Abstract
The concept of ‘xenon biosensor’ for magnetic resonance imaging (MRI) was first proposed by a Berkeley team in 2001, with evidence that hyperpolarized 129Xe bound to a biotin-labeled cryptophane can detect streptavidin at much lower concentrations (nM-μM) than is typical for contrast-enhanced MRI experiments. 129Xe biosensors have undergone many recent developments to address challenges in molecular imaging. For example, cryptophanes have been synthesized that exhibit 10-fold higher xenon affinity with distinct 129Xe magnetic resonance spectra. Also relevant are dendrimeric cryptophane assemblies and inorganic zeolites that localize many 129Xe atoms to rare targets. Finally, this article considers biosensors that produce measurable changes in 129Xe chemical shift based upon the activity of oligonucleotides, proteins, or enzymes, and includes the first cell studies.
Introduction
Hyperpolarized (hp) 129Xe biosensors were introduced in 2001 as novel contrast agents for magnetic resonance imaging (MRI) and spectroscopy (MRS) [1]. Xenon biosensors put a new (nuclear) spin on an old story: In 1973, Lauterbur [2] and Mansfield [3] obtained the first MR images from free induction decay (FID) data acquired in the presence of magnetic field gradients. Today, noninvasive proton (1H) MRI is one of the most widely used and versatile techniques for scanning deep tissue in the diagnosis of human disease. However, intrinsic 1H MRI signals typically provide poor detection sensitivity. Consequently, contrast agents must be introduced into patients for image enhancement, as occurs in more than half of the roughly 30 million MRI procedures performed annually in the U.S.
Contrast media based on gadolinium or iron-oxide particles have been reviewed extensively [4–5]. Efforts to enhance contrast further have produced ‘smart’ Gd3+ [6], iron-oxide [7], and also 19F-based agents [8] that provide signal in response to a biological trigger. However, fundamental limitations in sensitivity have motivated the investigation of alternate nuclei such as 129Xe, 13C, 83Kr, and 3He [9–12], which can be hyperpolarized to produce unpaired spins that result in much larger NMR signal.
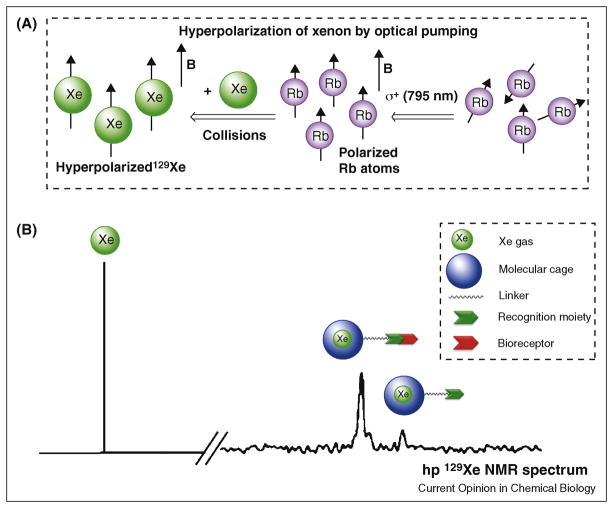
Here, we focus on molecular imaging with 129Xe gas, which is spin-½ and can achieve a hyperpolarized state with spin-lattice relaxation time (T1) of roughly 70 min. 129Xe hyperpolarization yields ~105 signal enhancement relative to the normal Boltzmann distribution of nuclear spins. The process of generating hp 129Xe through spin-exchange optical pumping (Figure 1A) [13] involves the transfer of angular momentum from circularly polarized light to an alkali metal (here we consider Rb). Polarized Rb subsequently interacts with the nuclear spin of xenon through dipolar coupling. Recently, large improvements in 129Xe hyperpolarization technology have achieved > 50% hp 129Xe at flow rates of 1–2 L per min, which enables human clinical applications [14].
Figure 1.
A) Process of optical pumping to produce hyperpolarized 129Xe. B) Schematic representation of hp 129Xe NMR spectrum showing (from left) resonances of free xenon gas in aqueous solution, Xe-encapsulated biosensor bound to corresponding bioreceptor, and Xe encapsulated in free biosensor. Legend shows components of biosensor: molecular cage, linker, and recognition moiety.
Hyperpolarized 129Xe offers potential advantages over conventional MRI contrast agents, namely: roughly 104–fold more NMR signal per nucleus than a corresponding proton, which takes into account the greater gyromagnetic ratio and isotopic abundance of 1H relative to 129Xe; lower toxicity than Gd3+ and most other paramagnetic metal ions, as xenon has low affinity for proteins and is relatively ‘inert’ in the body when introduced below the anesthetic threshold, which is 70% by volume of inhaled gas mixtures; exquisite NMR chemical shift sensitivity, with a greater than 200 ppm spectral window in aqueous solution, which facilitates the detection of multiple species simultaneously (i.e., multiplexing); and, significant polarizability, which contributes to xenon’s millimolar solubility in water [15] and promotes binding to molecular hosts via London dispersion forces. 129Xe biosensors (Figure 1B) that exploit xenon’s unique physical properties create exciting opportunities for molecular imaging. This article focuses on very recent advances in xenon biosensor design and application.
