Abstract
Although a family history of alcoholism is the strongest risk factor for developing alcohol dependence, there are few studies of the association between familial alcoholism and the human brain’s reward system activity. We used functional magnetic resonance imaging (fMRI) to determine how family history affects the brain’s response to subjects’ preferred alcoholic drink odors (AO) as compared to appetitive control odors (ApCO). Fourteen non-dependent heavy drinkers (HD) who were family history positive (FHP) participated, as did 12 HD who were family history negative (FHN). Subjects were imaged under both alcohol intoxication and placebo, using intravenous infusion and pharmacokinetic modeling to target a blood alcohol level of 50 mg%. Under placebo, HD-FHP had a larger medial frontal [AO > ApCO] effect than did HD-FHN. Alcohol intoxication dampened this response in the HD-FHP but potentiated it in the HD-FHN. This suggests that a family history of alcoholism and brain exposure to alcohol interact in heavy drinkers to differentially affect how the brain responds to alcohol cues.
Introduction
A family history of alcoholism doubles the odds of developing alcoholism (Hasin et al., 1997; Nurnberger et al., 2004). While environmental influences exert considerable influence in early adolescence, twin studies show an increasingly larger genetic influence by age 18 (Dick, Rose, & Kaprio, 2006), with a family history of alcoholism being a significant factor in the transition from abusive to dependent drinking (Hasin, Paykin, & Endicott, 2001). This familial history also comprises particular neurobiological signatures, as those with a family history of alcoholism are more likely to have smaller electrophysiological responses to salient stimuli (Begleiter & Porjesz, 1999; Polich, Pollock, & Bloom, 1994) and greater beta power in resting EEG (Rangaswamy et al., 2004). More recently, functional magnetic resonance imaging (fMRI) has shown the offspring of alcoholics to have smaller frontal responses to tasks requiring behavioral inhibition (Schweinsburg et al., 2004), a smaller amygdala response when perceiving fearful faces (Glahn, Lovallo, & Fox, 2007), smaller frontal and temporal responses when inferring others’ emotional states (Hill et al., 2007), and a larger response in the anterior cingulate and caudate during simulated gambling (Acheson et al., 2009). Bjork et al. (2008) used a monetary incentive task in adolescents with and without a family history of alcoholism (all of whom were healthy), but found no substantial differences between the groups in reward-related activation. While a number of studies have examined the human cerebral response to alcohol-related cues, particularly in alcoholics (e.g., Bragulat et al., 2008; Filbey et al., 2008b; Kareken et al., 2004; Myrick et al., 2008; Tapert et al., 2004; Wrase et al., 2007), very little research shows how familial alcoholism affects the brain response to alcohol-related cues— particularly in at-risk individuals who have yet to become dependent. In the closest study, Tapert et al. (2003) reported as a secondary finding greater frontal responses to pictures of alcoholic drinks in both control and alcohol use disordered teens (both dependent and abusive drinkers) with family histories of alcoholism when compared to those without such a family history.
Animal research suggests that selective breeding for alcohol preference might affect the heavily dopaminergic mesocorticolimbic reward system. For example, rodents selectively bred to prefer alcohol have reduced dopamine in the striatum (see Murphy et al., 2002; Strother et al., 2005) and medial prefrontal cortex (Engleman et al., 2006), but greater striatal dopaminergic responses to alcohol itself (Bustamante et al., 2008; also see Smith & Weiss, 1999; Weiss et al., 1993). In at least one case, alcohol-preferring rats (compared to Wistar rats) showed a greater dopaminergic response in the ventral striatum during alcohol anticipation (Katner, Kerr, & Weiss, 1996). In non-abusive drinkers without a family history of alcoholism there is greater striatal dopamine receptor availability (Volkow et al., 2006), suggesting a potential protective factor.
In this study, we examined how family history affects the brain’s response to alcohol’s olfactory cues in non-dependent, at-risk heavy drinkers. We also sought to determine how acute alcohol exposure affects the reward system’s response to alcohol’s conditioned cues by using clamped intravenous (IV) alcohol infusion— a method that prescribes a constant level of brain alcohol exposure throughout functional image acquisition, and avoids the highly variable time courses of breath alcohol concentrations that accompany oral consumption (O’Connor et al., 1998; Plawecki et al., 2007; Ramachandi et al., 2004; Ramchandani et al., 1999). We hypothesized that a family history of alcoholism would be associated with stronger responses to alcoholic drink aromas in the mesocorticolimbic reward system, and that a low-level of steady-state brain exposure to alcohol would potentiate these stimulus-induced responses (Bragulat et al., 2008). Such a potentiation could reflect a possible substrate for priming, when alcohol exposure increases desire to drink (De Wit, 1996; De Wit, 2000). We focused our hypotheses on the ventral tegmental area (VTA) and ventral striatum, and on the medial frontal brain regions to which the VTA and ventral striatum directly project (Chiba, Kayahara, & Nakano, 2001; Haber et al., 2006; Williams & Goldman-Rakic, 1998).
Methods
Subjects
Subjects were recruited and assessed using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994), the Timeline Followback interview (TFLB; Sobell et al., 1986) for habitual drinking, and the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al., 1993). Two samples of non-dependent heavy drinkers (HD) were acquired (Table 1), 14 of whom were family history positive for alcoholism (HD-FHP; at least two first or second degree relatives with probable alcoholism on the SSAGA family history module, excluding mothers to preclude possible fetal alcohol effects) and 12 of whom had no known family history of alcoholism (HD-FHN). None had been treated for alcohol disorders, had evidence of Axis-I psychiatric disorders, had neurological disorders of the brain, or failed olfactory screening. While differing in family history, there were no significant group differences in age (range 21 – 31 across all subjects), education, recent drinking (drinks per week and drinking day, heavy drinking days defined as >3/>4 for women/men), scores on the AUDIT, age of first and regular drinking, gender, and percentage of smokers (see Table 1). Although all subjects denied using illicit drugs, two FHN subjects tested positive for cannabinoids on the placebo day, one of whom continued to test positive on the alcohol day. One FHP subject tested positive for amphetamines on both placebo and alcohol days, and a second FHP subject tested positive for amphetamines only on the alcohol day. This latter subject was excluded from analyses of the alcohol session data because of clear acute intoxication and the subject’s difficulty detecting odors on that day. No subject otherwise exhibited behavioral signs of intoxication in either session. One HD-FHN subject could not be used for the alcohol session because of excess movement. All subjects were right handed. All voluntarily signed informed consent statements approved by the Indiana University School of Medicine IRB.
Table 1.
