Abstract
PURPOSE
To report normal macular thickness measurements and assess reproducibility of retinal thickness measurements acquired by a time domain optical coherence tomography (OCT)(Stratus [Carl Zeiss Meditec, Inc., Dublin, CA, USA]) and three commercially available spectral / Fourier domain OCT instruments (Cirrus HD-OCT [Carl Zeiss Meditec, Inc., Dublin, CA, USA], RTVue-100 [Optovue, Inc., Fremont, CA, USA], 3D OCT-1000 [Topcon, Inc., Paramus, NJ, USA]).
METHODS
Forty randomly selected eyes of 40 normal, healthy volunteers were imaged. Subjects were scanned twice during one visit and a subset of 25 was scanned again within 8 weeks. Retinal thickness measurements were automatically generated by OCT software and recorded after manual correction. Regression and Bland-Altman plots were used to compare agreement between instruments. Reproducibility was analyzed by using intraclass correlation coefficients (ICC), and incidence of artifacts was determined.
RESULTS
Macular thickness measurements were found to have high reproducibility across all instruments, with ICC values ranging 84.8–94.9% for Stratus OCT; 92.6–97.3% for Cirrus Cube; 76.4–93.7% for RTVue MM5, 61.1–96.8% for MM6; 93.1–97.9% for 3D OCT-1000 Radial, 31.5–97.5% for 3D Macular scans. Incidence of artifacts was higher in spectral / Fourier domain instruments, ranging 28.75 to 53.16%, compared to 17.46% in Stratus OCT. No significant age or gender trends were found in the measurements.
CONCLUSIONS
Commercial spectral / Fourier domain OCT instruments provide higher speed and axial resolution than the Stratus OCT, although they vary greatly in scanning protocols and are currently limited in their analysis functions. Further development of segmentation algorithms and quantitative features are needed to assist clinicians in objective use of these newer instruments to manage diseases.
Keywords: spectral, Fourier, optical coherence tomography, normal, macular thickness, reproducibility, artifact, segmentation
Introduction
Optical coherence tomography (OCT) is a diagnostic imaging technology that performs high resolution, cross-sectional imaging of biological tissues.1 In ophthalmology, qualitative assessment of retinal morphology via OCT is commonly used for detection and monitoring of diseases such as macular holes and age-related macular degeneration.2 Quantitative assessment is important in glaucoma and diabetic retinopathy, enabling objective monitoring of disease progression and response to therapy.3,4
Analogous to ultrasound, OCT measures light rather than acoustic waves. Light from a broadband light source is divided into a reference beam traveling a delay path and a sample beam that is directed onto the subject’s retina. Light backscattered by retinal structures interferes with light from the reference beam, and the interference of echoes is detected to create a measurement of light echoes versus depth. Standard OCT systems, such as the Stratus OCT [Carl Zeiss Meditec, Inc., Dublin, CA, USA], use time-domain detection, in which the reference mirror position and delay are mechanically scanned in order to produce axial scans (A-scans) of light echoes versus depth. Scan rates of 400 A-scans per second with an axial resolution of 8–10 μm in the eye are achieved with the Stratus OCT.
More recently, spectral / Fourier domain detection, in which a high-speed spectrometer is used to measure light echoes from all time delays simultaneously, has enhanced OCT capabilities. The reference mirror does not require mechanical scanning, and improved sensitivity enables dramatic improvements in sampling speed and signal-to-noise ratio.5–7 Spectral / Fourier domain detection, coupled with improvements in light sources, achieves axial scanning speeds of greater than 20,000 A-scans per second with an axial resolution of 5–7 μm in the eye. At least 7 commercial spectral / Fourier domain OCT systems have been released since 2006. Although studies have shown qualitative improvements using this new technology,8,9 quantitative evaluation of macular thickness needs to be validated prior to clinical use. Three models, the Cirrus HD-OCT [Carl Zeiss Meditec, Inc., Dublin, CA, USA], RTVue-100 [Optovue, Inc., Fremont, CA, USA], and 3D OCT-1000 [Topcon, Inc., Paramus, NJ, USA] were included in this study in order to report measurements of normal subjects and assess reproducibility.
Methods
Subjects
One eye was randomly selected for scanning from each of 41 healthy volunteers who were initially enrolled in this study. One subject was excluded from analysis after it was later revealed that he had a history of underlying ocular pathology. The mean age of subjects was 36.1 ± 15.9 (range, 20–82) years. Nineteen females and 21 males, consisting of 22 Caucasian, 13 Asian, 3 Hispanic, and 2 African-American subjects, were included.
Subjects were scanned twice on all 4 instruments in a random order during an initial visit, with at least 1 minute rest between scans and re-optimization of settings. A subset of 25 subjects was scanned during a second visit within 8 weeks. Inclusion criteria were best corrected visual acuity 20/40 or better and no history or evidence of retinal pathology, glaucoma, intraocular surgery or laser treatment. Spherical refractive error ranged between −7.5 and +0.50 diopters. No mydriatic treatment was used. The study was conducted in accordance with the ethical standards stated in the 1964 Declaration of Helsinki and approved by the Institutional Review Board of Tufts Medical Center with informed consent obtained prior to scan acquisition.