Xenon Biosensing: The Host Molecule
We define ‘xenon biosensor’ simply as a xenon-encapsulating agent attached to one or more bioactive molecules. Due to its filled valence electron shell, xenon resists forming covalent bonds; nonetheless, it shows modest affinity for hydrophobic cavities found in many organic molecules [16–19]. For example, the association constant, KA, is 200 M−1 for hemicarcerands [20] and 20 M−1 for α-cyclodextrin [17]. A calix[4]arene derivative exhibited a KA of only ~14 M−1 at 298 K in water [21] whereas for water-soluble cucurbit[6]uril, a xenon binding constant of 3000 M−1 was measured [18]. In fact, the non-covalent, dynamic association of 129Xe with these host molecules creates opportunities for xenon biosensing applications, as xenon can be hyperpolarized separately as the gas and subsequently introduced to the host molecule, at multiple time points for longitudinal studies.
Cryptophane-A, first reported in 1981 [22], is the most studied Xe-binding cage and is comprised of two cyclotriveratrylene caps joined by three ethyl linkers, which give an internal van der Waals volume of approximately 95 Ǻ3 (Figure 2). As a result of its connectivity, cryptophane-A has D3 symmetry and is chiral. Cryptophane-A is also commonly referred to as cryptophane-2,2,2, based on the three 2-carbon spacers. Cryptophane-A binds Xe reversibly with KA ~ 3,900 M−1 in C2D2Cl4 at 278 K [23]. In a series of related cryptophanes incorporating 3-carbon spacers, xenon affinity decreases with increasing size of the cavity [24].
Figure 2.
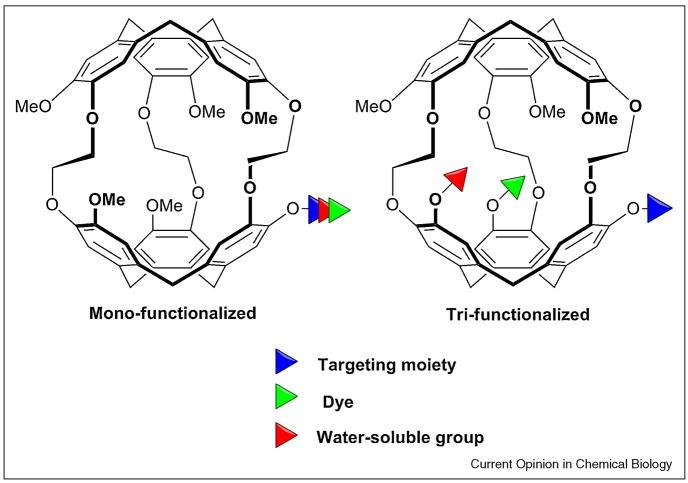
Alternate mono- and tri-functionalization approaches for attaching targeting moieties, dye molecules, and water-solubilizing groups to cryptophane-A.
Cryptophane-1,1,1 was recently synthesized [25], and exhibits the highest Xe association constant measured in organic solvent, KA = 10,000 M−1 at 293 K. This host has a calculated internal volume of 81 Å3, making the xenon volume (42 Å3) to host ratio equal to 0.52, which is very close to Mecozzi and Rebek’s prescribed 0.55 ratio for nonpolar host-guest complexes mediated purely by London forces [26]. Cryptophane-1,1,1 shows very slow decomplexation kinetics, which produces a narrow 129Xe NMR resonance useful for direct detection, but also hinders the Xe exchange needed for indirect detection [27]. As this example shows, both thermodynamics and kinetics impact the design of host molecules for xenon biosensing applications.
Several water-soluble cryptophanes have been synthesized to date, many with exceptional xenon-binding characteristics [28–30]. Huber’s water-soluble cryptophanes [28] replaced all six methoxy groups with OCH2COOH. The Xe affinity of hexa-acid cryptophane-A was measured to be 6800 M−1 at 293 K. Conformational changes were observed in the hexa-acid cryptophanes, including a non-xenon-binding crown-saddle isomer. Our lab subsequently developed synthetic protocols for attaching three carboxylates on just one of the cyclotriveratrylenes [29]. This creates a dipole moment along the axis of the cage that may interact favorably with the xenon electron cloud.
In order to perform more accurate and rapid measurements of xenon binding, our lab developed fluorescence quenching and isothermal titration calorimetry (ITC) assays [30]. Reacting tri-propargyl cryptophane with three azido-propionates gave a water-soluble cryptophane with association constant of 1.7 × 104 M−1 at 293 K [29]. ITC indicates that entropy is an important factor in aqueous xenon binding, as −TΔS is of similar magnitude to ΔH. As a result, xenon affinity increases with temperature: KA = 3.0 × 104 M−1 at 310 K. Importantly, xenon binding studies with the same compound in human plasma showed similar affinity (KA = 2.2 × 104 M−1 at 310 K), which supports use of cryptophanes for cellular imaging. In order to improve xenon binding further, a cryptophane was synthesized with the carboxylates placed closer to the cavity [30]. Presently, a tri-acetate cryptophane-A shows the highest affinity for xenon of any host molecule in water, KA = 3.3 × 104 M−1 at 293 K.