Subject Characteristics.
| FHN | FHP | |||||
|---|---|---|---|---|---|---|
| Heavy Drinkers (n= 12) | Heavy Drinkers (n= 14) | |||||
| Mean | (SD) | n (%) | Mean | (SD) | n (%) | |
| Age | 23.42 | (2.91) | 23.93 | (2.30) | ||
| Male | 9 (75%) | 7 (50%) | ||||
| Caucasian | 12 (100%) | 13 (93%) | ||||
| Education | 15.00 | (1.21) | 15.43 | (1.34) | ||
| Relatives w/Alcoholism | - | - | 3.14 | (1.51) | ||
| Drinks/Week | 17.11 | (9.68) | 18.08 | (6.87) | ||
| Drinks/Drinking Day | 5.70 | (1.52) | 5.53 | (2.52) | ||
| Heavy Drinking Days | 1.62 | (0.85) | 1.66 | (0.64) | ||
| AUDIT | 12.33 | (4.05) | 10.43 | (2.68) | ||
| Age at First Drink | 15.42 | (1.62) | 14.71 | (2.49) | ||
| Age of Regular Drinking | 18.42 | (1.68) | 19.00 | (1.30) | ||
| Smokers | 4 (33%) | 3 (23%) | ||||
Notes: FHN = Family history negative for alcoholism. FHP= Family history positive for alcoholism (>1 first or second degree relatives). Drinking data are from the Timeline Followback Interview. AUDIT = Alcohol Use Disorder Identification Test.
Procedure
Subjects participated in two imaging sessions during exposure to the aromas of the subjects’ individually preferred alcoholic drinks, as well as two sets of control odors. During each fMRI session, subjects underwent intravenous infusion of alcohol or placebo in randomized/counterbalanced order. To minimize expectations, subjects were told that they could receive alcohol or placebo during any imaging session (i.e., one session did not predict the other).
Olfactory stimuli
Odorants were delivered using an air-dilution olfactometer as previously described (Bragulat et al., 2008; Kareken et al., 2004). Three classes of odorants were used: Alcohol odors (AO, each subject’s two most frequently consumed alcoholic drinks), appetitive control odors (ApCO; chocolate and grape juice; McCormick & Company, Inc., Hunt Valley, MD), and non-appetitive odors (NApO) that represented stimuli not normally ingested, or evocative of ingestive behavior. As preliminary experience showed that some subjects found certain odors unpleasant, subjects chose two of four amongst grass, leather, lilac and Douglas fir; International Flavors & Fragrances, Union Beach, NJ). AO were the actual alcoholic drinks “bubbled” (rendered volatile by passing an airstream through the liquid) in two of the olfactometer’s vials. NApO and ApCO were chosen as prior data showed them to be approximately equal in intensity, pleasantness, and representativeness (Bragulat et al., 2008).
Stimulus training and craving
Before entering the scanner, subjects were familiarized with the odorants by smelling each (grouped by the stimulus classes of AO, NApO, ApCO) through the olfactometer while simultaneously viewing representative images on a computer monitor. Just prior to combined odor/picture cue-exposure (baseline), and again after each of the three stimulus classes, subjects answered questions probing mood and craving. Subjects rated desire to drink alcohol by responding to four items (#11, #18, #21, #32) from the Alcohol Craving Questionnaire (ACQ; Singleton, Tiffany, & Henningfield, 2000) on a visual analog scale (VAS; 1= strongly disagree, 7= strongly agree). Subjects similarly rated mood (“Right now, I feel angry, grouchy, annoyed, bad-tempered”, “Right now, I feel happy, energetic, full of pep, cheerful, vigorous”).
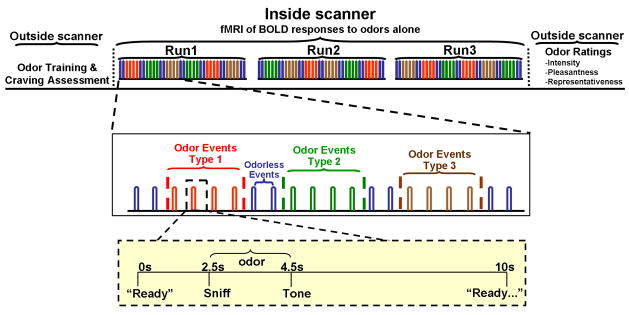
Activation paradigm (Figure 1)
Figure 1. Stimulation paradigm.
Subjects sniffed alcoholic odors (subject’s two most-preferred drinks), two non-appetitive odors (from grass, leather, and Douglas fir), or appetitive control odors (grape chocolate), as well as odorless air (sham stimuli) Each stimulus was delivered over 2 s odor valve openings, with auditory commands (yellow inset) instructing subjects to sniff Following a tone, subjects signaled their ability to detect an odor (“yes” or “no”) using a button response box. Each odorant in a given class was delivered twice in alternate order (e.g., beer, whiskey, beer, whiskey) over the course of two 40 s periods, with a 10 s stimulus onset asynchrony (SOA) between single odorant pulses. Although the stimuli were grouped in classes, responses were analyzed as events (timed to each valve opening, which initial testing showed to be the most sensitive approach). Three different stimulation sequences were randomized across the subjects, such that no stimulus class was repeated without an intervening class, and any one odor class was always followed by two odorless baseline events. Three functional imaging scans per subject session were performed, for a total of 24 odor events per odorant class.
Three functional scans of olfactory stimulation per subject-session were performed (24 odor events in each of the three stimulus classes of AO, ApCO and NApO plus 42 odorless control events). No images were presented during imaging, and subjects underwent olfactory stimulation with eyes closed. Subjects reported the presence (button 1) or absence (button 2) of an odorant on a response box, but were not asked to identify the odorants.
Odor ratings
After each imaging session, subjects were re-exposed to the odors. After smelling each odor, the subjects rated the odor’s intensity, pleasantness, and representativeness (how well the odor represented its intended source) on a 9-point VAS.
Alcohol Administration
Subjects were intravenously in fused with either alcohol (6% vol/vol) or saline (placebo) in counter-balanced order as previously described (Bragulat et al., 2008). Infusion pump rates were controlled by a computer, with the infusion profile customized for each individual to achieve the same time course of breath alcohol concentration (BrAC) for all subjects: A linear ascension to 50 mg% in 10 min, followed by constant exposure at 50 mg% throughout approximate 45 minute functional image acquisition. The placebo infusion employed the same pump-rate profile to be used/as was used in the individual’s alcohol session, but infused only saline. Prior to and after imaging sessions, BrAC was measured.