Scan Protocols
OCT imaging was performed with the Stratus OCT using time domain detection with 8–10 μm axial resolution and acquisition speeds of 400 axial scans per second. Scans were obtained with the macular thickness protocol, consisting of 6 consecutive, radial, equally-spaced, 6 mm linear scans with 512 sampling points (A-scans) each. In our clinic, this protocol is preferred over the fast macular thickness protocol due to its higher resolution (512 versus 128 A-scans per radial scan) and the ability to minimize motion artifacts by allowing the operator to select the best images for individual scans. All scans acquired had a signal strength of at least 7 (range, 7–10).
Spectral / Fourier domain OCT imaging was performed using 3 commercially-available instruments. The Cirrus HD-OCT had a 5 μm axial image resolution and imaging speed of 27,000 axial scans per second. On the Cirrus HD-OCT, the 5-line raster (5 horizontal line scans consisting of 4096 A-scans each, within a 6×1 mm2 area) and macular cube (128 horizontal line raster with 512 A-scans each, within a 6×6 mm2 area) protocols were used, with all scans having signal strength of at least 7 (range, 8.22–10.81). The RTVue-100 had a 5 μm axial image resolution and imaging speed of 26,000 axial scans per second. On the RTVue-100, the MM5 (5×5 mm2 grid of 11 horizontal and 11 vertical lines with 668 A-scans each and an inner 3×3 mm2 grid of 6 horizontal and 6 vertical lines with 400 A-scans each), MM6 (12 radial line scans with 1024 A-scans each, within 6 mm diameter), and 3D macula (128 line raster with 512 A-scans each, within 6×6 mm2) protocols were used, with all scans having signal strength of at least 40 (range, 40.4–79.4). The 3D OCT-1000 had a 6 μm axial image resolution and imaging speed of 18,000 axial scans per second. On the 3D OCT-1000, the radial (6 radial line scans with 1024 A-scans each) and 3D macular (128 line raster with 512 A-scans each, within 6×6 mm2) were used. Due to the early version of its software, a signal strength indicator (range, 29.88–80.04) was not available for the first 20 patients on the 3D OCT-1000 at time of acquisition.
Measurements
For all scan types analyzed, topographic surface maps were constructed using the automated software algorithms and displayed with numeric averages of the thickness measurements for each of the 9 map sectors as defined by the Early Treatment Diabetic Retinopathy Study (ETDRS).10 Final analysis was performed on scans after manually correcting for segmentation anomalies as described below. Central subfield thickness was defined as the average thickness in the central 1 mm subfield centered at the fovea. Inner and outer rings were divided into 4 quadrants, with the inner ring bounded by 1 mm and 3 mm concentric circles and the outer ring bounded by 3 mm and 5 or 6 mm concentric circles (Figure 1).
Fig 1.
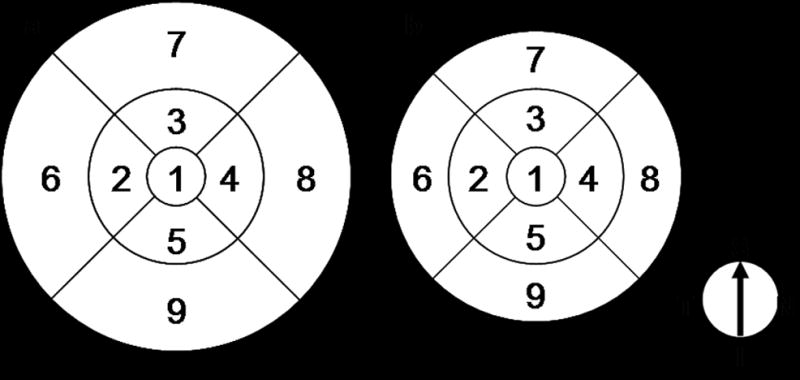
Nine regions of ETDRS map. All scans produce maps consisting of 1, 3, and 6 mm diameter concentric circles (a) except for the RTVue MM5, whose outer circle is 5 mm in diameter (b). Regions 2–5 comprise the parafoveal ring, while regions 6–9 comprise the perifoveal ring.
Each image from the radial scans was examined for error and manually corrected if an artifact was found. Due to the much larger quantity of data in raster and MM5 scans, topographic anomalies in the periphery were identified first using the ETDRS-like map and then correlated to the corresponding cross-sectional image, and only the central foveal 1 mm diameter region was manually corrected if necessary.
Throughout the duration of this study, software on all 3 spectral / Fourier domain instruments underwent numerous revisions and images were consequently reprocessed using updated algorithms. At the conclusion of the study, software version 4.0 for the Stratus OCT, version 3.0.0.67 of the research browser for the Cirrus HD-OCT, version 3.0.0.0 for the RTVue-100, and version 2.12 for the 3D OCT-1000 were used to generate the reported macular thickness measurements.
Statistical Analysis
Analysis of variance (ANOVA) using random-effects mixed models (Proc Mixed features, SAS version 9.1 [SAS Institute, Inc., Cary, NC, USA]) was performed to determine reproducibility in terms of intraclass correlation coefficients (ICC) and intervisit standard deviations (SD).11 ICC was the ratio of the intersubject component of variance to the total variance. Negative variance components were set to 0. Using our model, p-values (STATA 9.0 [StataCorp LP, College Station, TX, USA]) for the ICC’s were calculated for the subject component, as it was not possible to further split out the day component.
