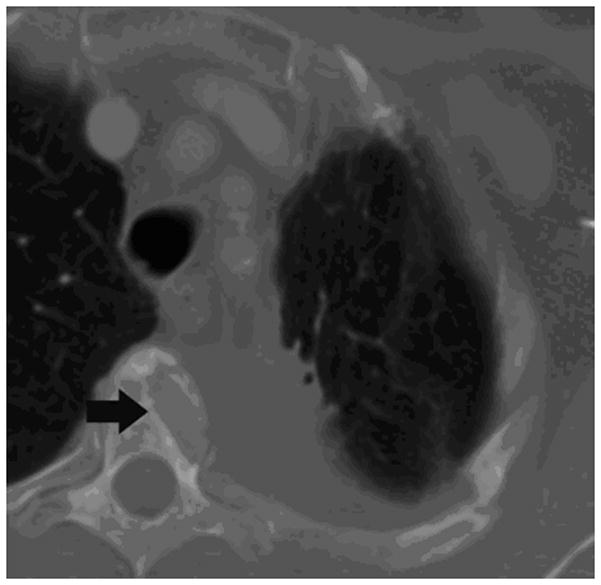
62-year old woman with T4 NSCLC status post prior radiation and chemotherapy. The patient had persistent unremitting pain. Supine CT image (a) shows the large lung mass involving the T4 vertebral body (arrow). RFA of bone-tumor interface was performed under CT-guided fluoroscopy (b,). The patient tolerated the procedure well and her pain improved dramatically. Follow-up CT images 2 years later in soft tissue (c) and bone windows (d) show mass necrosis (arrow) and partial remineralization of the bone destruction (arrow). The patient remains pain free over 2 years after RFA.
The maximal intratumoral temperature was recorded and a target intratumoral temperature greater than 60°C was required to be obtained as an indicator of adequate thermocoagulation. If the temperature exceeded 60°C, the RF electrode was withdrawn in increments of 1cm up to the length of the active tip (e.g., three 1cm increments for a 3cm active tip single electrode or 3cm for a 2.5cm active tip cluster electrode) while measuring the intratumoral temperature. If the temperature fell below 60°C and the RF electrode was still within the tumor, then another 4-minute treatment was performed at the new position. If after the first 4-minute treatment the maximum intratumoral temperature did not exceed 60°C, then an additional 4-minute treatment was performed at the same position. This could be repeated for a maximum time of 12 minutes (3 treatments) at any given electrode position. After the entire longitudinal dimension of the tumor is treated with a series of overlapping temperature-based treatments, the RF electrode is positioned into a new portion of tumor such that the electrode shaft is 1.5–2cm away from the longitudinal axis of the previous series of treatments. This is repeated until these cylinder-shaped treatment regions have encompassed the volume of the tumor mass. Post-RFA procedure, vital signs were monitored for a minimum of 2 hours.
Assessments
Pain assessment was measured before and after RFA using a modified Memorial Pain Assessment Card (MPAC).21 To eliminate a potential placebo effect and the regression-to-the mean phenomenon, daily pain scales were performed for 5 days prior to the procedure and 14 days after the procedure by giving the patient data sheets to record their responses. The initial two week post ablation MPAC data was only accepted if recorded the same day as the scheduled reporting. The one and three month follow-up data were acquired in the clinic setting during the imaging follow-up. A two week window was allowed for this appointment. In a small number of cases where participants were unable to attend these appointments within the window, the assessments sheets were mailed. This system has been shown to be a valid, reliable and sensitive measure of cancer pain. Four different measurement scales were utilized: pain relief (0: no relief, 100: complete relief), patient mood (0: worse, 100: best), pain intensity (0: least possible, 100: worst possible), and pain severity (1: no pain, 8: excruciating). To simplify statistical analysis the pain severity scale was created by transforming the 8 pain descriptors into a number scale based upon the severity of pain description. Patients underwent stabilization of narcotic usage one week prior to RFA. Narcotics usage, dosage and frequency were recorded in a pain medication diary and recorded daily starting from one week pre-RFA to daily for 14 days post-treatment as well as one month and three months. Patients were evaluated over a three-month period of time.
Toxicities from RFA were graded according to the National Cancer Institute’s Common Toxicity Criteria (version 3.0).
The follow-up contrast enhanced CT and MRI examinations were evaluated for the size of the treated tumor and the size of the ablation defect in three dimensions. The volume of the treated tumor and ablation volume were calculated by using a prolate ellipse equation (height × width × length × 0.52) and the percentage of the tumor volume that was ablated was calculated.