Subjects orally rated subjective responses to the alcohol infusion on the “high” (operationally to subjects as, “up-stimulated, feeling good”) and “intoxicated” (“drunk, tipsy, inebriated”) items of the Subjective High Assessment Scale (SHAS; Schuckit et al., 2000). Before starting the infusion pump, subjects used a uniform baseline of zero, with ratings subsequently varying from the 0 baseline to a maximum of 100 (the most “high” or “intoxicated” ever). Ratings were obtained at baseline, after the calculated BrAC target before functional imaging, between each fMRI scan, and once after the last scan. After imaging, subjects left the scanner, and with the infusion pumps running a BrAC measurement was obtained.
Image acquisition and analysis
Whole-brain blood oxygenation level dependent (BOLD) imaging was conducted on a Siemens 3T Magnetom Trio scanner across three functional scans. A whole-brain high resolution anatomical image volume (1.0mm × 1.0mm × 1.2mm voxel dimension) was first collected using a 3D magnetization prepared rapid gradient echo (MPRAGE) sequence for anatomic registration of the functional images. In three functional runs, blood oxygenation level dependent images of 37 slices covering a 111mm superior-inferior extent of the brain were acquired over a 402s period, using a gradient echo echo-planar imaging sequence that incorporated a 3D prospective acquisition correction (acquisition matrix = 96 × 96, voxel size = 2.5mm × 2.5mm × 3.0mm; For 12 subjects (7 HD-FHP, 5 HD-FHN): 134 measurements, TR/TE = 3000/40ms, flip angle = 90°, no acceleration, slice thickness = 2.5mm with 0.5mm interslice gap; For 14 subjects (7 HD-FHP, 7 HD-FHN): 174 measurements, TR/TE = 2250/30ms, flip angle = 78°, GRAPPA acceleration factor = 2, slice thickness = 3.0mm with no inter-slice gap These minor acquisition differences, which were balanced across groups, were necessary given an upgrade to the Trio. Direct whole-brain voxel-wise testing of the two acquisitions showed no significant differences in BOLD activation to olfactory stimulation across all three odorant sets (p < 0.05, false discovery rate corrected).
Data were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, University College, London). Functional volumes were corrected for slice acquisition timing differences and rigid-body realigned to the initial volume of the first functional imaging scan to account for residual movement after prospective motion correction. Each subject’s high resolution anatomic image was co-registered to the reference functional volume, segmented into gray, white and CSF tissue, and nonlinear spatial transformation parameters from this segmentation were subsequently applied to transform functional image volumes into the Montreal Neurological Institute (MNI) coordinate space (isotropic 2 mm voxels). Normalized functional image volumes were smoothed by a 6 mm full-width at half-maximum (FWHM) isotropic Gaussian kernel.
Discrete 2 s periods of odorant (or sham) valve events (Figure 1) were modeled in a within subject (fixed-effects) general linear model using as basis functions SPM’s canonical hemodynamic response function (HRF). Initial testing showed that piriform and orbitofrontal responses to odorants (compared to odorless sniffing) were maximized when the HRF onset was delayed by one second after the sniff instruction, with time and dispersion derivatives of the HRF accounting for slight variations in response onset and duration. Movement parameters from realignment were included as regressors to account for residual movement-induced effects. A high-pass filter with a cut-off of 1/128 Hz was applied to each voxel’s time series to remove low frequency noise; auto-regression was not used due to the long inter-stimulus interval (Della-Maggiore et al., 2002). This within subject model yielded contrast images of activation within an odorant condition (AO, NApO, and ApCO) for each subject, with each odorant set contrasted against sniffing of an odorless control event (i.e, control valve opening without odorant delivery). This permitted quantifying the extent to which the BOLD response from an odorant class was different from stimulation (auditory commands, sniffing, attentional processing, motor response) without a chemosensory stimulus.
Random effects analysis of these contrast images in a priori regions of interest (ROI) employed a Group(2) × Odor(2) × Condition(2) random effects, linear mixed model analysis of variance in SPSS 17.0 for Windows (SPSS Inc., Chicago, IL). “Group” represents the two heavy drinking groups who differ in family history, “Odor” refers to effects from AO and ApCO (each contrasted against the odorless control events), while “Condition” indicates alcohol and placebo infusion. We concentrated analyses on AO and ApCO, which represent two classes of appetitive stimuli. As alcohol can prime desire to both drink and eat (e.g., Caton, Bate, & Hetherington, 2007; De Wit, 1996; Yeomans, Hails, & Nesic, 1999) NApO (odors that represent stimuli that are not ingested) were reserved to determine if alcohol altered the olfactory sensory response in primary olfactory cortex.
Our analysis of specific a priori ROIs stems from hypotheses specific to the mesocorticolimbic reward pathway from the VTA/striatum to frontal cortex (which animal research implicates as sensitive to selection for high drinking). This approach has the additional benefit of reducing the number of comparisons. Left and right medial frontal ROIs (Figure 2) were defined to anatomically approximate the medial prefrontal (mPFC) and ventromedial prefrontal (vmPFC) regions to which the VTA and ventral striatum project (Chiba, Kayahara, & Nakano, 2001; Haber et al., 2006; Williams & Goldman-Rakic, 1998). These ROIs encompass activation from reward-associated stimuli in multiple studies (Filbey et al., 2008b; Hare et al., 2008; Kable & Glimcher, 2007; McClure et al., 2007; Myrick et al., 2008; Schott et al., 2008), including a study by our group (Bragulat et al., 2008). The ROIs defining the medial prefrontal areas have rostro-caudal extents spanning +56 mm to +36 mm in MNI space. mPFC has a superior extent of +14 mm and an inferior extent of −6 mm, while vmPFC spans −6 mm to −22 mm. Lateral extents were defined by conjoining the ROI boxes with the gray matter voxels in a smoothed (6 mm FWHM) gray matter mask (Figure 2). The VTA was approximated with an 8 mm diameter sphere centered on [0, −18, −12] (also see Kareken et al., 2004). The ventral striatum (Figure 2, right) was defined using rules developed by Malawi et al. (2001). As the caudate head also projects to the medial frontal area (Chiba, Kayahara, & Nakano, 2001; Haber et al., 2006), we also defined caudate head ROIs spanning from +26 mm rostrally to 0 mm (the anterior commissure) caudally, approximating areas mapped by Haber et al. (2006). The MarsBar utility (Brett, et al, 2002; http://marsbar.sourceforge.net/) was used to extract the mean AO and ApCO contrast value (i.e., the output of the subject-specific fixed effect model) from each ROI, within each of the subjects.
Figure 2.