Bland-Altman plots (R v 2.6 [Free Software Foundation, Inc., Boston, MA, USA]) were used to assess agreements of foveal thicknesses. Regression plots were graphed to generate conversion factors comparing various spectral / Fourier domain OCT instruments to Stratus OCT. R2 values were calculated for these regression plots to measure the quality of the model, along with the minimum and maximum limits of the 95% confidence intervals. The relationship between foveal thickness and gender and age was examined using two-tailed t-tests and linear regression analysis respectively (Microsoft Excel version 2002 SP3 [Microsoft Corporation, Redmond, WA, USA]).
Artifacts
The incidence of artifacts, defined as inaccurate segmentation of retinal layers, was compared across instruments. Error types were classified based on prior work on Stratus OCT.12 “Any artifact” indicates scans with any error, all of which, aside from “out of register” errors, required manual correction.
Artifacts were classified into 6 categories (Figure 2).
Fig 2.
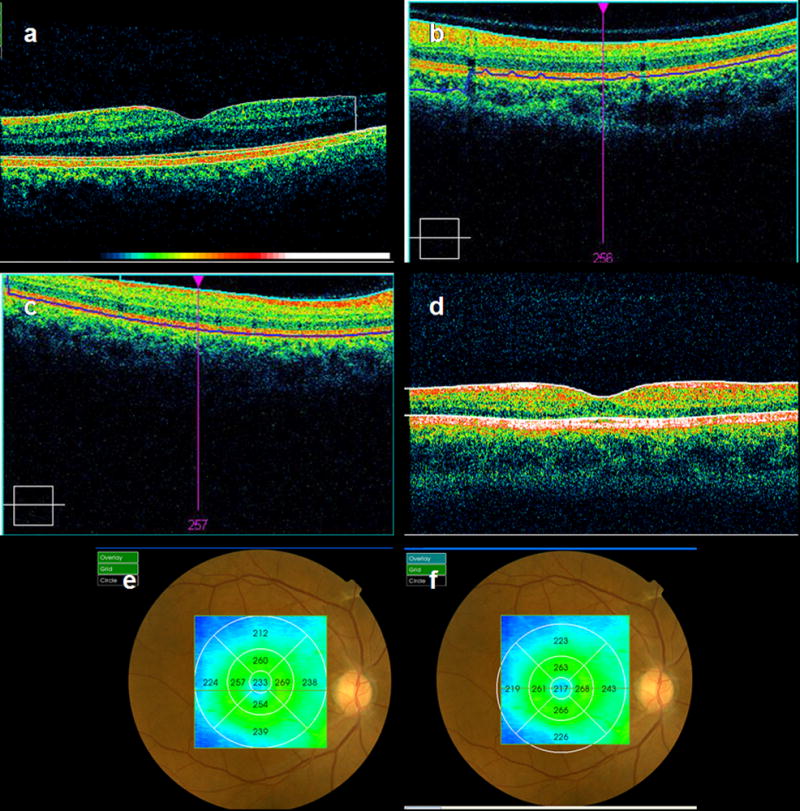
Demonstration of error types, including errors in segmentation of inner retina (a); outer retina (b); out-of-register, inner, and outer retina scan error (c); cut-edge artifact (d); and off-center fixation error causing inaccurate foveal thickness measurement shown before (e) and after manual correction (f).
“Inaccurate foveal thickness” denotes scans where the average thickness of the central subfield changed 1 μm or more after manual correction.
“Inner retina misidentification” indicates scans with inaccurate segmentation of the internal limiting membrane.
“Outer retina misidentification” indicates scans with errors in segmentation of the outer boundary used for retinal thickness calculations.
“Out of register” artifacts are those in which the scan was shifted superiorly, resulting in a truncated inner retina.
“Off center” artifacts occurred when the foveal center was misidentified as being >0.25 mm away from the true fovea based on the topographic map.
“Cut edge” artifacts occurred when the edge of the scan was truncated.
Manual correction of artifacts included deleting offending images on Stratus OCT if error was present, redrawing boundary lines on spectral / Fourier domain OCT images if necessary, and shifting the ETDRS grid on the 3D OCT-1000 for fixation errors. Measurements from peripheral subfields that were shifted out of the scan range as a result were excluded from analysis.
Results
Several scan types, including the Cirrus 5-line raster and RTVue 3D macular protocols, could not yet be quantified and were thus excluded from analysis. For the other scans, mean thicknesses of map subfields acquired from all first visits after manual post-processing (Table 1) revealed systematic differences in spectral / Fourier domain instruments compared to Stratus OCT. Standard deviations were universally highest at the fovea. Macular thickness measurements were generally thinnest at the fovea and thickest in the parafoveal superior and nasal quadrants.
Table 1.