Statistical Analysis
Measurements for pain intensity, pain, and mood were each modeled with a mixed effects model with time as the predictor of primary interest. A random patient effect was assumed to account for correlation between repeated observations from the same subject. The reduction in pain markers at 1 month and 3 month post-RFA was estimated from these models along with 95% confidence intervals. To estimate the effect of RFA on pain severity reduction (an ordinal variable), cumulative logistic regression22 was fitted, which is the standard choice for ordinal response variable with multiple categories. To account for potential confounders, important covariates such as age, performance status, primary cancer site, anatomical location, narcotic score, tumor size, prior radiation therapy and percentage of tumor volume ablated were included in the regression models. To calculate the number of patients who had increased pain after the RFA procedure, the mean MPAC Pain Scale assessment score for each patient for the 14 days immediately following the RFA treatment, the single score at 1 month or the single score at 3months, was subtracted from the mean pain score for each patient for the 5 days preceding the RFA treatment. A negative value was indicative of increased pain following the RFA procedure. To assess the effect of missing data on the outcome, different models were fitted according to different missing data mechanisms and the results were compared. Specifically, the sensitivity of the model fitting was assessed to three missing data assumptions: (1) data missing completely at random (MCAR), where missing data do not depend on covariates or outcomes; (2) data missing at random (MAR), where missing data may depend on covariates and observed outcomes but not on unobserved outcomes; and (3) data missing not at random (MNAR), where missing data depend on unobserved outcomes.23 To account for MNAR, a pattern mixture (PM) model was fitted, where separate models corresponding to each missing data pattern were fit and the results were combined statistically to form the final analysis result.24 All reported p-values are two-sided. All computations were carried out with commercially available statistical software (SAS Version 9.1, Cary, NC).
RESULTS
Study population
Sixty-six participants were entered on the study. Six patients (9%) were ineligible due to primary bone cancer (n=2), chemotherapy within 14 days (n=2), ibuprofen within 24 hours (n=1), and radiation to the RFA site within 30 days of the procedure (n=1). Among the 60 eligible participants, three were medically unstable and did not undergo RFA. 1 patient did not complete the RFA procedure due to uncontrolled pain and 1 patient had EKG changes that prevented completion of the procedure. Thus, a total of 55 participants underwent RFA and were included in the primary analysis.
Patient and tumor characteristics are shown in Table 1. The median age was 62 (34–85). The mean treated tumor size was 5.2 +/−s.d. 0.23cm (range 2.0–8.0cm). Lung, renal and colon cancer were the most common types of cancers treated. The anatomical regions treated were the pelvis, chest wall, spine and extremity. Of all the tumors treated 9 had an intact cortex requiring bone needle access prior to coaxially inserting the RF electrode.
Table 1.
Patient Characteristics
| Median age: | 62 (34–85) |
| Male:Female: | 29:26 |
| Tumor size | 5.2+/− s.d. 0.23cm (2–8cm) |
| Primary Cancer | |
| Lung | 17 (30.9%) |
| Kidney | 10 (18.2%) |
| Colon | 10 (18.2%) |
| Breast | 4 (7.3%) |
| Prostate | 2 (3.6%) |
| Other | 12 (21.8%) |
| Region | |
| Pelvis | 22 (40%) |
| Chest wall | 20 (36.4%) |
| Spine | 8 (14.5%) |
| Extremity | 5 (9.1%) |
| Baseline pain values scale (0–100) | |
| Pain Intensity | |
| N | 54 |
| Minimum | 51 |
| Median | 54.00 |
| Maximum | 91 |
| Mean (sd) | 54.41 (18.53) |
| Pain Reduction | |
| N | 54 |
| Minimum | 3 |
| Median | 41.70 |
| Maximum | 95 |
| Mean (sd) | 44.12 (17.27) |
| Mood | |
| N | 55 |
| Minimum | 51 |
| Median | 46.40 |
| Maximum | 84 |
| Mean (sd) | 46.96 (17.97) |
| Pain Severity-Tursky Scale (1–8) | |
| N | 53 |
| Minimum | 4 |
| Median | 6 |
| Maximum | 8 |
| Mean (sd) | 5.55 (0.97) |
Of the 55 participants who completed RFA, 13 (23.6%) did not have the 1-month follow-up measurement and 23 (41.8%) did not have 3-month follow-up measurement. All 55 patients had completed at least one of the initial and follow-up pain and mood measures so they were included in the statistical analysis. The reasons for missing follow-up data were: MPAC data returned beyond allowable time window (2) patient could not be reached despite multiple attempts to acquire data (7), participant too ill to continue due to admission to hospice, intensive care unit or nursing home (6), participant death (4), and participant refusal (4).
Toxicities
All toxicities ≥ grade 2 are shown in Table 2. Only three patients had grade 3 toxicities including pain from RFA (n=1), neuropathic pain (n=1) and foot drop (n=1). The grade 3 neuropathic pain was related to an ablation of a metastasis to the right pelvis whereby a self-limited thermal injury to the pudendal nerve was observed which resolved at the one month follow-up visit. The extensor weakness of the foot occurred secondary to an ablation in the acetabular region. The patient had an 8cm mass which was previously radiated and already had preexisting leg weakness, avascular necrosis of the femoral head and severe osteoarthritis. This patient complained of increased leg weakness the second day after the ablation which improved with physical therapy. The patient remained pain free for two years after the ablation and was ambulating with a cane. There were no episodes of significant infection or bleeding. There were no identifiable cardiac or pulmonary toxicities.
Table 2.