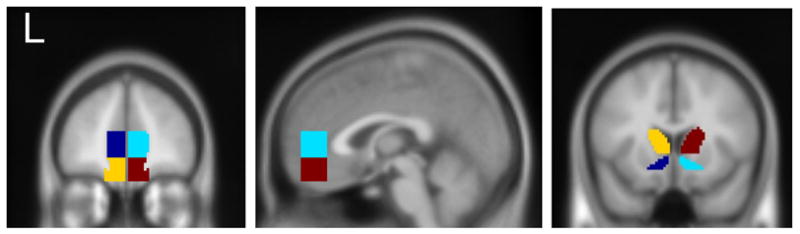
Stereotactically defined ROIs. Left and middle panels; Left and right medial (dark blue. cyan, respectively) and ventromedial prefrontal (yellow, burgundy). Right panel. Left and right ventral striatal (dark blue, cyan) and caudate (yellow, burgundy).
Results
Subjective Responses to Olfactory Stimuli
Odorant characteristics
Tested in a Group(2) × Odor(3) × Condition(2) linear mixed model covering all three odorant classes present during imaging, there was a Odor main effect for intensity (F= 4.60, p = 0.02), but without a Group main effect or a Group × Condition interaction. t-contrasts showed no significant differences in intensity between AO (7.52, SD= 0.95) and ApCO (7.25, SD= 1.12) or NApO (6.78, SD = 1.39), with the intensity difference being between the two control odorant classes (ApCO and NApO; p < 0.05). All stimulus classes were equally pleasant (AO= 7.07, SD= 1.46; NApO= 6.95, SD= 1.23; ApCO =7.22, SD= 1.14), without significant group or infusion interactions. With an Odor main effect for representativeness (F= 6.25, p = 0.007), planned comparisons showed that AO (7.92, SD= 1.01) was perceived as slightly but significantly more representative than ApCO (6.97, SD =1.45). However, and of importance to the group comparisons of imaging results, there were no other main effects or interactions between Group and Condition.
Odorant detection
Of the 114 events over all three imaging runs, the total correct (correct hits + correct rejections) was high in both placebo (111.52, SD= 3.46) and alcohol (110.30, SD= 7.88) infusions. Non-parametric analysis showed no between-group differences in either infusion condition (Kolmogorov-Smirnov tests), and no differences related to infusion (Wilxocon Signed Rank tests) within group.
Mood and Craving
Analyzed in a Group(2) × Stimulus(4) × Condition(2) mixed linear model (where “Stimulus” comprises baseline, and AO, ApCO, and NApO odor/picture presentation during stimulus familiarization), there was a small significant main effect for Stimulus on positive mood (F= 4.23, p < 0.05), but no interaction with Group or Condition. Paired differences between mood after AO exposure (4.48, SD= 1.11) and the remaining conditions (NApO= 4.35, SD= 1.06; ApCO= 4.36, SD= 1.08; Baseline= 4.33, SD= 0.95; p’s < 0.05) were nevertheless small in absolute magnitude. There were no significant main effects or interactions on negative mood (grand mean 1.25, SD = 0.49).
There was a significant Stimulus effect (F = 11.95, p < 0.001) on craving, but no interactions with Group or Condition. Paired comparisons showed significantly higher craving after exposure to AO (2.65, SD= 1.30) than after baseline (1.98, SD= 1.08) or the two control odorant classes (NApO= 1.86, SD= 1.01; ApCO= 1.99, SD= 0.984; p < 0.001).
Intravenous Infusions
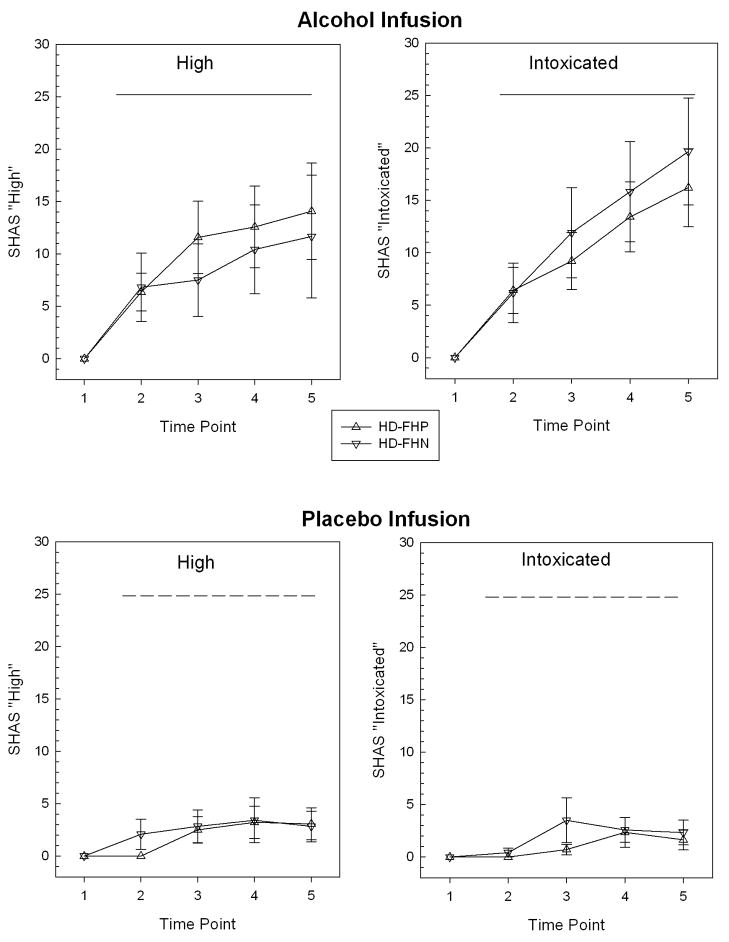
Alcohol infusion resulted in BrAC values at the end of the imaging interval that closely approximated the 50mg% target (HD-FHP= 0.048, SD= .007; HD-FHN= 0.051, SD= 0.006), with no group differences.SHAS responses during infusion changed little from baseline during placebo-saline infusion (Figure 3, bottom), and the groups were not significantly different from one another during alcohol infusion.
Figure 3.
Perceived “high” and “intoxication” (mean ± standard error) under intravenous infusion during functional imaging. Time points are relative (1 = baseline before infusion pump start; 2= at model peak breath alcohol; 3 – 5= after each of three olfactory stimulation fMRI scans. Horizontal bar marks period of steady-state infusion (dash= placebo saline; solid= ethanol at modeled to be 50 mg%).