Comparison of mean thickness measurements for all subjects collected during first visit.*
| Stratus Macular Thickness | Cirrus Cube 512×128 | RTVue MM5 | RTVue MM6 | 3D OCT- 1000 Radial | 3D OCT- 1000 3D Macular | |
|---|---|---|---|---|---|---|
| Fovea | 203±17 | 262±16 | 267±15 | 256±15 | 227±17 | 231±16 |
| Parafovea | ||||||
| Temporal | 262±14 | 306±10 | 317±9 | 308±13 | 279±10 | 280±10 |
| Superior | 276±13 | 320±12 | 331±12 | 324±11 | 292±12 | 293±12 |
| Nasal | 278±13 | 323±12 | 330±11 | 324±11 | 294±12 | 296±12 |
| Inferior | 274±11 | 316±11 | 327±10 | 318±10 | 287±10 | 288±10 |
| Perifovea | ||||||
| Temporal | 219±12 | 255±9 | 283±11 | 265±10 | 233±10 | 234±16 |
| Superior | 239±13 | 274±13 | 296±14 | 278±13 | 248±13 | 249±13 |
| Nasal | 255±14 | 293±13 | 305±14 | 291±14 | 264±13 | 266±13 |
| Inferior | 225±14 | 264±11 | 289±13 | 267±12 | 238±11 | 240±12 |
Measurements were acquired after manual correction of artifacts and shown in μm ± standard deviation.
Bland-Altman plots of central subfield measurements (Figure 3) showed that values obtained on spectral / Fourier domain OCT were greater than corresponding measurements from Stratus OCT. 95% limits of agreement spanned 23.76 μm for Cirrus HD-OCT, 26.52 μm for RTVue MM5, 31.64 μm for RTVue MM6, 21.96 μm for 3D OCT-1000 Radial, and 21.72 μm for 3D OCT-1000 3D Macular.
Fig 3.
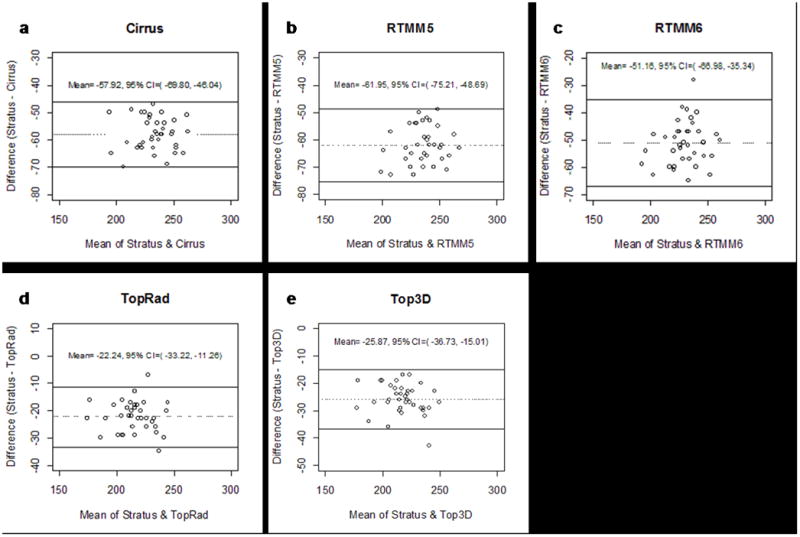
Bland-Altman plots of central subfield thickness, displayed as the difference between Cirrus HD-OCT (a); RTVue-100 MM5 (b); RTVue-100 MM6 (c); 3D OCT-1000 Radial (d); 3D OCT-1000 3D Macular (e) and Stratus OCT versus the average of the 2 measurements. Lines indicating average mean difference with 95% confidence limits are shown.
Figure 4 presents linear regression plots of central subfield thickness measured with different spectral / Fourier domain instruments versus the Stratus OCT. Measurements correlated and fit well to a linear model. Figure 4 shows y-intercept values ranging from ~70 to 90 μm in the Cirrus and RTVue to ~25 to 30 μm in the 3D OCT-1000. The slopes vary between ~0.8 in the RTVue to 1.0 in the 3D OCT-1000. Table 2 presents the r2 values derived from the regression plots. The high r2 values indicate that the linear regression plots are of high quality. Minimum and maximum values for the width of the 95% confidence intervals for each instrument were in the 19–25 μm range.
Fig 4.
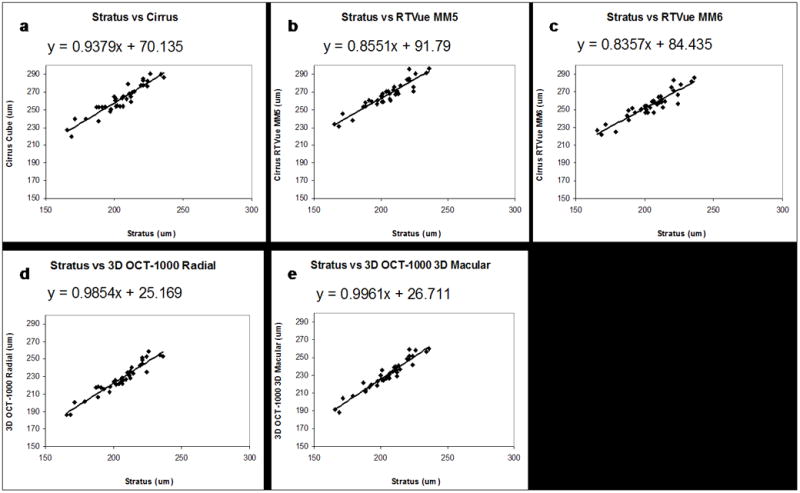
Regression plots of central subfield thickness measured using Cirrus HD-OCT (a); RTVue-100 MM5 (b); RTVue-100 MM6 (c); 3D OCT-1000 Radial (d); 3D OCT-1000 3D Macular (e) compared to Stratus OCT. The differences in y-intercepts reflect differences in measurement algorithms.