Table of All Adverse Events
| Days since RFA | Grade | Description | Related to RFA |
|---|---|---|---|
| 6 | GRADE 2 | Neuropathic pain | Probable |
| 32 | GRADE 3 | Other neurology | Unrelated |
| 41 | GRADE 5 | Progressive systemic tumor | Unrelated |
| 83 | GRADE 3 | Pelvic pain | Unrelated |
| 2 | GRADE 2 | Gastrointestinal | Unrelated |
| 22 | GRADE 5 | Progressive systemic tumor | Unrelated |
| 17 | GRADE 2 | Skin grounding | Definite |
| 1 | GRADE 3 | Neuropathic pain | Definite |
| 181 | GRADE 5 | Progressive systemic tumor | Unrelated |
| 30 | GRADE 2 | Infection with neutropenia | Unknown |
| 13 | GRADE 2 | Arrhythmia | Unknown |
| 72 | GRADE 2 | Bone pain | Unknown |
| 35 | GRADE 2 | Neuropathic pain | Unknown |
| 35 | GRADE 2 | Pelvic pain | Unknown |
| 25 | GRADE 5 | Progressive systemic tumor | Unrelated |
| 80 | GRADE 5 | Progressive systemic tumor | Unrelated |
| 43 | GRADE 4 | Bone pain – disabling | Unlikely |
| 106 | GRADE 2 | Bone fracture | Possible |
| 2 | GRADE 3 | Foot drop | Definite |
Effect of RFA on pain reduction
Statistical analysis showed that the estimated effects of RFA in pain reduction were not sensitive to assumptions of the missing data mechanism. Therefore, we reported the analyses results based on the missing-at-random23 assumption for clarity and ease of interpretation. Pain scales are reported using a 100 point scale. RFA had a statistically significant effect in reducing pain at both 1- and 3-month follow-up for all 4 pain assessment measures (Fig. 1). The average increase in pain relief from pre-RFA to 1-month follow-up is 26.27 (95% CI, 17.65 to 34.89, P<0.0001) and the increase from pre-RFA to 3-month follow-up is 16.38 (95% CI, 3.37 to 29.39, P=0.02). The average increase in mood from pre-RFA to 1-month follow-up is 19.89 (95% CI, 11.85 to 27.93, P<0.0001) and the increase from pre-RFA to 3-month follow-up is 14.93 (95% CI, 5.03 to 24.83, P=0.005). The average decrease in pain intensity from pre-RFA to 1-month follow-up is 26.92 (95% CI, 17.67 to 36.17, P<0.0001) and the decrease from pre-RFA to 3-month follow-up is 14.16 (95% CI, 2.93 to 25.39, P=0.02). The odds of being in lower pain severity at 1-month follow-up is 14.03 (95% CI, 2.33 to 25.73, P<0.0001) times higher than that at pre-RFA, and the odds at 3-month follow-up is 8.00 (95% CI, 0.85 to 15.15, P<0.001) times higher than that at pre-RFA. These results are summarized in Table 3. Tumor size had a statistically significant effect on pain severity. Holding the other covariates as fixed, for every 1mm increase in tumor size the odds of being in higher pain severity was on average 1.38 (95% CI: 1.11–1.72) times higher. The mean volume of ablated tumor was 17.5cc and the mean tumor volume was 29cc for a mean percent ablated tumor volume of 60%. The correlation of pain relief, mood and pain intensity with the covariate of volume of ablated tumor was not statistically significant at 9.10 (95% CI −10.96 to 29.17, P=0.38), −12.33 (95% CI −28.35 to 3.69, P=0.14) and 10.62 (95% CI −8.10 to 29.35, P=0.27), respectively. Previous radiotherapy to the site (specific treatment to palliate the painful bone metastasis) did not statistically correlate with a reduction in pain intensity, improvement in mood and increase in pain relief at 1.45 (95% CI −12.07 to 14.98, P=0.83), 4.5 (95% CI −6.4 to 15.40, P=0.42) and −6.44 (95% CI −19.08 to −6.44, P=0.32), respectively. Increased pain during the two weeks following the RFA procedure, one month and three month follow-up period compared to pre-RFA levels occurred in 27%, 17% and 29% of patients, respectively.
Figure 1.