Alcohol effects on olfactory system activation
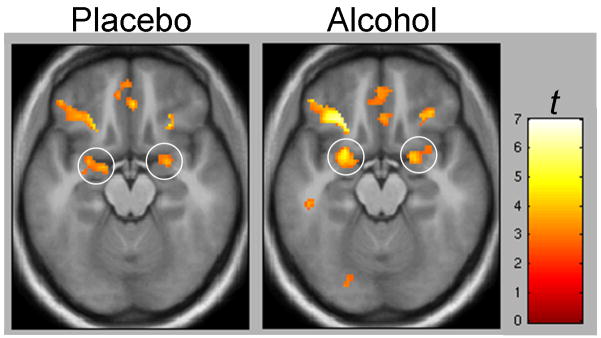
As the BOLD effect depends on blood flow and alcohol is vasoactive, we determined if steady state alcohol infusion affected the olfactory sensory system response. For this we used a voxel-by-voxel factorial model as implemented in SPM5 to define the boundaries of functional ROIs for primary olfactory (piriform) cortex by examining the [NApO > odorless sniffing] olfactory main effect (height threshold p < 0.001, extent threshold k > 5 voxels). Mean activity from these functionally defined volumes was extracted and compared to mean activity under alcohol infusion in a 2(Condition) × 2(Group) mixed model. Neither left nor right piriform responses showed significant main effects or interactions (p’s ≥ 0.50). This suggests that the primary olfactory cortex BOLD response to odorants with little appetitive significance was unchanged by alcohol infusion. Voxel-wise testing in a factorial (Condition × Group) model similarly showed no significant differences (p > 0.05, uncorrected) in either piriform or orbitofrontal (associative olfactory) cortex (see Figure 4).
Figure 4.
Voxel-wise heat maps (display height, p < 0.005; extent threshold. k > 25) of olfactory sensory system activation [NApO > odorless sniffing] under intravenous saline and steady-state alcohol (targeted breath level = 0.050) Circles = piriform (primary olfactory) cortex activation. Also note lateral orbitofrontal (olfactory association cortex) activation. Random effects analyses show no significant betwecn-session differences (see text for details).
fMRI
Stimulus Effects
Within the a priori ROIs, there were statistically significant Odor main effects (reflecting significant BOLD differences between AO and ApCO) in the left (F= 13.54, p ≤ 0.001) and right (F= 9.62, p ≤ 0.005) mPFC ROIs, and in the left (F= 15.76, p ≤ 0.001) and right (F= 15.99, p ≤ 0.001) vmPFC ROIs. Neither the ventral striatum nor the caudate heads showed a significant Odor main effect (p’s > 0.50), but there was a significant Odor effect in the VTA (F= 5.16, p ≤ 0.05). In all cases, these significant main effects reflected a greater AO than ApCO response (p’s 0.005 for frontal ROIs, p ≤ 0.05 for the VTA).
Condition Effects
There were no statistically significant main effects (all p > 0.15) in any of the ROIs for the Condition (infusion) term, except for a borderline effect in the left (p = 0.06) ventral striatum, reflecting a higher olfactory response under placebo. Condition also did not interact with Odor (all p > 0.50). Both findings suggest that the alcohol infusion did not (as with piriform cortex; see above) systematically alter the olfactory response in mesocorticolimbic reward areas.
Group Effects
There were no significant main effects of Group, and no significant Group × Odor interactions (all p > 0.10) to suggest that the BOLD response to odors was overall greater in one group when collapsed across the infusion conditions.
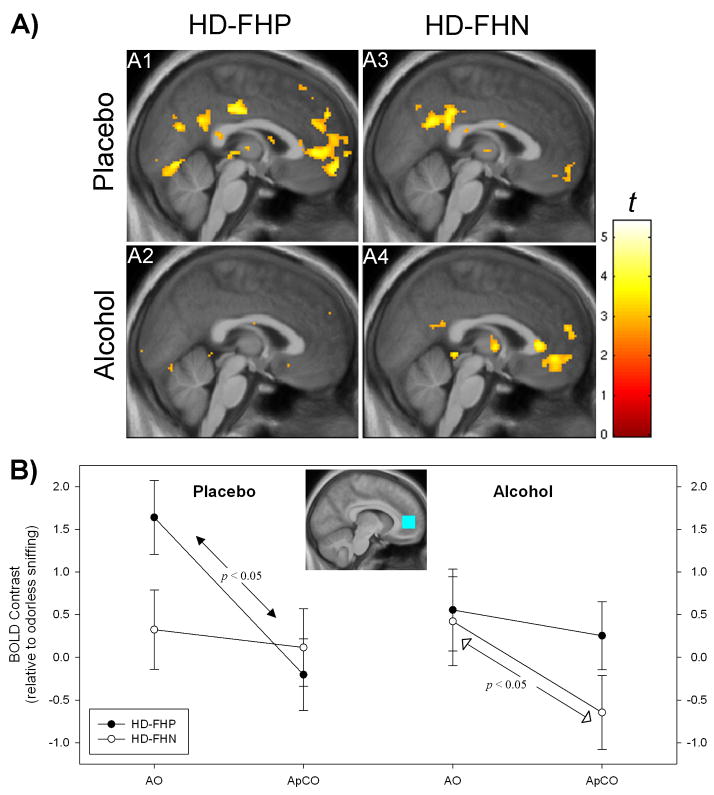
However, there was a significant three-way Group × Odor × Condition interaction in the right mPFC ROI (F= 4.82, p < 0.05). As visualized in voxel-wise maps (Figure 5A) and in plots of the ROI means (Figure 5B), the interaction was such that under placebo, the HD-FHP had a significantly greater BOLD response to AO than to ApCO, while the same contrast within the HD-FHN was insignificant. Under alcohol, this differential odorant response became insignificant in the HD-FHP, but significant in the HD-FHN.
Figure 5.
A) Voxel-wise effects of [AO > ApCO] in subject groups under placebo and alcohol (targeted breath alcohol = 0.050) x = 2 mm right. Figure display threshold, p < 0.005, Coordinates for peak medial frontal effects exceeding p< 0.001, uncorrected, k > 10 voxels: A1[−6, 56, −6], A2[not significant], A3[no significant], A4[4,44, −6] and −10,42, −8]. B) Nature of significant Group × Odor × Condition interaction in right medial prefrontal cortex (ROI depicted in inset; text for more details). Odor effects are relative to odorless sniffing (i e., a negative value indicates a larger BOLD response to odorless sniffing than to the odorant). Left. Under placebo, AO was significantly greater than ApCO only in the HD-FHP. Right. Under alcohol, the contrast between AO and ApCO was significant in HD-FHN, but not in HD-FHP. See text for abbreviations.