Table 2.
R2 values and 95% confidence interval (CI) widths calculated for regression plot equations of spectral /Fourier domain versus Stratus OCT measurements of central subfield thickness.
| r-squared | 95% CI Min | 95% CI Max | |
|---|---|---|---|
| Cirrus Cube 512×128 | 0.89 | 23.58 | 25.22 |
| RTVue MM5 | 0.88 | 22.42 | 23.98 |
| RTVue MM6 | 0.85 | 24.16 | 25.83 |
| 3D OCT-1000 Radial | 0.92 | 20.37 | 21.79 |
| 3D OCT-1000 3D Macular | 0.93 | 19.48 | 20.84 |
All scan types demonstrated high reproducibility after manual correction (Table 3). The fovea, superior and nasal quadrants generally demonstrated the highest reproducibility, with combination of highest ICC and lowest intervisit SD. With the exception of the perifoveal temporal subfield of the 3D OCT-1000 Macular scans, all ICC’s were >60% for all instruments and most were in the >80% range. All ICCs were statistically significantly larger than 0 (p<0.01 for each ICC).
Table 3.
Reproducibility of macular thickness measurements by scan type.*
| Stratus Macular Thickness | Cirrus Cube 512×128 | RTVue MM5 | RTVue MM6 | 3D OCT-1000Radial | 3D OCT-1000 3D Macular | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICC* | SD† | ICC | SD | ICC | SD | ICC | SD | ICC | SD | ICC | SD | |
| Fovea | 92.02 | 3.32 | 97.25 | 1.91 | 88.54 | 3.44 | 90.75 | 1.76 | 97.88 | 1.10 | 96.59 | 0.71 |
| Parafovea | ||||||||||||
| Temporal | 94.93 | 2.15 | 92.61 | 2.44 | 82.64 | 2.60 | 61.09 | 0.00 | 96.41 | 0.00 | 82.06 | 0.00 |
| Superior | 94.63 | 1.67 | 95.30 | 2.10 | 92.07 | 2.31 | 92.72 | 2.19 | 94.96 | 1.28 | 95.87 | 1.21 |
| Nasal | 84.53 | 2.80 | 94.85 | 2.13 | 93.73 | 1.69 | 92.6 | 0.00 | 93.7 | 1.13 | 94.61 | 1.73 |
| Inferior | 93.99 | 1.67 | 94.34 | 1.86 | 76.43 | 3.09 | 92.14 | 1.14 | 95.26 | 1.18 | 92.28 | 0.00 |
| Perifovea | ||||||||||||
| Temporal | 89.38 | 1.98 | 93.28 | 1.56 | 82.44 | 0.00 | 91.94 | 2.01 | 93.11 | 0.00 | 31.47 | 2.43 |
| Superior | 92.17 | 2.42 | 94.41 | 2.31 | 80.12 | 0.00 | 96.78 | 0.00 | 94.73 | 1.71 | 95.71 | 1.43 |
| Nasal | 91.16 | 1.19 | 96.93 | 1.91 | 92.2 | 2.76 | 85.41 | 0.00 | 95.25 | 0.00 | 97.46 | 1.45 |
| Inferior | 84.8 | 0.00 | 95.55 | 1.41 | 88.82 | 2.47 | 95.36 | 1.22 | 94.33 | 1.15 | 64.38 | 0.00 |
ICC = intraclass correlation coefficient expressed as % (p<0.01 for each ICC)
SD = intervisit standard deviation expressed in μm.
No statistically significant difference was found in central subfield thickness between sexes on any of the instruments, although mean thicknesses in men were greater than in women (Table 4). No gender differences were found in thickness of the other 8 subfields (p>0.05, data not shown). By linear regression analysis, no relationship was found between age and foveal thickness (p>0.05, data not shown).
Table 4.
Central foveal subfield thickness measurements according to gender.*
| Scan Type | Men (n=21) Mean ± SD | Women (n=19) Mean ± SD | p-value |
|---|---|---|---|
| Stratus Macular Thickness | 208±14 | 201±19 | 0.240 |
| Cirrus Cube 512×128 | 266±16 | 258±17 | 0.156 |
| RTVue MM5 | 271±14 | 263±16 | 0.127 |
| RTVue MM6 | 258±14 | 253±16 | 0.267 |
| 3D OCT-1000 Radial | 230±16 | 223±18 | 0.199 |
| 3D OCT-1000 3D Macular | 234±16 | 226±18 | 0.151 |
Measurements are expressed μm ± standard deviation.
Artifact incidence and corresponding signal strengths are reported in Table 5. Signal strengths were determined automatically by the software on each instrument. Although the incidence of spectral / Fourier OCT scans with artifacts was relatively high, ranging from 28.75 to 53.16%, most segmentation errors occurred in the periphery and did not affect central subfield measurements. “Cut edge” artifacts occurred only in Stratus OCT scans. To assess the degree of error caused by eccentric fixation, 3D OCT-1000 scans were further examined. 75% of radial scans and 100% of 3D macular scans containing an “off center” artifact had consequent inaccurate foveal thickness measurements, deviating from the accurate thickness by 5.50 and 9.67 μm respectively on average.