Importantly for multiplexing studies, the known cryptophanes produce very different 129Xe NMR chemical shifts [25,28,31]. Among Huber’s hexa-acid cryptophanes in water, the encapsulated 129Xe shows a single resonance that shifts ~30 ppm upfield with increasing size of the cavity: 222-cage, 64 ppm; 223-cage, 52 ppm; 233-cage, 42 ppm; 333-cage, 35 ppm [28]. A similar trend is observed for the corresponding organic-soluble cryptophanes in C2D2Cl4 [13]. This range of chemical shifts will enable multiplexing experiments that employ different cryptophanes to target various biomarkers. Furthermore, these data suggest that as the cavity becomes smaller, the electron cloud of the xenon feels a greater perturbation from the cage, leading to a larger chemical shift. Interestingly, the Xe@cryptophane-1,1,1 peak is at 31.1 ppm, fully 36 ppm upfield from Xe@cryptophane-2,2,2 in C2D2Cl4. Although this appears to violate the volume-chemical shift trend, cryptophane-1,1,1 lacks the six methoxy substituents on the phenyl rings. This suggests that incorporating electron-donating and withdrawing substituents on the cryptophane should allow efficient stereoelectronic modulation of 129Xe NMR chemical shift for molecular imaging applications.
Xenon Biosensing: The Targeting Molecule
Attached to the host is the targeting molecule, which may be a recognition moiety with high affinity and specificity for a single analyte, e.g., biotin-streptavidin [1,32], peptide-antigen [33], DNA-DNA hybrid [34], or affinity tag for a specific cancer biomarker [35–36]. Attachment of a substrate molecule has the potential to allow even more sensitive detection of an enzyme via the conversion of multiple substrates to products [37]. However, activity-based enzyme sensors must ensure that 129Xe is sufficiently near to the substrate to detect product formation. By the same token, the host molecule should not interfere with enzyme activity.
A 129Xe-biosensor complex freely diffusing in solution typically results in a single NMR resonance (Figure 1B) that does not distinguish between cryptophane enantiomers. Binding to a biological receptor typically produces one or more additional 129Xe peaks. To date, many xenon biosensors have relied on a monofunctionalized cryptophane approach [32–33,35,37]. Newer approaches for tri-substituting cryptophane-A facilitate the introduction of additional targeting moieties, water solubilizing groups or dye labels for fluorescence microscopy (Figure 2) [30,36]. Such flexibility is useful for tuning the biological and spectroscopic properties of the xenon biosensor.
Hyperpolarized 129Xe Biodetection: Overview
It is now possible to collect MR images of inhaled hp 129Xe in the lungs, brain, and other organs of the body [38–41]. However, 129Xe MRI biosensors will require even greater sensitivity to achieve in vivo molecular imaging. Two different biodetection strategies are possible: 1) Target localization, with maximal hp 129Xe localized to the target and minimal background signal elsewhere in the specimen; 2) Multiplexing, whereby multiple xenon biosensors identify several biomarkers. Colocalization of different 129Xe MR spectroscopic signals should help to identify unusual cellular biochemistry, as might be associated with a disease state.
Xenon delivery strategies have sought to maximize the signal-to-noise ratio of the hyperpolarized 129Xe MR signal, by minimizing the decay of polarization during transport or by dilution [38,42]. The T1 of 129Xe varies widely in solution, depending on its molecular environment [1,43–46]: T1 = 4 s in deoxygenated blood, T1 = 13 s in oxygenated blood, T1 = 20–40 s in cryptophanes, and T1 ≈ 1000 s in deuterated saline solution [43,47]. Direct inhalation is the most clinically feasible method of delivering hyperpolarized 129Xe in humans, and some organs, such as the lungs and brain are accessible in this way [48]. In other cases, direct intravenous [42] or arterial [38] injection of hyperpolarized 129Xe is feasible, particularly in rodents. Thus, it is practical to deliver hp 129Xe for sensitive in vivo MRI detection following introduction of the biosensor. Experiments with radioisotope 133Xe in pigs confirmed that xenon is cleared from the lungs and other organs, with retention only in fatty tissues after two hours [49]. Thus, introduction of fresh hp 129Xe will allow longitudinal studies, provided the biosensor is retained.
Hyperpolarized 129Xe Biodetection: Synthetic Strategies
Water-soluble dendrimers have been shown to encapsulate multiple cryptophanes, while presenting recognition moieties that deliver the cargo to a specific target [50]. The published approach exploited electrostatic and hydrophobic interactions to incorporate the cryptophanes, which avoided synthetic steps and the creation of cryptophane diastereomers that might produce additional 129Xe NMR resonances. This invites exploration of polymeric systems that carry a larger payload of cryptophane and improve bioavailability. Relevant examples are micelles and liposomes that can enhance MRI contrast by encapsulating large numbers of paramagnetic species [51].
Another interesting approach for localizing xenon is the use of nanoporous materials. Lerouge prepared silica-based, 65-nm zeolites that contained many pores capable of binding hp 129Xe with a fairly long T1 [52]. Biocompatibility and colloidal stability of these nanoparticles were achieved by functionalization with PEG chains and a peptide targeting biological receptors; localization in a mouse was studied by 111In scintigraphy. It may be possible to create zeolite structures with enhanced xenon-binding and spectroscopic characteristics, as needed for hp 129Xe MRI studies.
Hyperpolarized 129Xe Biodetection: NMR Strategies
Sensitive NMR methods for detecting hp 129Xe have been developed, as elaborated in a recent review [53]. Most promisingly, a hp 129Xe chemical exchange saturation transfer (hyper-CEST) strategy allows the selective depolarization of biosensor-bound hp 129Xe using pulsed excitation [27]. This leads to the accumulated depolarization of bulk hp 129Xe through rapid exchange of xenon atoms between “cryptophane-bound” and “free” states. Monitoring depletion of the “free” hp 129Xe NMR signal provides roughly 103 signal enhancement, based on the large pool of aqueous xenon (~ 3 mM at 1 atm and 310 K) [15] compared to the typically micromolar concentration of 129Xe@biosensor that can be delivered to cells and other biological targets. However, some challenges remain. For example, it will be difficult to achieve a constant polarization level in vivo. Moreover, a decrease of spatial resolution is likely, due to xenon diffusion during the long saturation time.