Discussion
Three principal findings emerged from this study: 1) Compared to appetitive control odors, aromas of preferred alcoholic drinks produced extensive medial prefrontal activation in heavy drinkers. 2) Right medial prefrontal activation by AO (compared to ApCO) separated HD-FHP from HD-FHN under placebo. 3) Intravenously infused and clamped (50 mg%) alcohol dampened the contrast between the odor classes in HD-FHP’s medial prefrontal cortex, but enhanced this stimulus class difference in the HD-FHN.
The locus of medial prefrontal activation from AO in the present study was highly similar to that in our previous study of alcoholic drink odors (Bragulat et al., 2008), as well as to studies of visually displayed food for which subjects bid money (Hare et al., 2008; Hare, Camerer, & Rangel, 2009), of the subjective value of monetary reward in a delayed discounting task (Kable & Glimcher, 2007), and of immediate monetary choice in another delayed discounting paradigm (McClure et al., 2004). Reward cue-related activation in these medial prefrontal areas also correlates with perceived stimulus “value” when selecting visually displayed food items (Hare, Camerer, & Rangel, 2009). The right medial prefrontal contrast between AO and ApCO was most pronounced in the HD-FHP under placebo, where it differentiated the HD-FHP from equivalently heavy drinking individuals without a family history of alcoholism. This finding suggests a possible effect of familial history in reward cue processing in these heavy drinking subjects, and builds upon reported alterations in the reward system using animal models of familial alcoholism. For example, a selectively bred alcohol preference is associated with reduced dopamine content in the ventral striatum (see Murphy et al., 2002) and medial prefrontal (Engleman et al., 2006) and cingulate cortex (Zhou et al., 1995). Lower frontal serotonin— a neurotransmitter implicated in inhibitory control and impulsivity (Pattij & Vanderschuren, 2008)— has been found, as well (Gongwer et al., 1989; Murphy et al., 1987). Human electrophysiology shows that the P3 response to novel stimuli is lower in individuals with a family history of alcoholism (Polich, Pollock, & Bloom, 1994), with some studies finding this most apparent frontally (Ehlers et al., 2001; also see Finn, Ramsey, & Earleywine, 2000; O’Connor et al., 1994). Employing fMRI of a simulated gambling task, Acheson et al. (2009) found that subjects with a family history of alcoholism had significantly greater anterior cingulate and caudate head responses than FHN subjects, even though both performed equivalently. However, there was also a trend for the FHP to drink more, and overall drinking across groups was closer to social levels. In that case, such findings could represent FHP subjects who are survivors of alcoholism risk.
The present study comprised heavy drinkers. While a family history of alcoholism confers a genetic proclivity for alcoholism, heavy recent drinking is an emerging expression of that proclivity and constitutes a significant increased risk for a lifetime alcohol disorder (Hasin et al., 1997). As both groups in our study were well matched in recent drinking, HD-FHP’s greater medial frontal response to alcoholic drink aromas is more likely to represent something beyond risk survival. Thus, while those with a family history of alcoholism may have lower electrophysiological responses to novel stimuli (Ehlers et al., 2001; Finn, Ramsey, & Earleywine, 2000; O’Connor et al., 1994; Polich, Pollock, & Bloom, 1994), such individuals may also possess frontal areas that respond more strongly to stimuli associated with alcohol (also see Tapert et al., 2003). Longitudinal follow-up would be required to determine whether such a functional difference predicts future dependence. Whether a family history of alcoholism in social drinkers (a group not studied here) results in similar pattern of medial prefrontal responses to alcohol cues also remains to be determined.
Ventral striatal activity was conspicuously absent, although there was a significant difference between odor classes within the VTA that did not differ between groups. Alcoholic drink cues activate the ventral striatum in some (e.g., Bragulat et al., 2008; Kareken et al., 2004; Myrick et al., 2004; Myrick et al., 2008), but not all alcohol cue exposure studies (e.g., George et al., 2001; Hermann et al., 2006; Park et al., 2007; Schneider et al., 2001; Tapert et al., 2004). The reasons are unclear, but there are some theoretical possibilities. First, unlike work in animals, human imaging studies of cue availability rarely include the possibility of contingently obtaining reward (alcohol) as a function of cue exposure— a facet which might dampen ventral striatal responses that encode any learned reward-cue association. In this vein, Bjork and Hommer (2007) showed that passively received monetary rewards did not provoke significant ventral striatal BOLD responses, whereas the anticipation of making an instrumental response to obtain money did— particularly if the reward contingency was uncertain. Similarly, Elliott et al. (2004) found that instrumental acts for rewards modulated the putamen’s response to monetary gain, with greater activity when the instrumental response was required. Interestingly, however, ventral striatal activity was not elicited under any condition in the Elliott et al. study. Second, ventral striatal (and midbrain) responses may be more closely associated with unanticipated events (appetitive or aversive) and reward prediction, while medial frontal cortex is more directly involved in coding for the reinforcing value represented by the stimulus (Hare et al., 2008; Imperato et al., 1992; Imperato, Cabib, & Puglisi-Allegra, 1993; Jensen et al., 2007; Joshua et al., 2008; Kalivas & Duffy, 1995).
We initially hypothesized that alcohol would potentiate the frontal response to its cues, as we had previously found in a smaller sample of individuals who varied in their family history of alcoholism (Bragulat et al., 2008). The picture emerging from this study was more complex, as alcohol dampened the mPFC response to AO in HD-FHP, while potentiating it in HD-FHN. This could suggest that a genetic predisposition to alcoholism biases the medial frontal response to rewarding stimuli in general (Acheson et al., 2009) or to alcohol’s cues specifically. Once alcohol has been obtained, this response may diminish. By contrast, heavy drinkers without any obvious genetic component may be less cue-responsive until acute intoxication, when the medial frontal coding of reward value strengthens. Thus, genetic history (as inferred from family history) may differentially influence the state in which reward cue processing is most active. Support for such a concept comes from rodent studies that distinguish between approach and consumption (Czachowski & Samson, 1999), and where dopaminergic manipulation affects approach more than consumption (Czachowski et al., 2002; Czachowski, Chappell, & Samson, 2001). Moreover, genetic selection for drinking can affect appetitive and consumptive features differently (Czachowski & Samson, 2002). In humans, stimulus-provoked craving (appetitive drive) is also higher in individuals with the A118G variant of the OPRM1 (μ-opiod receptor) gene (van den Wildenberg et al., 2007). Similarly, subjects who tasted alcohol during fMRI had greater vmPFC fMRI activation if they possessed the DRD4 VNTR dopamine receptor gene variant or the A118G OPRM1 polymorphism (Filbey et al., 2008a). In our subjects, however, there were no overt group differences in craving.