Table 5.
Artifacts and signal strength of all scans acquired in first visit.
| Scan Type | Signal Strength* | Any Artifact | Inaccurate CFT† | IRM‡ | ORM** | Out of Register | Off Center | Cut Edge |
|---|---|---|---|---|---|---|---|---|
| Stratus Macular Thickness | 8.83±1.22 | 17.46% | 4.76% | 12.70% | 0.00% | 1.59% | 6.35% | 6.35% |
| Cirrus Cube 512×128 | 9.93±0.42 | 43.75% | 0.00% | 15.00% | 36.25% | 8.75% | 0.00% | 0.00% |
| RTVue MM5 | 61.76±9.10 | 32.50% | 6.25% | 22.50% | 1.25% | 7.50% | 11.25% | 0.00% |
| RTVue MM6 | 62.72±9.74 | 38.75% | 8.75% | 35.00% | 7.50% | 0.00% | 6.25% | 0.00% |
| 3D OCT-1000 Radial | 60.54±9.29 | 28.75% | 15.00% | 26.25% | 1.25% | 5.00% | 5.00% | 0.00% |
| 3D OCT-1000 3D Macular | 50.64±9.95 | 53.16% | 17.72% | 34.18% | 12.66% | 18.99% | 7.59% | 0.00% |
Signal strengths are determined by automated software on each instrument.
CFT = central subfield foveal thickness
IRM = inner retinal misidentification
ORM = outer retinal misidentification.
Discussion
Macular thickness measurements13,14 and their reproducibility11,15,16 have been studied extensively in the past for Stratus OCT, while few studies have examined measurements for the new spectral / Fourier domain instruments.17,18 Our data agrees with prior published thickness and reproducibility measurements obtained on Stratus OCT and 3D OCT-1000 and adds data from the Cirrus HD-OCT and RTVue-100. Several scan types, however, could not yet be quantified, and currently a normative database is only available for the RTVue MM5 protocol.
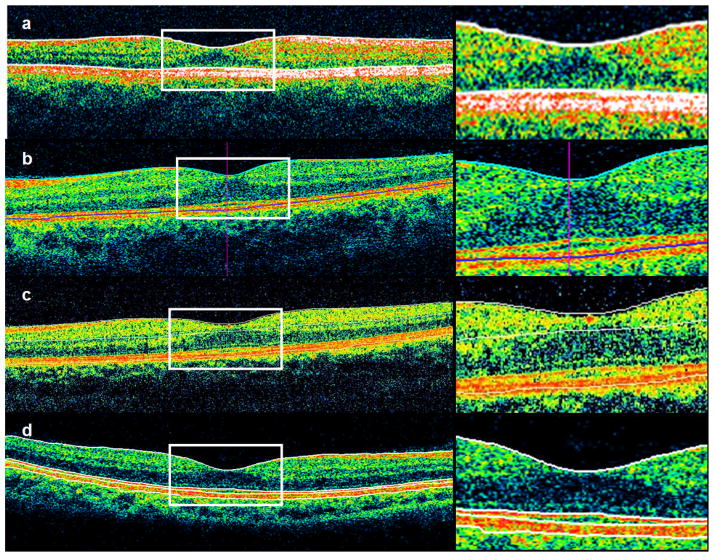
Although some confusion exists in the literature regarding the resolution of outer retinal layers by OCT and their in vivo correlation,19 recent work using a prototype ultrahigh-resolution OCT has better clarified interpretation of these layers. At 5–7 μm axial resolution, the outer retina can be visualized as three bands in OCT images presumed to correspond to the inner / outer segment (IS/OS) junction, the photoreceptor outer segment tips, and the RPE. All 4 commercial OCT instruments define the inner retinal boundary as the internal limiting membrane (ILM), but they differ in defining the outer retinal boundary. Stratus OCT measures retinal thickness to the IS/OS junction of photoreceptors, while Cirrus HD-OCT and RTVue-100 include the outer segments and measure closer to the retinal pigment epithelium (RPE). The 3D OCT-1000 measures to the photoreceptor outer segment tips (Figure 5), which is between the RPE and the IS/OS junction. A previous study showed that the distance between the inner IS/OS boundary and the outer RPE/Bruch’s membrane boundary is between 60 and 80 μm and varies with eccentricity.20 This suggests that systematic discrepancies in retinal thickness measurements between instruments may be partially accounted for by the choice of outer retinal boundary.
Fig 5.
Demonstration of macular thickness segmentation algorithms in different instruments. All instruments define the inner boundary as the internal limiting membrane, but there is variation in outer boundaries. Stratus OCT measures to the IS/OS junction (a), Cirrus HD-OCT (b) and RTVue-100 (c) both measure close to the RPE, and the RTVue also draws a boundary at the inner plexiform layer. 3D OCT-1000 measures to the photoreceptor outer segment tips (d), shown as the third line from the top. This instrument also draws boundaries at the IS/OS junction (second line) and posterior RPE boundary (fourth line), which are not included in retinal thickness measurements.