Biological Applications: Achieving Large 129Xe NMR Chemical Shifts
The initial Berkeley biotin biosensor gave a hp 129Xe NMR peak at 70 ppm. Addition of 40 nanomoles of streptavidin produced a new hp 129Xe NMR peak ~2.5 ppm downfield [1]. Shorter tethers that enforce the streptavidin-cryptophane interaction shifted the 129Xe NMR spectrum by as much as 4 ppm [54–56]; however, the 129Xe NMR resonances also became considerably broadened (> 1 ppm), which limited biodetection. The origin of the 129Xe NMR chemical shift, as well as the appearance of multiple “bound” peaks in some systems, have been investigated computationally by Jameson [55,57]. It is now evident that cryptophane-biotin diastereomers can produce overlapping 129Xe NMR spectra. An additional complication is that streptavidin (a dimer of dimers) binds multiple biotins, which may promote Xe-cryptophane:Xe-cryptophane interactions.
We subsequently designed an enzyme-responsive 129Xe biosensor for the detection of a cancer biomarker, matrix metalloproteinase-7 [37]. The probe was designed such that proteolysis of a peptide containing three positively charged residues caused a change in the electrostatic environment of 129Xe, which could possibly affect 129Xe NMR chemical shift. 129Xe NMR peaks of 61.9 and 62.5 ppm for the intact peptide diastereomers gave upfield shifts of 0.5 and 0.3 ppm upon proteolysis. Although these Δδ values are too small for in vivo detection, this work illustrated the potential for studying enzyme activity.
Roy et al. subsequently designed a cryptophane biosensor for in vitro DNA detection [34]. In order to avoid formation of diastereomers, a chiral (−)-cryptophane-A was synthesized and covalently attached to a 20-mer DNA probe. The NMR spectrum of hp 129Xe with this biosensor alone or in the presence of noncomplementary DNA gave a peak at 68.5 ppm. Addition of the complementary DNA strand produced a new resonance at 67.0 ppm that clearly identified the DNA target in solution. Although unique DNA sequences are rare targets in the cell nucleus, this illustrates the utility of enantiopure cryptophanes for biosensing applications. More recently, Schlundt introduced a peptide-labeled 129Xe biosensor that binds to a major histocompatibility complex (MHC) class II protein [33]. In this case, a 1-ppm downfield shift was observed upon protein binding, and larger chemical shift changes may be possible using a shorter linker. This work demonstrated sensitive biodetection using hyper-CEST NMR techniques.
Finally, our lab has developed 129Xe biosensors for a second cancer biomarker, carbonic anhydrase (CA), which serves as a useful model system for elucidating the origin of 129Xe NMR chemical shifts [36]. Tri-propargyl cryptophane was substituted with linkers varying between 6 and 8 bonds to CA-specific p-benzenesulfonamide ligand to yield non-diastereomeric biosensors with a single 129Xe NMR resonance. These Xe biosensors were designed to bind CA isozymes I and II by considering the depth of the active-site channel, the size of the cryptophane, and the propensity of related compounds to bind CA. X-ray crystallographic studies confirmed binding of benzenesulfonamide to Zn2+ at the CAII active site (Figure 3A), with the 8-bond-linked cryptophane positioned at the mouth of the protein channel [58].
Figure 3.
A) X-ray crystal structure of CAII bound to benzenesulfonamide-8-bond-linker cryptophane, only one enantiomer shown. Data reveal sulfonamidate coordination to Zn2+ (gray atom), and Xe (green atom) bound inside cryptophane. B) Laser-polarized 129Xe NMR spectra: (i) 7-bond-linker biosensor free in solution; (ii) biosensor bound to CAI and (iii) CAII.
The biosensors complexed with CA I and II yielded “bound” hyperpolarized 129Xe NMR resonances of narrow linewidth at approximately 64 ppm that were shifted between 3.0 and 7.5 ppm downfield upon CA binding. These chemical shift differences clearly distinguished between CA I and II in solution. One of the most intriguing findings from this work came with the 7-bond-linker biosensor, which showed one “bound” resonance for CAI and distinctively two “bound” resonances for CAII (Figure 3B).
Biological Applications: Xenon Biosensors in Cells
Our lab has synthesized Xe biosensors targeting cancer biomarkers and performed cell studies [35]. Mono-propargyl cryptophane reacted with azido-peptides in a copper(I)-catalyzed [3+2] cycloaddition. Delivery of cryptophane into human cancer and normal cell lines was achieved with cationic cell penetrating peptides. A tetraRGD peptide targeting αvβ3 integrin increased specificity of cryptophane cell uptake (Figure 4). The peptido-cryptophanes were determined to be relatively nontoxic at the micromolar concentrations needed for hp 129Xe NMR experiments.
Figure 4.
Cryptophane-tetraRGD biosensor targeting αvβ3 integrin. Inset: Uptake of Cy3-labeled biosensor in (A) NCI-H1975 and (B) HFL-1 cells.