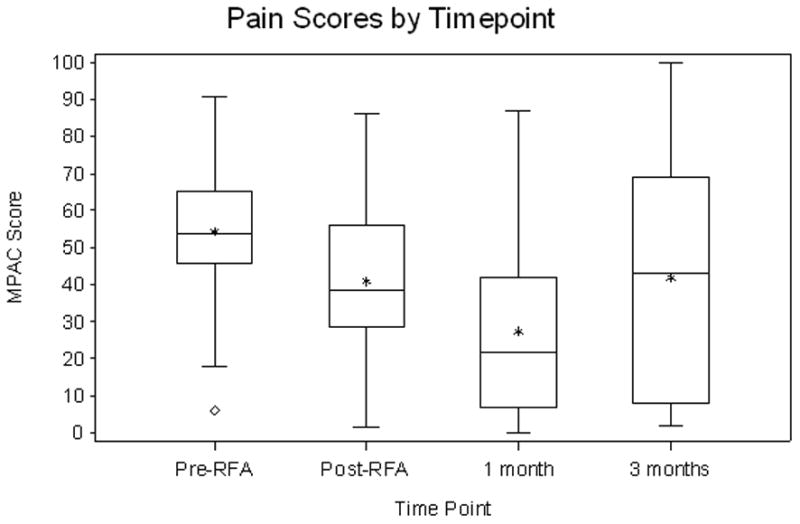
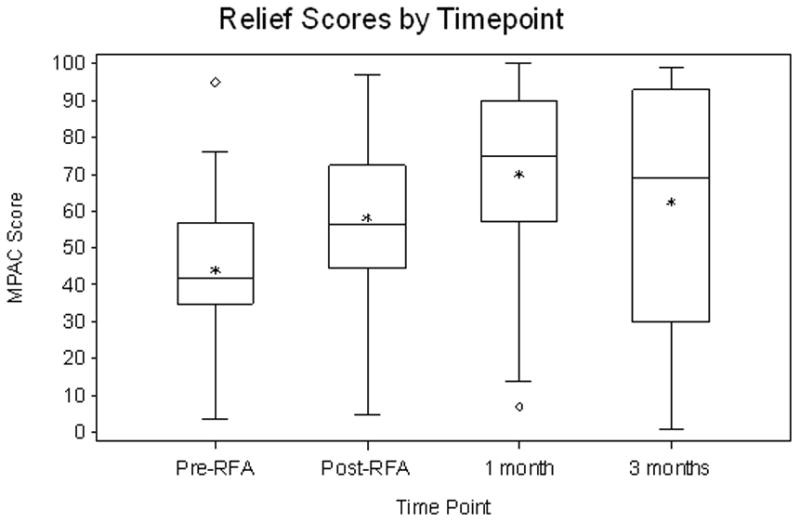
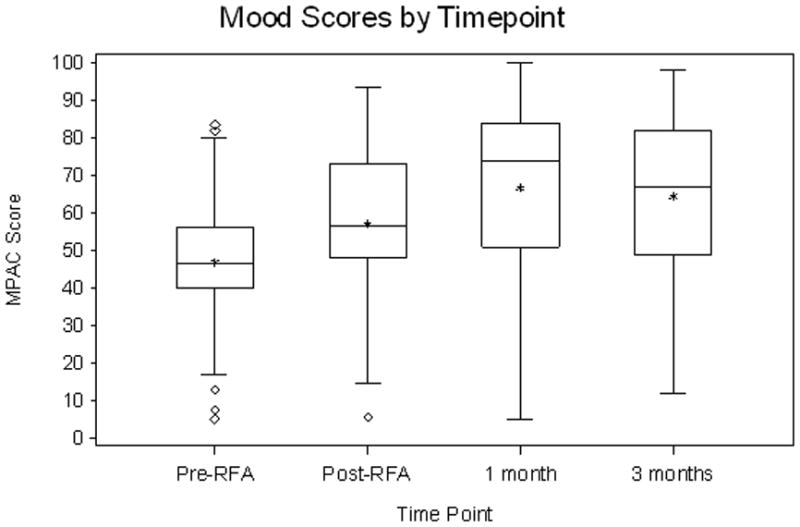
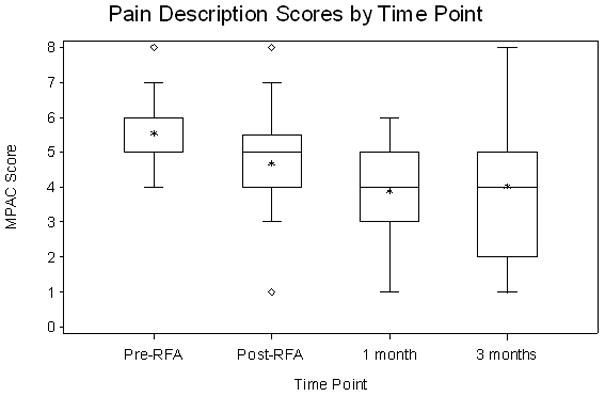
Summary box plots of the changes in pain score (a), pain relief (b), mood (c) and pain description (d).
Table 3.
Statistical Analysis
| Pre-RFA to 1-month follow-up (s.e.) | Pre-RFA to 3-month follow-up (s.e.) | |
|---|---|---|
| Pain MPAC (0–100) | −26.92 (4.72) [n=41] | −14.16 (5.73) [n=31] |
| Pain relief | 26.27 (4.40) [n=42] | 16.38 (6.64) [n=32] |
| Mood | 19.89 (4.10) [n=43] | 14.93 (5.05) [n=33] |
| Pain severity- | 14.03 (5.97) [n=42] | 8.00 (3.65) [n=32] |
DISCUSSION
Of approximately 965,000 new cancer cases each year in the United States, approximately 30–70% will develop skeletal metastases. Given the high prevalence of carcinomas of the breast, lung and prostate, these cancers account for more that 80% of cases of metastatic bone disease. Bone metastases lead to significant morbidity due to pain, pathologic fracture, and neural compression.
