Abstract
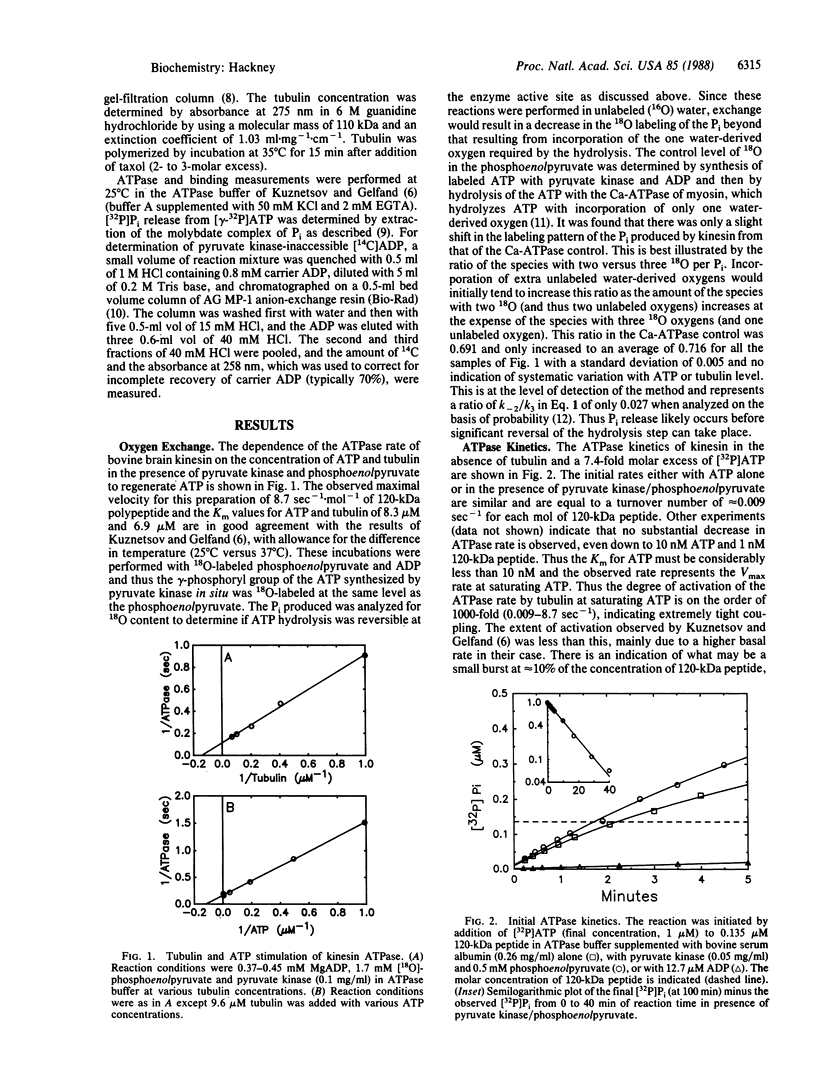
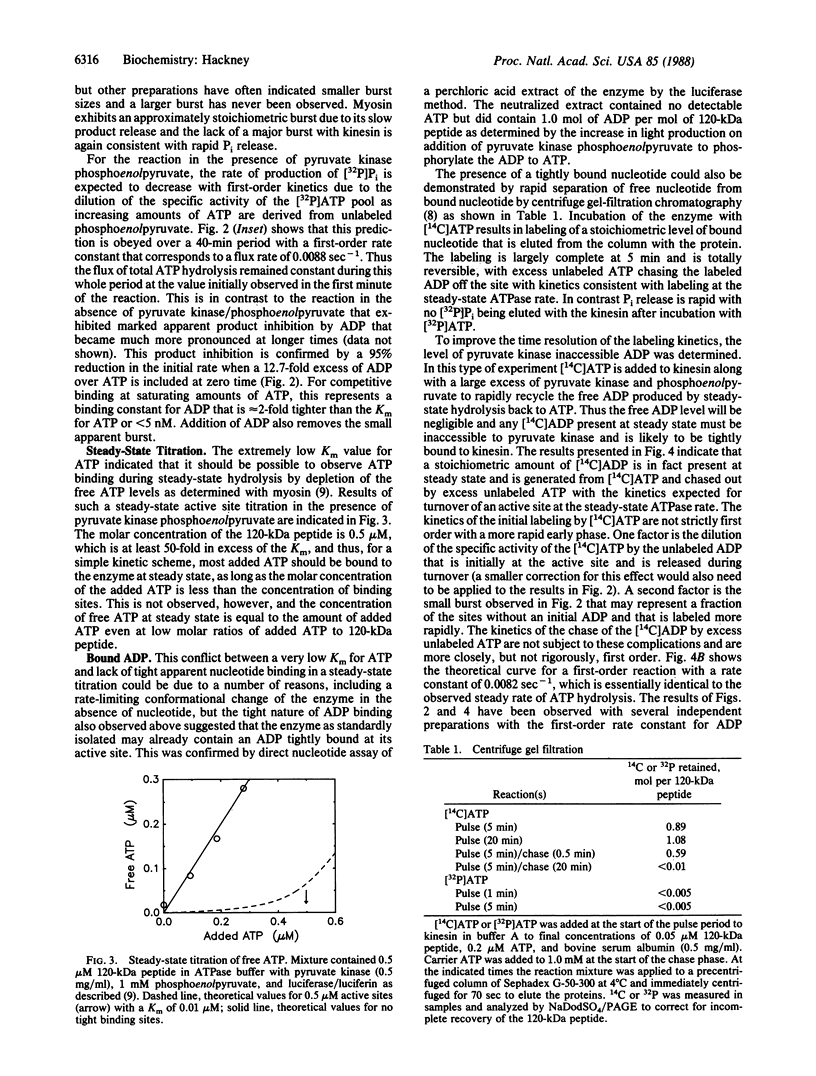
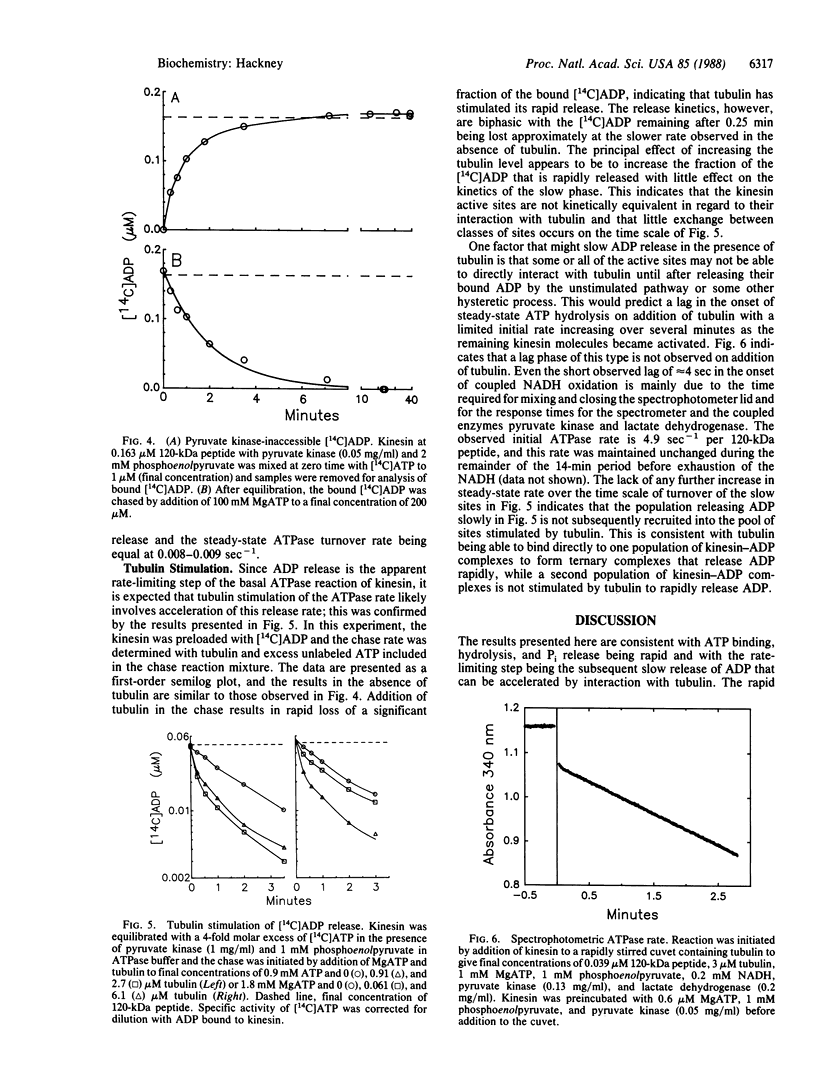
The ATPase rate of kinesin isolated from bovine brain by the method of S.A. Kuznetsov and V.I. Gelfand [(1986) Proc. Natl. Acad. Sci. USA 83, 8530-8534)] is stimulated 1000-fold by interaction with tubulin (turnover rate per 120-kDa peptide increases from approximately equal to 0.009 sec-1 to 9 sec-1). The tubulin-stimulated reaction exhibits no extra incorporation of water-derived oxygens over a wide range of ATP and tubulin concentrations, indicating that Pi release is faster than the reversal of hydrolysis. ADP release, however, is slow for the basal reaction and its release is rate limiting as indicated by the very tight ADP binding (Ki less than 5 nM), the retention of a stoichiometric level of bound ADP through ion-exchange chromatography and dialysis, and the reversible labeling of a bound ADP by [14C]ATP at the steady-state ATPase rate as shown by centrifuge gel filtration and inaccessibility to pyruvate kinase. Tubulin accelerates the release of the bound ADP consistent with its activation of the net ATPase reaction. The detailed kinetics of ADP release in the presence of tubulin are biphasic indicating apparent heterogeneity with a fraction of the kinesin active sites being unaffected by tubulin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hackney D. D., Clark P. K. Steady state kinetics at high enzyme concentration. The myosin MgATPase. J Biol Chem. 1985 May 10;260(9):5505–5510. [PubMed] [Google Scholar]
- Hackney D. D. Theoretical analysis of distribution of [18O]Pi species during exchange with water. Application to exchanges catalyzed by yeast inorganic pyrophosphatase. J Biol Chem. 1980 Jun 10;255(11):5320–5328. [PubMed] [Google Scholar]
- Kuznetsov S. A., Gelfand V. I. Bovine brain kinesin is a microtubule-activated ATPase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8530–8534. doi: 10.1073/pnas.83.22.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Penningroth S. M., Rose P. M., Peterson D. D. Evidence that the 116 kDa component of kinesin binds and hydrolyzes ATP. FEBS Lett. 1987 Sep 28;222(1):204–210. doi: 10.1016/0014-5793(87)80220-x. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Boyer P. D. Effect of actin concentration on the intermediate oxygen exchange of myosin; relation to the refractory state and the mechanism of exchange. Biochemistry. 1978 Dec 12;17(25):5417–5422. doi: 10.1021/bi00618a015. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985 Aug;42(1):39–50. doi: 10.1016/s0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lee J. C. Preparation of tubulin from brain. Methods Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]