Conclusion: A Bright and Undetermined Future for 129Xe MRI Biosensing
129Xe MRI biosensors have been designed to bind xenon with high affinity and target oligonucleotides or proteins [33–34,36–37]. Such compounds have the potential to detect various diseases, by localizing hp 129Xe to a tissue and/or by providing multiplexed detection of different biomarkers. Improved capabilities for hp 129Xe detection and chemical shift resolution are also coming from new 129Xe hyperpolarization technology [13], organic and inorganic assemblies for localizing large numbers of 129Xe atoms [50,52], and hyper-CEST imaging strategies [27]. Uptake of xenon biosensors in cells and mice has been verified [35,52]. In addition, proof-of-concept MRI studies have demonstrated the ability to resolve spatially cryptophanes that are targeted to labeled beads, different compartments or solvents [27,59–61]. Intravenously injected hp 129Xe-saturated saline was shown to be a useful probe for pulmonary perfusion or gas exchange evaluation in the lung [62], further validating 129Xe MRI as a sensitive method for imaging lung diseases [63]. With these rapid technological advances, it should now be possible to detect specific bio-receptors in cells and animals via contrast-enhanced hp 129Xe MRI and MRS. Experimental validation appears to be close at hand, which will guide the continued development of xenon biosensors for molecular imaging and help to identify the best in vivo applications for this technology.
Acknowledgments
We acknowledge our colleagues and researchers world-wide for their contributions to the field of xenon biosensing. Special thanks go to Julie Aaron for supplying Figure 3A. Work on this manuscript was supported by the DOD (W81XWH-04-1-0657), NIH (1R33CA110104), and Camille and Henry Dreyfus Teacher-Scholar Award to I.J.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Recent papers of particular interest are highlighted as:
• of special interest
•• of outstanding interest
- 1.Spence MM, Rubin SM, Dimitrov IE, Ruiz EJ, Wemmer DE, Pines A, Yao SQ, Tian F, Schultz PG. Functionalized xenon as a biosensor. Proc Natl Acad Sci USA. 2001;98:10654–10657. doi: 10.1073/pnas.191368398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauterbur PC. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 3.Mansfield P, Grannell PK. NMR ‘diffraction’ in solids? J Phys C: Solid State Phys. 1973;6:L422–L426. [Google Scholar]
- 4.Chan KW-Y, Wong W-T. Small molecular gadolinium(III) complexes as MRI contrast agents for diagnostic imaging. Coord Chem Rev. 2007;251:2428–2451. [Google Scholar]
- 5.Slotkin J, Cahill K, Tharin S, Shapiro E. Cellular magnetic resonance imaging: Nanometer and micrometer size particles for noninvasive cell localization. Neurotherapeutics. 2009;4:428–433. doi: 10.1016/j.nurt.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urbanczyk-Pearson LM, Femia FJ, Smith J, Parigi G, Duimstra JA, Eckermann AL, Luchinat C, Meade TJ. Mechanistic investigation of beta-galactosidase-activated MR contrast agents. Inorg Chem. 2008;47:56–68. doi: 10.1021/ic700888w. [DOI] [PubMed] [Google Scholar]
- 7.Perez JM, Josephson L, Weissleder R. Use of magnetic nanoparticles as nanosensors to probe for molecular interactions. Chembiochem. 2004;5:261–264. doi: 10.1002/cbic.200300730. [DOI] [PubMed] [Google Scholar]
- 8.Mizukami S, Takikawa R, Sugihara F, Shirakawa M, Kikuchi K. Dual-function probe to detect protease activity for fluorescence measurement and 19F MRI. Angew Chem-Int Ed. 2009;48:3641–3643. doi: 10.1002/anie.200806328. [DOI] [PubMed] [Google Scholar]
- 9.Mugler JP, Driehuys B, Brookeman JR, Cates GD, Berr SS, Bryant RG, Daniel TM, deLange EE, Downs JH, Erickson CJ, et al. MR imaging and spectroscopy using hyperpolarized Xe-129 gas: Preliminary human results. Magn Res Med. 1997;37:809–815. doi: 10.1002/mrm.1910370602. [DOI] [PubMed] [Google Scholar]
- 10.Golman K, Petersson JS. Metabolic imaging and other applications of hyperpolarized C- 13. Academic Radiol. 2006;13:932–942. doi: 10.1016/j.acra.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Clevelanda ZI, Pavlovskayaa GE, Elkinsb ND, Stupica KF, Repineb JE, Meersmann T. Hyperpolarized 83Kr MRI of lungs. J Magn Reson. 2008;195:232–237. doi: 10.1016/j.jmr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins SR, Levin DL, Emami K, Kadlecek S, Yu JS, Ishii M, Rizi RR. Advances in magnetic resonance imaging of lung physiology. J Appl Physiol. 2007;102:1244–1254. doi: 10.1152/japplphysiol.00738.2006. [DOI] [PubMed] [Google Scholar]
- 13.Berthault P, Huber G, Desvaux H. Biosensing using laser-polarized xenon NMR/MRI. Prog Nucl Magn Reson Spec. 2008;55:35–60. [Google Scholar]