Apart from narcotic administration external beam radiotherapy is the primary modality for palliation of painful osseous metastases. The Radiation Therapy Oncology Group study by Tong et al8 measured cancer patient’s response to radiation therapy with a pain scale and narcotic requirement scale of 1–4. Two hundred and sixty-six of the 1016 patients studied had a solitary metastasis. The study showed a complete response rate of 54% and a partial response rate of 90%. There was a 30% relapse rate within the patients who survived at least 12 weeks and patients in the study with lung cancer or with severe constant pain at the outset tended not to improve after radiation. Madsen reported a response rate of only 48% when measuring a patient’s pain using a visual analog scale.9 As pointed out by a review of all published reports by Ratanatharathorn et al.16 the relapse after initial response is frequent, the pain relief in all studies is poor and the practices of radiation therapy need to be improved.
Surgical therapy is applied in certain instances where mechanical strengthening is necessary such as an impending fracture. These therapies are often unsuccessful in pain reduction and patients may require significant doses of narcotics. Therefore, a more effective modality of local treatment for bone metastases could substantially improve quality of life. The life expectancy of patients with osseous metastatic disease is limited with an average median survival of between 3–6 months. Therefore, finding an effective local therapy that can be done at a single outpatient sitting would be beneficial.
Percutaneous image-guided procedures for providing local tumor ablative therapy such as ethanol injection,24 vertebroplasty25 and RFA26–27 have shown some promise in the treatment of metastatic bone lesions. Percutaneous RFA is a technique that was originally pioneered decades ago for the treatment of trigeminal neuralgia.28 For the treatment of bone lesions the technique involves placing an electrode under CT guidance directly into the metastasis. The electrode is coupled to a radiofrequency generator and causes tissue necrosis by heating of adjacent tissues. The potential advantages of RFA versus other destructive methods are multifold: cell death is immediate, lesion size can be accurately controlled, lesion temperature can be monitored, electrode placement can be achieved with a percutaneous image-guided procedure, and radiofrequency lesions can be performed under local anesthesia and conscious sedation.
Today RFA is commonly used in the musculoskeletal system for treatment of intractable back pain due to failed back syndrome29 and chronic back pain due to facet joint osteoarthritis.30 In addition CT-guided RFA has been shown to be a cost effective surgical alternative in the treatment of osteoid osteomas.27 Several early studies specifically looked at RFA’s ability to reduce pain from osseous metastatic disease. Callstrom et al19 published a single-arm, paired comparison, observational study involving 12 patients with a single painful osteolytic metastasis; each patient served as their own control. Radiation therapy or chemotherapy had failed to provide pain relief. All treated lesions were osteolytic, with a combination of bone destruction and soft tissue mass. A single lesion was treated in all 12 patients. The size of the treated lesion ranged from 1 to 11 cm. One patient with a large lesion was treated in two separate sessions 6 weeks apart, and the remaining 11 patients were treated in a single RF session. Before RF ablation, the mean worst pain score in a 24-hour period in the 12 patients was 8.0 (range 6 to10). At 4 weeks post–RF treatment, the recorded mean worst pain had decreased to 3.1 (P<0.001). No major complications from RF ablation were observed in these 12 patients. The ACRIN trial is consistent with a study by Goetz et al30 which is a follow-up study from Callstroms et al’s study19 that demonstrated pain reduction in 41 of 43 patients (95%) following RFA. In that trial, a pre-procedural pain score of 7.9 was reported followed by decreasing mean worst pain scores to 4.5 (P<0.0001) at week 4, 3.0 (P<0.0001) at week 12, and 1.4 (P=0.0005) at week 24 post-RF treatment. This industry sponsored study reports highly significant and rapid reductions in pain scores as well as improvements in the quality of life for patients following RF ablation of painful bone metastases. In the ACRIN trial we collected but did not analyze the brief pain inventory scale (BPI-SF). The mean worst pain pre RFA was 7.86 and the mean worst pain at two weeks, one and three months post-RFA was 6.61, 4.93 and 4.97 respectively.
This NCI-sponsored Clinical Trials Cooperative Group phase II study demonstrates that radiofrequency ablation (RFA) can effectively palliate pain from bone metastases in patients with advanced solid tumors 1 and 3 months after the procedure. Pain was assessed by the MPAC prior to RFA. Statistically significant improvement was seen in all four measures of the MPAC: pain relief, patient mood, patient intensity and pain severity. The MPAC was chosen over other pain assessment instruments because it is simple, takes less than 30 seconds to complete and it is an efficient means of quantifying pain.31–33 Evaluating not only pain severity, but improvement in pain and mood makes it more suitable for medically ill patients undergoing treatment.