The linear regression analysis shown in Figure 4 shows the central subfield thickness measured by the Stratus OCT versus other instruments. The y-intercept values for the Stratus versus Cirrus and RTVue measurements are consistent with the fact that the Cirrus and RTVue measure retinal thickness to the RPE, while the Stratus measures to the IS/OS junction. The smaller y-intercept values of 25 to 30 μm for the 3D OCT-1000 are consistent with the 3D OCT-1000 measuring retinal thickness to the photoreceptor outer segment tips (between the RPE and IS/OS junction). At the same time, if the variation in retinal thickness between subjects occurs predominantly in retinal layers outside the outer segments, one would expect that the linear regression would have a slope of 1. However, the slopes vary between ~0.8 for the RTVue to 1.0 for the 3D OCT-1000. Variations in slope are difficult to explain solely based on the different reference boundary convention in the different instruments and may arise because of differences in the conversion factors used to convert echo time delay to distance (index of refraction) or more subtle differences in the segmentation algorithms used in the different instruments. However, since the details of segmentation algorithms are proprietary to the manufacturers, it is difficult to ascertain the reason for these differences.
Although an attempt was made to generate an equation to convert spectral / Fourier domain OCT and Stratus OCT measurements interchangeably (Figure 4), the spans of 95% confidence intervals for these regression plots (Table 2) indicate that such a conversion factor should not currently be used unless one can accept a predictive error of up to ~26 μm per central subfield thickness measurement. Differences in mean thickness measurements between machines can vary by over 20 μm (Figure 3), likely due to variability introduced by the comparison of different outer retinal layers.
As a result of improved delineation of these outer retinal layers, the standard for thickness calculations may change as the community decides which boundaries to use. Currently, however, no consensus exists. Each layer has different advantages and disadvantages when being considered as the posterior retinal boundary. The inner boundary of the IS/OS junction has high contrast and is relatively straightforward to detect in normal eyes. On the other hand, using this boundary excludes the photoreceptor outer segments from thickness measurements and may cause errors when the photoreceptors are either elevated or impaired. The inner border of RPE might be advantageous for quantifying diseases affecting inner retinal layers but sparing the RPE, such as macular holes, cystoid macular edema, or separation of sensory layers secondary to vitreomacular traction. On the other hand, the outer border of RPE might be more beneficial in diseases involving the RPE, such as central serous chorioretinopathy, pigmented epithelial detachment, or choroidal neovascularization.
Since there is no universal standard for analysis of retinal layer segmentation errors, the method used by Ray et al. was adapted for artifact analysis. Other classification schemes involving measurements of artifact length and distance from the fovea21 were not used due to the high volume of images and limitations in graphical user interface of current analysis software. Although this classification scheme does not directly assess the degree of error, the “inaccurate foveal thickness” category provides an indirect and clinically relevant measurement of whether the artifacts affect this important region. It should be noted that thickness measurements from the Stratus are based on interpolated data from only 6 radial scans, compared to measurements acquired from up to 128 line scans on the spectral / Fourier domain instruments. Consequently, a segmentation artifact on a single line scan on the Stratus is likely to have a much larger impact on the final thickness measurement than an equivalent artifact on a single spectral / Fourier domain line scan. A more rigorous analysis comparing induced errors in volume using third-party software would better elucidate the actual significance of these segmentation artifacts, but within the current limits of the software at this time, it was not possible to extract the raw figures from all instruments for comparison.
The highest reproducibility in foveal subfield thickness, characterized by highest ICC and lowest SD, was found in Cirrus HD-OCT and 3D OCT-1000 scans. This may be partly due to the fact that no “off center” artifacts were found in any Cirrus scans, although it is unclear why the same subjects had fixation errors on the other instruments. The 3D OCT-1000 was the only instrument that allowed repositioning of the ETDRS grid to compensate for fixation errors, and it is possible that this additional post-processing step helped achieve higher reproducibility. “Off center” artifacts, due to eccentric fixation, were likely to result in inaccurate central subfield thickness when present, although the difference in measurement was relatively small. By comparison, Campbell et al. reported 44.5% error in foveal thickness measurement with only 0.50 mm decentration22, and Ray et al. found that “off center” artifacts always produced incorrect foveal measurements on Stratus OCT. Intervisit changes in measurement due to eccentric fixation may potentially be minimized by registration of OCT scans to landmark features such as retinal vessels and optic nerve head or by employing eye tracking, as in the Heidelberg Spectralis HRA+OCT [Heidelberg Engineering, Inc., Vista, CA, USA].
Although some studies have shown differences in macular thickness based on gender23, age24, and race25, no statistically significant difference by these parameters was found on any of the instruments in our study. This may be due to the smaller sample size or different population involved in this study.
It should be noted that signal strength numbers across instruments are not comparable, as each instrument uses a proprietary scale and criteria to determine image quality. Direct, objective comparison of image quality would only be possible by exporting raw data from each instrument and using third-party software to compare scans.
There are limitations to the current study. Scans were acquired by four experienced operators, who may have slight variations in technique. To minimize this effect on individual subjects, volunteers were scanned during both visits by the same operator. The addition of the “quality factor” feature for image quality on the 3D OCT-1000 may improve acquisition quality, which would likely decrease segmentation artifacts. Dilation of subjects would likely improve image quality as well. Lastly, patients with vitreoretinal disorders causing gross deviations from normal retinal contour or cataracts degrading image quality will likely have a higher incidence of artifacts. It is important to note that this study reports reproducibility estimates in normal eyes, and segmentation algorithms may perform differently in eyes with retinal pathologies. This study serves as a baseline to compare with future studies on patients with these diseases.