- 14.Ruset IC, Ketel S, Hersman FW. Optical pumping system design for large production of hyperpolarized 129Xe. Phys Rev Lett. 2006;96 doi: 10.1103/PhysRevLett.96.053002. 053002-053001-053004. [DOI] [PubMed] [Google Scholar]
- 15.Clever HL. Solubility Data Series. Vol. 2. New York: Pergamon Press; 1979. [Google Scholar]
- 16.Rathore R, Lindeman SV, Rao K, Sun D, Kochi JK. Guest penetration deep within the cavity of calix[4]arene hosts: The tight binding of nitric oxide to distal (cofacial) aromatic groups. Angew Chem-Int Ed. 2000;39:2123–2127. doi: 10.1002/1521-3773(20000616)39:12<2123::aid-anie2123>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Bartik K, Luhmer M, Heyes SJ, Ottinger R, Reisse J. Probing molecular cavities in alpha-cyclodextrin solutions by xenon NMR. J Magn Reson B. 1995;109:164–168. [Google Scholar]
- 18.Kim BS, Ko YH, Kim Y, Lee HJ, Selvapalam N, Lee HC, Kim K. Water soluble cucurbit[6]uril derivative as a potential Xe carrier for Xe-129 NMR-based biosensors. Chem Commun. 2008:2756–2758. doi: 10.1039/b805724a. [DOI] [PubMed] [Google Scholar]
- 19.Ripmeester JA, Ratcliffe CI, Tse JS. The nuclear magnetic-resonance of Xe-129 trapped in clathrates and some other solids. J Chem Soc-Faraday Trans I. 1988;84:3731–3745. [Google Scholar]
- 20.Robbins TA, Knobler CB, Bellew DR, Cram DJ. Host-guest complexion .67. A highly adaptive and strongly binding hemicarcerand. J Am Chem Soc. 1994;116:111–122. [Google Scholar]
- 21.Fukutomi J, Adachi Y, Kaneko A, Kimura A, Fujiwara H. Inclusion complex formation of thiacalix[4]arene and Xe in aqueous solution studied by hyperpolarized Xe-129 NMR. J Incl Phenom Macrocyclic Chem. 2007;58:115–122. [Google Scholar]
- 22.Gabard J, Collet A. Synthesis of a (D3)-Bis(cyclotriveratrylenyl) macrocage by stereospecific replication of a (C3) -subunit. J Chem Soc-Chem Commun. 1981:1137–1139. [Google Scholar]
- 23.Bartik K, Luhmer M, Dutasta J-P, Collet A, Reisse J. 129Xe and 1H NMR study of the reversible trapping of xenon by cryptophane-A in organic solution. J Am Chem Soc. 1998;120:784–791. [Google Scholar]
- 24.Brotin T, Dutasta JP. Cryptophanes and their complexes--Present and future. Chem Rev. 2009;109:88–130. doi: 10.1021/cr0680437. [DOI] [PubMed] [Google Scholar]
- 25 •.Fogarty HA, Berthault P, Brotin T, Huber G, Desvaux H, Dutasta JP. A cryptophane core optimized for xenon encapsulation. J Am Chem Soc. 2007;129:10332–10333. doi: 10.1021/ja073771c. The authors report the smallest synthesized cryptophane cage, cryptophane-111, which exhibits the highest association constant to date for Xe in organic solvents, 10000 M−1 at 293 K. [DOI] [PubMed] [Google Scholar]
- 26.Mecozzi S, Rebek J. The 55% solution: A formula for molecular recognition in the liquid state. Chem Eur J. 1998;4:1016–1022. [Google Scholar]
- 27 ••.Schroder L, Lowery TJ, Hilty C, Wemmer DE, Pines A. Molecular imaging using a targeted magnetic resonance hyperpolarized biosensor. Science. 2006;314:446–449. doi: 10.1126/science.1131847. A new technique, hyper-CEST, was introduced to improve the hp 129Xe MRI signal. Accumulation of depolarized nuclei occurs via selective saturation of biosensor-encapsulated 129Xe and subsequent chemical exchange with the free xenon. [DOI] [PubMed] [Google Scholar]
- 28.Huber G, Brotin T, Dubois L, Desvaux H, Dutasta JP, Berthault P. Water soluble cryptophanes showing unprecedented affinity for xenon: Candidates as NMR-based biosensors. J Am Chem Soc. 2006;128:6239–6246. doi: 10.1021/ja060266r. [DOI] [PubMed] [Google Scholar]
- 29.Hill PA, Wei Q, Eckenhoff RG, Dmochowski IJ. Thermodynamics of xenon binding to cryptophane in water and human plasma. J Am Chem Soc. 2007;129:9262–9263. doi: 10.1021/ja072965p. [DOI] [PubMed] [Google Scholar]
- 30 •.Hill PA, Wei Q, Troxler T, Dmochowski IJ. Substituent effects on xenon binding affinity and solution behavior of water-soluble cryptophanes. J Am Chem Soc. 2009;131:3069–3077. doi: 10.1021/ja8100566. The highest association constant for xenon in buffer, KA ~ 33,000 M−1 at 293 K, was obtained for cryptophane functionalized with three nearby carboxylates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brotin T, Devic T, Lesage A, Emsley L, Collet A. Synthesis of deuterium-labeled cryptophane-A and investigation of Xe@Cryptophane complexation dynamics by 1D-EXSY NMR experiments. Chem Eur J. 2001;7:1561–1573. doi: 10.1002/1521-3765(20010401)7:7<1561::aid-chem1561>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 32.Spence MM, Ruiz EJ, Rubin SM, Lowery TJ, Winssinger N, Schultz PG, Wemmer DE, Pines A. Development of a functionalized xenon biosensor. J Am Chem Soc. 2004;126:15287–15294. doi: 10.1021/ja0483037. [DOI] [PubMed] [Google Scholar]