Of the 55 patients completing RFA, 13 (23.2%) did not have the 1-month follow-up measurement and 23 (41.1%) did not have the 3-month follow-up measurement. This data loss is typical for a follow-up study and is a limitation of this study and other studies of its kind. The inability to obtain more complete 3-month follow-up was due to general patient deterioration due to widespread metastatic disease. This is not uncommon for palliative studies and similar to a published drop-out rate of 32% in a recent randomized trial looking at short versus long course radiotherapy and its effects on bone pain from metastatic disease.34 In the study by Goetz et al the drop out rate was 47%30. The majority of patients on this study had progressive, refractory solid tumors that had progressed after multiple chemotherapy and radiation regimens. Despite this limitation, we were able to demonstrate statistically significant pain relief. In order to assess the effect of missing data on the outcome, we considered all possible missing data mechanisms and corresponding models were fitted. Our statistical analyses showed that the estimated effects of RFA in pain reduction were not sensitive to assumptions of the missing data mechanism.35 Hence, statistical analyses indicated that missing data did not have a systematic effect on the estimates of pain reduction.
RFA was well tolerated and the observed toxicity rate was low. Only 3 out of 55 participants (5.4%) experienced grade 3 adverse events related to RFA (95% CI: 1.9% to 17.0%). The upper confidence limit is lower than the 30% rate defined pre-study as being unacceptable which was used to determine the study sample size. Two factors may have contributed to the low toxicity. General endotracheal anesthesia or deep sedation with monitored anesthesia care was not utilized when treating tumors close to major motor nerves. This allowed sensorimotor testing during ablations where tumors were in close proximity to a major neurovascular bundle. In fact 27 of the patients had tumors within 3cm of a major neurovascular bundle yet there was only one motor nerve deficit. Neuropathic pain can occur when treating tumors near sensory nerves due to the direct toxic effect of the heat on the nerve and may occur later due to periablational inflammatory tissue that extends adjacent to a nerve. This occurred as a grade 2 in two patients (day 6 and day 35 post RFA) and a grade 3 in one patient (day one post RFA). In our experience the neuropathic pain is self limited and can be treated with gabapentin and usually resolves or improves as was the case in all three patients. Pain immediately after RFA that was greater than the baseline pre-RFA pain was observed in 27% of patients. Four patients had worse pain at either the one or three month follow-up period. No retreatment with RFA was allowed as part of this trial. Despite allowing patients to have chemotherapy 2 weeks after RFA the mood and pain control data were statistically significant. A total of 14 patients had chemotherapy greater than 14 days after RFA with a mean start date of one month after RFA. The one month pain relief was greater than the 3 month pain relief and allowing chemotherapy administration after RFA may partially account for less pain relief at the three month follow-up period. In comparison to the Goetz et al trial our pain relief was not as pronounced. This can be explained by several factors including eligibility criteria and differences in tumor types. In the Goetz et al trial the patients had to have a life expectancy of greater than 2 months and had to have had previous treatment to the tumor site of which 74% had previous radiotherapy to the tumor site. In our trial we did not have these two eligibility criteria and the number of patients who had previous radiotherapy to the site for bone pain palliation was only 13/55(23.6%). When we specifically looked at the small subset of patients who did have previous radiotherapy we did not see a statistically significant difference in pain relief, mood or pain intensity compared with those patients who did not initially receive radiotherapy to their painful bone metastasis. Combination radiotherapy and ablation has been reported to be more efficacious than one modality alone36 as applied to palliation of chest wall masses. This difference in the number of painful sites previously radiated could explain the differences in pain relief. In the Goetz et al trial there were a large majority of other tumors (19/42) some of which have more favorable biology (e.g desmoid, paraganglioma, meningioma, thyroid, prostate, breast) and very few lung cancer metastases (4/42) treated whereas in the ACRIN trial there were three times more lung metastases (17/55) treated which are known to be more aggressive and difficult to treat. If we combine the percentage of lung, colon and renal metastases treated the ACRIN trial had 68% versus 53% in the Goetz et al trial. Based upon these differences it is not surprising that the magnitude of pain relief was less in the ACRIN trial.
In conclusion, this study demonstrates that RFA for bone metastases can be safely performed and achieves palliation for bone pain metastases in a cooperative group setting. It represents a novel treatment option for patients with solid tumors that have metastasized to the bone and further analysis in a randomized controlled trial is warranted.
Acknowledgments
Funding/Support: The trial was conducted by the American College of Radiology Imaging Network (ACRIN) and funded by the national Cancer Institute (NCI). ACRIN receives funding from the National Cancer Institute through the grants U01 CA079778 and U01 CA080098.