There are limitations to this study. Polysubstance use is prevalent amongst those with family histories of alcoholism (Nurnberger et al., 2004). Although we strove to exclude other substance abuse, some subjects who denied drug use ultimately tested positive for illicit drugs. However, eliminating the HD-FHP subject who tested positive for stimulants on the day of the placebo session did not change the significance of the Group × Odor × Condition interactions. Even after eliminating all four HD subjects who tested positive at any session, voxel-wise testing continued to show the same trends (HD-FHP with a larger right mPFC [AO > ApCO] response under placebo than HD-FHN at [6, 66, 0] at p < 0.001, uncorrected; HD-FHN with greater responses than HD-FHP in the left vmPFC [−12, 42, −8], p = 0.001). Thus, the limited illicit drug use in this sample did not principally drive the effects. Power constraints also preclude assessing interactions with gender or nicotine use.
In conclusion, frontal regions thought to process reward value may respond differently to alcohol’s classically conditioned cues in subjects with a family history of alcoholism. While alcohol appears to dampen medial frontal responses to alcohol cues in HD-FHP, it may enhance it in HD-FHN. Genetic background may therefore determine when, and under what circumstances, cues activate the reward network. How this affects drinking remains to be determined.
Acknowledgments
Supported by R01 AA014605 (DAK), R21 AA018020 (DAK), the Indiana Alcohol Research Center P60 AA007611, and the General Clinical Research Center at Indiana University School of Medicine, MO1 RR000750. We gratefully acknowledge the support of Dr. John Nurnburger (Department of Psychiatry), Michele Beal, Victoria Stapleton and Courtney Robbins (Department of Radiology), the statistical consultation of Jaroslaw Harezlak, Ph.D. and Susan Perkins, Ph.D. (Department of Medicine, Division of Biostatistics), and Stephen Warrenburg of International Flavors & Fragrances.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Robinson JL, Glahn DC, Lovallo WR, Fox PT. Differential activation of the anterior cingulate cortex and caudate nucleus during a gambling simulation in persons with a family history of alcoholism: Studies from the Oklahoma Family Health Patterns Project. Drug and Alcohol Dependence. 2009;100:17–23. doi: 10.1016/j.drugalcdep.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcoholism: Clinical and Experimental Research. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: A factorial fMRI investigation. Behavioural Brain Research. 2007;177:165–170. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J, Knutson B, Hommer D. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103:1308–1319. doi: 10.1111/j.1360-0443.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Talavage T, Davidson D, O’Connor SJ, Kareken DA. Alcohol Sensitizes Cerebral Responses to the Odors of Alcoholic Drinks: An fMRI Study. Alcoholism: Clinical and Experimental Research. 2008;32:1124–1134. doi: 10.1111/j.1530-0277.2008.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Bustamante D, Quintanilla ME, Tampier L, Gonzalez-Lira V, Israel Y, Herrera-Marschitz M. Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. European Journal of Pharmacology. 2008;591:153–158. doi: 10.1016/j.ejphar.2008.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton SJ, Bate L, Hetherington MM. Acute effects of an alcoholic drink on food intake: Aperitif versus co-ingestion. Physiology & Behavior. 2007;90:368–375. doi: 10.1016/j.physbeh.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Research. 2001;888:83–101. doi: 10.1016/s0006-8993(00)03013-4. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcoholism: Clinical and Experimental Research. 2002;26:1653–1661. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of Raclopride in the Nucleus Accumbens on Ethanol Seeking and Consumption. Alcoholism: Clinical and Experimental Research. 2001;25:1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Breakpoint Determination and Ethanol Self-Administration Using an Across-Session Progressive Ratio Procedure in the Rat. Alcoholism: Clinical and Experimental Research. 1999;23:1580–1586. [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- De Wit H. Priming effects with drugs and other reinforcers. Experimental and Clinical Psychopharmacology. 1996;4:5–10. [Google Scholar]
- De Wit H. Laboratory-based assessment of alcohol craving in social drinkers. Addiction. 2000;95:S165–S169. doi: 10.1080/09652140050111735. [DOI] [PubMed] [Google Scholar]
- Della-Maggiore V, Chau W, Peres-Neto PR, McIntosh AR. An Empirical Comparison of SPM Preprocessing Parameters to the Analysis of fMRI Data. NeuroImage. 2002;17:19–28. doi: 10.1006/nimg.2002.1113. [DOI] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, Kaprio J. The Next Challenge for Psychiatric Genetics: Characterizing the Risk Associated with Identified Genes. Annals of Clinical Psychiatry: The official Journal of the American Academy of Clinical Psychiatrists. 2006;18:223–231. doi: 10.1080/10401230600948407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Visual P3 findings in Mission Indian youth: relationship to family history of alcohol dependence and behavioral problems. Psychiatry Research. 2001;105:67–78. doi: 10.1016/s0165-1781(01)00313-4. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, William Deakin JF. Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. NeuroImage. 2004;21:984–990. doi: 10.1016/j.neuroimage.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Ray L, Smolen A, Claus ED, Audette A, Hutchison KE. Differential neural response to alcohol priming and alcohol taste cues is associated with DRD4 VNTR and OPRM1 genotypes. Alcoholism: Clinical and Experimental Research. 2008a;32:1113–1123. doi: 10.1111/j.1530-0277.2008.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the Taste of Alcohol Elicits Activation of the Mesocorticolimbic Neurocircuitry. Neuropsychopharmacology. 2008b;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR, Ramsey SE, Earleywine M. Frontal EEG Response to Threat, Aggressive Traits and a Family History of Alcoholism: A Preliminary Study(*) Journal of Studies on Alcohol. 2000;61:38. doi: 10.15288/jsa.2000.61.38. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced Amygdala Activation in Young Adults at High Risk of Alcoholism: Studies from the Oklahoma Family Health Patterns Project. Biological Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongwer MA, Murphy JM, McBride WJ, Lumeng L, Li TK. Regional brain contents of serotonin, dopamine and their metabolites in the selectively bred high- and low-alcohol drinking lines of rats. Alcohol. 1989;6:317–320. doi: 10.1016/0741-8329(89)90089-x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-Related Cortical Inputs Define a Large Striatal Region in Primates That Interface with Associative Cortical Connections, Providing a Substrate for Incentive-Based Learning. Journal of Neuroscience. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-Control in Decision-Making Involves Modulation of the vmPFC Valuation System. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the Role of the Orbitofrontal Cortex and the Striatum in the Computation of Goal Values and Prediction Errors. Journal of Neuroscience. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin D, Paykin A, Endicott J. Course of DSM-IV Alcohol Dependence in a Community Sample: Effects of Parental History and Binge Drinking. Alcoholism: Clinical and Experimental Research. 2001;25:411–414. [PubMed] [Google Scholar]