- 33 •.Schlundt A, Kilian W, Beyermann M, Sticht J, Guenther S, Höpner S, Falk K, Roetzschke O, Mitschang L, Freund C. A xenon-129 biosensor for monitoring MHC-peptide interactions. Angew Chem Int Ed Engl. 2009;48:4142–4145. doi: 10.1002/anie.200806149. A major histocompatibility complex (MHC) class II protein was targeted with a specific peptide ligand attached to cryptophane-A. A chemical shift difference of approximately 1 ppm was reported between free and MHC-bound biosensors by hp 129Xe NMR spectroscopy. [DOI] [PubMed] [Google Scholar]
- 34 •.Roy V, Brotin T, Dutasta J-P, Charles M-H, Delair T, Mallet F, Huber G, Desvaux H, Boulard Y, Berthault P. A cryptophane biosensor for the detection of specific nucleotide targets through xenon NMR spectroscopy. ChemPhysChem. 2007;8:2082–2085. doi: 10.1002/cphc.200700384. A cryptophane-DNA oligonucleotide biosensor was synthesized for in vitro DNA detection. The hp 129Xe NMR spectrum showed that in the presence of the complementary strand, the bound xenon signal was shifted upfield by ~1.5 ppm, whereas it remained unchanged in the presence of the non-complementary strand. [DOI] [PubMed] [Google Scholar]
- 35 ••.Seward GK, Wei Q, Dmochowski IJ. Peptide-mediated cellular uptake of cryptophane. Bioconj Chem. 2008;19:2129–2135. doi: 10.1021/bc8002265. Cellular delivery of cryptophane into human cancer and normal cell lines was first reported. Peptide-labeled cryptophanes were determined to be relatively nontoxic by MTT assay at the micromolar concentrations required for hp 129Xe NMR experiments. A tetra(RGD)-functionalized cryptophane showed cell uptake mediated by integrin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36 ••.Chambers JM, Hill PA, Aaron JA, Han ZH, Christianson DW, Kuzma NN, Dmochowski IJ. Cryptophane xenon-129 nuclear magnetic resonance biosensors targeting human carbonic anhydrase. J Am Chem Soc. 2009;131:563–569. doi: 10.1021/ja806092w. Biosensors complexed with CA I and II yielded “bound” hyperpolarized 129Xe NMR resonances of narrow linewidth that were shifted between 3.0 and 7.5 ppm downfield compared to the “free” biosensor. The NMR spectra clearly distinguished between CA I and II in solution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei Q, Seward GK, Hill PA, Patton B, Dimitrov IE, Kuzma NN, Dmochowski IJ. Designing Xe-129 NMR biosensors for matrix metalloproteinase detection. J Am Chem Soc. 2006;128:13274–13283. doi: 10.1021/ja0640501. [DOI] [PubMed] [Google Scholar]
- 38.Duhamel G, Choquet P, Grillon E, Lamalle L, Leviel JL, Ziegler A, Constantinesco A. Xenon-129 MR imaging and spectroscopy of rat brain using arterial delivery of hyperpolarized xenon in a lipid emulsion. Magn Reson Med. 2001;46:208–212. doi: 10.1002/mrm.1180. [DOI] [PubMed] [Google Scholar]
- 39.Ruppert K, Mata JF, Brookeman JR, Hagspiel KD, Mugler JP. Exploring lung function with hyperpolarized Xe-129 nuclear magnetic resonance. Magn Reson Med. 2004;51:676–687. doi: 10.1002/mrm.10736. [DOI] [PubMed] [Google Scholar]
- 40.Driehuys B, Cofer GP, Pollaro J, Mackel JB, Hedlund LW, Johnson GA. Imaging alveolar-capillary gas transfer using hyperpolarized Xe-129 MRI. Proc Natl Acad Sci USA. 2006;103:18278–18283. doi: 10.1073/pnas.0608458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41 ••.Driehuys B, Pollaro J, Cofer GP. In vivo MRI using real-time production of hyperpolarized Xe-129. Magn Res Med. 2008;60:14–20. doi: 10.1002/mrm.21651. 129Xe MRI was successfully applied to evaluate pulmonary perfusion or gas exchange in the lung of rats, using intravenously injected hp 129Xe-saturated saline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodson BM, Song YQ, Taylor RE, Schepkin VD, Brennan KM, Chingas GC, Budinger TF, Navon G, Pines A. In vivo NMR and MRI using injection delivery of laser-polarized xenon. Proc Natl Acad Sci USA. 1997;94:14725–14729. doi: 10.1073/pnas.94.26.14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkatesh AK, Zhao L, Balamore D, Jolesz FA, Albert MS. Evaluation of carrier agents for hyperpolarized xenon MRI. NMR Biomed. 2000;13:245–252. doi: 10.1002/1099-1492(200006)13:4<245::aid-nbm635>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Luhmer M, Goodson BM, Song YQ, Laws DD, Kaiser L, Cyrier MC, Pines A. Study of xenon binding in cryptophane-A using laser-induced NMR polarization enhancement. J Am Chem Soc. 1999;121:3502–3512. [Google Scholar]
- 45.Cherubini A, Bifone A. Hyperpolarised xenon in biology. Prog Nucl Magn Reson Spec. 2003;42:1–30. [Google Scholar]