We are indebted to the many people at the headquarters of the American College of Radiology Imaging Network and at the recruiting sites for their important contributions to the study as well as the oncologists at the clinical sites; to Gary Dorfman, MD, Cornell Medical Center for protocol development; Valley Lab/Covidien for technical assistance; Sujaya Rao, MD Anderson Cancer Center, for data collection; Lisa Nelson, University of Alabama Medical Center, for data collection; S. Nahum Goldberg, M.D., Beth Israel Deaconess Medical Center, for data collection; Ronald J. Zagoria, M.D., Wake Forest University Baptist Medical Center, for data collection; Daniel B. Brown, M.D., Thomas Jefferson University Hospital for data collection, Adam C. Zoga, M.D, Thomas Jefferson University Hospital for data collection, Sridhar Shankar, M.D., University of Massachusetts Memorial Medical Center for data collection, Wendy Smith, RT(R)(CV), Rhode Island Hospital, for data collection; Cynthia Cobb, RT(R)(CT), Rhode Island Hospital, for data collection; Evelyn Stainthorpe, Abrams Cancer Center of the University of Pennsylvania, for data collection; Bruce Hillman, MD, ACRIN, for administrative assistance; Mitchell Schnall, MD, PhD, ACRIN, for administrative assistance; Steven King, MS, CHE, ACRIN, for administrative assistance; Charles Apgar, MBA, ACRIN, for administrative assistance; Anthony Levering, RT (R)(CT)(MR), ACRIN, for data collection; Robert Sole, R.T.(RCTC), ACRIN, for data collection; Nancy Fredericks, MBA, ACRIN, for administrative assistance; Maria Oh, ACRIN, for administrative assistance; C. Rex Welsh, M.A., ACRIN, for data collection; Timothy Welsh, ACRIN, for data collection; Fraser Wilton, C.E.T., ACRIN, for data collection; Cheryl Crozier, RN, ACRIN for data collection; Mary Kelly-Truran, RN, ACRIN, for data collection; Chris Steward, BS, RT (R)(CV), ACRIN, for data collection and the following members of the American College of Radiology Biostatistics Center: Constantine Gatsonis, Ph.D. for editorial assistance and Meredith Blevins for statistical assistance
Footnotes
Financial Disclosures: Drs. Dupuy and Ahrar both receive speaking honoraria from Covidien
References
- 1.Stoll BA, Parbhoo S, editors. Bone Metastasis: Monitoring and Treatment. New York: Raven Press; 1983. [Google Scholar]
- 2.Twycross RG. Management of pain in skeletal metastases. Clin Ortho. 1995;312:187–196. [PubMed] [Google Scholar]
- 3.Janjan NA. Radiation for bone metastases: conventional techniques and the role of systemic pharmaceuticals. Cancer. 1997;80:1628–1645. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1628::aid-cncr13>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Quilty PM, Kirk D, Bolger JJ, Dearnaley DP, Lewington VJ, Mason MD, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol. 1994;31:33–40. doi: 10.1016/0167-8140(94)90411-1. [DOI] [PubMed] [Google Scholar]
- 5.Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double blind placebo-controlled trial. J Clin Oncol. 1998;16:1574–1581. doi: 10.1200/JCO.1998.16.4.1574. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi GB, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1999;12:1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 7.Conte PF, Latreille J, Mauriac L, Calabresi F, Santos R, Campos D, et al. Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: results from a multinational randomized controlled trial. The Aredia Multinational Cooperative Group. J Clin Oncol. 1996;14:2552–2559. doi: 10.1200/JCO.1996.14.9.2552. [DOI] [PubMed] [Google Scholar]
- 8.Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases; final results of the study by the radiation therapy oncology group. Cancer. 1982;50:893–899. doi: 10.1002/1097-0142(19820901)50:5<893::aid-cncr2820500515>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Madsen EL. Painful bone metastasis: efficacy of radiotherapy assessed by the patients: a randomized trial comparing 4Gy versus 10Gy x 2. Int J Radiat Oncol Biol Phys. 1983;9:1775–1779. doi: 10.1016/0360-3016(83)90343-7. [DOI] [PubMed] [Google Scholar]
- 10.Blitzer PH. Reanalysis of then RTOG study of the palliation of symptomatic osseous metastasis. Cancer. 1984;55:1468–1472. doi: 10.1002/1097-0142(19850401)55:7<1468::aid-cncr2820550708>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Price P, Hoskin PJ, Easton D, Austin D, Palmer SG, Yarnold JR. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol. 1986;6:247–255. doi: 10.1016/s0167-8140(86)80191-8. [DOI] [PubMed] [Google Scholar]
- 12.Arcangeli G, Micheli A, Arcangeli G, Gainnarelli D, La Pasta O, Tollis A, et al. The responsiveness of bone metastases to radiotherapy: the effect of site, histology and radiation dose on pain relief. Radiother Oncol. 1989;14:95–101. doi: 10.1016/0167-8140(89)90053-4. [DOI] [PubMed] [Google Scholar]
- 13.Cole DJ. A randomized trial of a single treatment versus conventional fractionation in the palliative radiotherapy of painful bone metastases. Clin Oncol. 1989;1:59–62. doi: 10.1016/s0936-6555(89)80035-4. [DOI] [PubMed] [Google Scholar]