- Hasin D, Van Rossem R, McCloud S, Endicott J. Alcohol dependence and abuse diagnoses: validity in community sample heavy drinkers. Alcoholism: Clinical and Experimental Research. 1997;21:213–219. [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of Cue-induced Brain Activation of Abstinent Alcoholics by a Single Administration of Amisulpride as Measured With fMRI. Alcoholism: Clinical and Experimental Research. 2006;30:1349–1354. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Hill S, Kostelnik B, Holmes B, McDermott M, Diwadkar V, Keshavan M. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcoholism: Clinical and Experimental Research. 2007;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Research. 1992;577:194–199. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- Imperato A, Cabib S, Puglisi-Allegra S. Repeated stressful experiences differently affect the time-dependent responses of the mesolimbic dopamine system to the stressor. Brain Research. 1993;601:333–336. doi: 10.1016/0006-8993(93)91732-8. [DOI] [PubMed] [Google Scholar]
- Jensen J, Smith AJ, Willeit M, Crawley AP, Mikulis DJ, Vitcu I, Kapur S. Separate brain regions code for salience vs. valence during reward prediction in humans. Human Brain Mapping. 2007;28:294–302. doi: 10.1002/hbm.20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua M, Adler A, Mitelman R, Vaadia E, Bergman H. Midbrain Dopaminergic Neurons and Striatal Cholinergic Interneurons Encode the Difference between Reward and Aversive Events at Different Epochs of Probabilistic Classical Conditioning Trials. Journal of Neuroscience. 2008;28:11673–11684. doi: 10.1523/JNEUROSCI.3839-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Research. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AEK, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li TK. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: Preliminary findings. Alcoholism: Clinical and Experimental Research. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- Katner SN, Kerr TM, Weiss F. Ethanol anticipation enhances dopamine efflux in the nucleus accumbens of alcohol-preferring (P) but not Wistar rats. Behavioural Pharmacology. 1996;7:669–674. [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time Discounting for Primary Rewards. Journal of Neuroscience. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate Neural Systems Value Immediate and Delayed Monetary Rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Murphy JM, McBride WJ, Lumeng L, Li TK. Contents of monoamines in forebrain regions of alcohol-preferring (P) and -nonpreferring (NP) lines of rats. Pharmacology Biochemistry and Behavior. 1987;26:389–392. doi: 10.1016/0091-3057(87)90134-1. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behavior Genetics. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of Naltrexone and Ondansetron on Alcohol Cue-Induced Activation of the Ventral Striatum in Alcohol-Dependent People. Archives of General Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Wiegand R, Bucholz K, O’Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B. A family study of alcohol dependence - Coaggregation of multiple disorders in relatives of alcohol-dependent probands. Archives of General Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcoholism: Clinical and Experimental Research. 1998;22:202–210. [PubMed] [Google Scholar]
- O’Connor S, Bauer L, Tasman A, Hesselbrock V. Reduced P3 amplitudes are associated with both a family history of alcoholism and antisocial personality disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:1307–1321. doi: 10.1016/0278-5846(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn JH, Suk JA, Kim SH, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol and Alcoholism. 2007;42:417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJMJ. The neuropharmacology of impulsive behaviour. Trends in Pharmacological Sciences. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, DeCarlo R, Li T-K, O’Connor SJ. Improved transformation of morphometric measurements for a priori parameter estimation in a physiologically-based pharmacokinetic model of ethanol. Biomedical Signal Processing and Control. 2007;2:97–110. doi: 10.1016/j.bspc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Ramachandi VA, O’Connor SJ, Neumark Y, Zimmerman U, Morzorati S, De Wit H. The Alcohol Clamp: Applications, Challenges and New Directions-- An RSA 2004 Symposium Summary. Alcoholism: Clinical and Experimental Research. 2004;30:155–164. doi: 10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcoholism: Clinical and Experimental Research. 1999;23:617–623. [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Kuperman S, Rohrbaugh J, O’Connor SJ, Bauer LO, Reich T, Begleiter H. Resting EEG in offspring of male alcoholics: beta frequencies. International Journal of Psychophysiology. 2004;51:239–251. doi: 10.1016/j.ijpsycho.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. American Journal of Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Duzel E, Bauer A. Mesolimbic Functional Magnetic Resonance Imaging Activations during Reward Anticipation Correlate with Reward-Related Ventral Striatal Dopamine Release. Journal of Neuroscience. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Tsuang J, Hesselbrock V, Bucholz K. Response to alcohol in daughters of alcoholics: a pilot study and a comparison with sons of alcoholics. Alcohol & Alcoholism. 2000;35:242–248. doi: 10.1093/alcalc/35.3.242. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido CARM, Brown SA, Tapert SF. An fMRI Study of Response Inhibition in Youths with a Family History of Alcoholism. Annals of the New York Academy of Sciences. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Tiffany ST, Henningfield JE. Alcohol Craving Questionnaire (ACQ-NOW): Background, Scoring, and Administration. Baltimore, MD: Intramural Research Program, National Institute on Drug Abuse; 2000. [Google Scholar]
- Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. Journal of Pharmacology and Experimental Therapeutics. 1999;288:1223–1228. [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addictive Behaviors. 1986;11:149–161. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Strother WN, Lumeng L, Li TK, McBride WJ. Dopamine and serotonin content in select brain regions of weanling and adult alcohol drinking rat lines. Pharmacology Biochemistry and Behavior. 2005;80:229–237. doi: 10.1016/j.pbb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Baratta MV, Brown SA. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addictive Behaviors. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg E, Wiers R, Dessers J, Janssen R, Lambrichs E, Smeets H, van Breukelen G. A functional polymorphism of the mu-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcoholism: Clinical and Experimental Research. 2007;31:1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High Levels of Dopamine D2 Receptors in Unaffected Members of Alcoholic Families: Possible Protective Factors. Archives of General Psychiatry. 2006;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. Journal of Pharmacology & Experimental Therapeutics. 1993;267:250–258. [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cerebral Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Hails NJ, Nesic JS. Alcohol and the appetizer effect. Behavioural Pharmacology. 1999;10:151–161. doi: 10.1097/00008877-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Zhang JK, Lumeng L, Li T-K. Mesolimbic dopamine system in alcohol-preferring rats. Alcohol. 1995;12:403–412. doi: 10.1016/0741-8329(95)00010-o. [DOI] [PubMed] [Google Scholar]