- 46.Wolber J, Rowland IJ, Leach MO, Bifone A. Perfluorocarbon emulsions as intravenous delivery media for hyperpolarized xenon. Magn Reson Med. 1999;41:442–449. doi: 10.1002/(sici)1522-2594(199903)41:3<442::aid-mrm3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 47.Bifone A, Song YQ, Seydoux R, Taylor RE, Goodson BM, Pietrass T, Budinger TF, Navon G, Pines A. NMR of laser-polarized xenon in human blood. Proc Natl Acad Sci USA. 1996;93:12932–12936. doi: 10.1073/pnas.93.23.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welsh RC, Chupp TE, Coulter KP, Rosen MS, Swanson SD. Magnetic resonance imaging with laser-polarized Xe-129. Nucl Instrum Methods Phys Res A-Accel Spectrom Det Assoc Equip. 1998;402:461–463. [Google Scholar]
- 49.Marx T, Schmidt M, Schirmer U, Reinelt H. Xenon anaesthesia. J R Soc Med. 2000;93:513–517. doi: 10.1177/014107680009301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mynar JL, Lowery TJ, Wemmer DE, Pines A, Frechet JMJ. Xenon biosensor amplification via dendrimer-cage supramolecular constructs. J Am Chem Soc. 2006;128:6334–6335. doi: 10.1021/ja061735s. [DOI] [PubMed] [Google Scholar]
- 51.Mulder WJM, Strijkers GJ, van Tilborg GAF, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 52 •.Lerouge F, Melnyk O, Durand JO, Raehm L, Berthault P, Huber G, Desvaux H, Constantinesco A, Choquet P, Detour J, et al. Towards thrombosis-targeted zeolite nanoparticles for laser-polarized Xe-129 MRI. J Mater Chem. 2009;19:379–386. Very slow exchange phenomenon between free xenon and xenon embedded in pores of zeolite colloidal nanoparticles functionalized with a peptide is observed and hp 129Xe exhibits fairly long relaxation times inside the pores. This is the first paper to look at the distribution of a xenon biosensor in vivo. [Google Scholar]
- 53.Harel E, Schroder L, Xu S. Novel detection schemes of nuclear magnetic resonance and magnetic resonance imaging: Applications from analytical chemistry to molecular sensors. Annu Rev Anal Chem. 2008;1:133–163. doi: 10.1146/annurev.anchem.1.031207.113018. [DOI] [PubMed] [Google Scholar]
- 54.Lowery TJ, Garcia S, Chavez L, Ruiz EJ, Wu T, Brotin T, Dutasta JP, King DS, Schultz PG, Pines A, et al. Optimization of xenon biosensors for detection of protein interactions. Chembiochem. 2006;7:65–73. doi: 10.1002/cbic.200500327. [DOI] [PubMed] [Google Scholar]
- 55.Ruiz EJ, Sears DN, Pines A, Jameson CJ. Diastereomeric Xe chemical shifts in tethered cryptophane cages. J Am Chem Soc. 2006;128:16980–16988. doi: 10.1021/ja066661z. [DOI] [PubMed] [Google Scholar]
- 56.Moule AJ, Spence MM, Han SI, Seeley JA, Pierce KL, Saxena S, Pines A. Amplification of xenon NMR and MRI by remote detection. Proc Natl Acad Sci USA. 2003;100:9122–9127. doi: 10.1073/pnas.1133497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sears DN, Jameson CJ. Theoretical calculations of the Xe chemical shifts in cryptophane cages. J Chem Phys. 2003;119:12231–12244. [Google Scholar]
- 58 •.Aaron JA, Chambers JM, Jude KM, Costanzo LD, Dmochowski IJ, Christianson DW. Structure of a 129Xe-cryptophane biosensor complexed with human carbonic anhydrase II. J Am Chem Soc. 2008;130:6942–6943. doi: 10.1021/ja802214x. The first reported X-ray crystal structure of cryptophane bound to protein (CA) confirms tight binding of biosensor with CAII and the encapsulation of one xenon atom inside of functionalized cryptophane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schröder L, Chavez L, Meldrum T, Smith M, Lowery TJ, Wemmer DE, Pines A. Temperature-controlled molecular depolarization gates in nuclear magnetic resonance. Angew Chem-Int Ed. 2008;47:4316–4320. doi: 10.1002/anie.200800382. [DOI] [PubMed] [Google Scholar]
- 60.Hilty C, Lowery TJ, Wemmer DE, Pines A. Spectrally resolved magnetic resonance imaging of a xenon biosensor. Angew Chem-Int Ed. 2006;45:70–73. doi: 10.1002/anie.200502693. [DOI] [PubMed] [Google Scholar]
- 61 •.Berthault P, Bogaert-Buchmann A, Desvaux H, Huber G, Boulard Y. Sensitivity and multiplexing capabilities of MRI based on polarized Xe-129 biosensors. J Am Chem Soc. 2008;130:16456–16457. doi: 10.1021/ja805274u. Discrimination via high spatial resolution 129Xe MRI images was achieved between xenon in two different hosts dissolved in two immiscible media by using a gradient echo sequence with interleaved frequency selection. [DOI] [PubMed] [Google Scholar]
- 62.Driehuys B, Moller HE, Cleveland ZI, Pollaro J, Hedlund LW. Pulmonary perfusion and xenon gas exchange in rats: MR imaging with intravenous injection of hyperpolarized Xe-129. Radiology. 2009;252:386–393. doi: 10.1148/radiol.2522081550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santyr GE, Lam WW, Parra-Robles JM, Taves TM, Ouriadov AV. Hyperpolarized noble gas magnetic resonance imaging of the animal lung: Approaches and applications. J Appl Phys. 2009;105:102004. [Google Scholar]