- 14.Poulter CA, Cosmatos D, Rubin P, Urtasun R, Cooper JS, Kuske RR, et al. A report of RTOG 8206: a phase III study of whether the addition of single dose hemibody irradiation to standard fractionated local field irradiation is more effective that local field irradiation alone in the treatment of symptomatic osseous metastases. Int J Radiat Oncol Biol Phys. 1992;23:207–214. doi: 10.1016/0360-3016(92)90563-w. [DOI] [PubMed] [Google Scholar]
- 15.Arcangeli G, Giovinazzo G, Saracino B, D’Angelo L, Giannarelli D, Arcangeli G, et al. Radiation therapy in the management of symptomatic bone metastases: the effect of total dose and histology on pain relief and response duration. Int J Radiat Oncol Biol Phys. 1998;42:1119–1126. doi: 10.1016/s0360-3016(98)00264-8. [DOI] [PubMed] [Google Scholar]
- 16.Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44:1–18. doi: 10.1016/s0360-3016(98)00510-0. [DOI] [PubMed] [Google Scholar]
- 17.Agarawal JP, Swangsilpa T, van der Linden Y, Rades D, Jeremic B, Hoskin PJ. The role of external beam radiotherapy in the management of bone metastases. Clin Oncol (R Coll Radiol) 2006;18:747–60. doi: 10.1016/j.clon.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Cleeland CS. The measurement of pain from metastatic bone disease: capturing the patient’s experience. Clin Cancer Res. 2006;12:6236s–6242s. doi: 10.1158/1078-0432.CCR-06-0988. [DOI] [PubMed] [Google Scholar]
- 19.Callstrom MR, Charboneau JW, Goetz MP, Rubin J, Wong GY, Sloan JA, et al. Painful metastases involving bone: feasibility of percutaneous CT and US-guided radio-frequency ablation. Radiology. 2002;224:87–97. doi: 10.1148/radiol.2241011613. [DOI] [PubMed] [Google Scholar]
- 20.Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: a multi-center study. J Clin Oncol. 2004;22:300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 21.Fishman B, Pasternak S, Wallenstein SL, House RW, Holland JC, Foley KM. The memorial pain assessment card. A valid instrument for the evaluation of cancer pain. Cancer. 1987;60:1151–1158. doi: 10.1002/1097-0142(19870901)60:5<1151::aid-cncr2820600538>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Little RJA, Rubin DB. Statistical analysis with missing data. New York: Wiley; 2002. [Google Scholar]
- 23.Hedeker D, Gibbons RD. Application of random-effects pattern mixture models for missing data in longitudinal studies. Psychol Methods. 1997;2:64–78. [Google Scholar]
- 24.Gangi A, Kastler BA, Klinkert A, Dietemann JL. Injection of alcohol into bone metastases under CT guidance. J Comput Assist Tomogr. 1994;18:932–935. doi: 10.1097/00004728-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Levine SA, Perin LA, Hayes D, Hayes WS. An evidence-based evaluation of percutaneous vertebroplasty. Manag Care. 2000;9:56–60. 63. [PubMed] [Google Scholar]
- 26.Dupuy DE, Hong RJ, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: temperature distribution in the spinal canal. Am J Roentgenol. 2000;175:1263–1266. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal DI, Hornicek FJ, Wolf MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am. 1998;80:815–821. doi: 10.2106/00004623-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Sweet WH, Wepsic JG. Controlled thermocoagulation of trigeminal ganglion and rootlets for differential destruction of pain fibers. J Neurosurg. 1974;40:143–156. doi: 10.3171/jns.1974.40.2.0143. [DOI] [PubMed] [Google Scholar]
- 29.Sluijter ME. The use of radiofrequency lesions for pain relief in failed back patients. Int Disabil Studies. 1988;10:37–43. doi: 10.3109/09638288809164058. [DOI] [PubMed] [Google Scholar]
- 30.Ogsbury JS, Simons H, Lehman RAW. Facet denervation in treatment of low back syndrome. Pain. 1977;2:257–263. doi: 10.1016/0304-3959(77)90006-9. [DOI] [PubMed] [Google Scholar]
- 31.Von Roenn JH, Cleeland CS, Gonin R, Hatfield AK, Pandya KJ. Physician attitudes and practice in cancer pain management: a survey from the eastern cooperative oncology group. Ann Intern Med. 1993;119:121–126. doi: 10.7326/0003-4819-119-2-199307150-00005. [DOI] [PubMed] [Google Scholar]
- 32.Moryl N, Coyle N, Foley KM. Managing an acute pain crisis in a patient with advanced cancer: this is as much of a crisis as a code. JAMA. 2008;299:1457–1467. doi: 10.1001/jama.299.12.1457. [DOI] [PubMed] [Google Scholar]
- 33.Kutner JS, Smith MC, Corbin L, Hemphill L, Benton K, Mellis BK, et al. Massage therapy versus simple touch to improve pain and mood in patients with advanced cancer: a randomized trial. Ann Intern Med. 2008;149:369–379. doi: 10.7326/0003-4819-149-6-200809160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 35.Agresti A. An Introduction to Categorical Data Analysis. New York: Wiley; 2007. [Google Scholar]
- 36.Grieco CA, Simon CJ, Mayo-Smith WW, Dipetrillo TA, Ready NE, Dupuy DE. Image-guided percutaneous thermal ablation for the palliative treatment of chest wall masses. Am J Clin Oncol. 2007;30:361–367. doi: 10.1097/COC.0b013e318033e76a. [DOI] [PubMed] [Google Scholar]