In conclusion, commercial spectral / Fourier domain OCT instruments vary greatly in scanning protocols and are currently limited in analysis functions, although they uniformly possess higher speed and axial image resolution compared to Stratus OCT. Clinicians should be aware that differences in system calibration, scan protocols, and segmentation algorithms may contribute to disparities in thickness measurements between instruments. To minimize clinical confusion in OCT measurements, user selection of layers for quantification or a consensus among manufacturers in defining retinal thickness boundaries as well as segmentation algorithms is needed. Other features that may aid in more objective analyses of diseases include options for quantification of subretinal fluid volumes, drusen, macular holes, tumors, and normative databases. Finally, due to the high incidence of segmentation artifacts, post-processing options for manual correction such as redrawing boundaries and shifting the ETDRS grid are necessary. Manual correction can be labor-intensive, however, and therefore impractical in a busy clinical setting. Manufacturers and software developers should make every effort to minimize this through refinement of segmentation algorithms. More flexibility, involving a combination of manual adjustment and automatic segmentation based on disease, may be necessary to attain this goal. Although we have not demonstrated a quantitative improvement in spectral / Fourier domain OCT over Stratus OCT, we expect that the higher acquisition speed and resolution should reduce errors from eye motion and improve measurement accuracy and reproducibility as further developments in software become available.
Acknowledgments
Support
This work was supported in part by a Research to Prevent Blindness Challenge grant to the New England Eye Center/Department of Ophthalmology -Tufts University School of Medicine, NIH contracts RO1-EY11289-23, R01-EY13178-07, P30-EY008098, Air Force Office of Scientific Research FA9550-07-1-0101 and FA9550-07-1-0014.
Footnotes
Disclosures
J.G. Fujimoto receives royalties from intellectual property owned by MIT and licensed to Carl Zeiss Meditec, Inc. and has stock options in Optovue, Inc.. J.S. Schuman receives research support and royalties from intellectual property licensed to Carl Zeiss Meditec, Inc.. J.S. Duker receives research support from Carl Zeiss Meditech, Inc., Optovue, Inc., and Topcon Medical Systems, Inc..
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102(2):217–229. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 3.Hee MR, Puliafito CA, Wong C, et al. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995;113(8):1019–1029. doi: 10.1001/archopht.1995.01100080071031. [DOI] [PubMed] [Google Scholar]
- 4.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113(5):586–596. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 5.de Boer JF, Cense B, Park BH, et al. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett. 2003;28(21):2067–2069. doi: 10.1364/ol.28.002067. [DOI] [PubMed] [Google Scholar]
- 6.Leitgeb R, Hitzenberger CK, Fercher AF. Performance of fourier domain vs. time domain optical coherence tomography. Opt Express. 2003;11(8):889–894. doi: 10.1364/oe.11.000889. [DOI] [PubMed] [Google Scholar]
- 7.Choma MA, Sarunic MV, Yang CH, Izatt JA. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003;11(18):2183–2189. doi: 10.1364/oe.11.002183. [DOI] [PubMed] [Google Scholar]
- 8.Gupta V, Gupta P, Singh R, et al. Spectral-domain Cirrus high-definition optical coherence tomography is better than time-domain Stratus optical coherence tomography for evaluation of macular pathologic features in uveitis. Am J Ophthalmol. 2008;145(6):1018–1022. doi: 10.1016/j.ajo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto A, Hangai M, Yoshimura N. Spectral-domain optical coherence tomography with multiple B-scan averaging for enhanced imaging of retinal diseases. Ophthalmology. 2008;115(6):1071–1078. doi: 10.1016/j.ophtha.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Early Treatment Diabetic Retinopathy Study Research Group. ETDRS Report Number 7: Early Treatment Diabetic Retinopathy Study Design and Baseline Patient Characteristics. Ophthalmology. 1991;98:741–756. doi: 10.1016/s0161-6420(13)38009-9. [DOI] [PubMed] [Google Scholar]
- 11.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using Stratus OCT. Invest Ophthalmol Vis Sci. 2004;45(6):1716–1724. doi: 10.1167/iovs.03-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray R, Stinett SS, Jaffe GJ. Evaluation of image artifact produced by optical coherence tomography of retinal pathology. Am J Ophthalmol. 2004;139(1):18–29. doi: 10.1016/j.ajo.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 13.Browning DJ, Fraser CM. Regional patterns of sight-threatening diabetic macular edema. Am J Ophthalmol. 2005;140:117–124. doi: 10.1016/j.ajo.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Chan A, Duker JS, Ko TH, et al. Normal macular thickness measurements in healthy eyes using stratus optical coherence tomography. Arch Ophthalmol. 2006;124:193–198. doi: 10.1001/archopht.124.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polito A, Del Borello M, Isola M, et al. Repeatability and reproducibility of fast macular thickness mapping with stratus optical coherence tomography. Arch Ophthalmol. 2005;123:1330–1337. doi: 10.1001/archopht.123.10.1330. [DOI] [PubMed] [Google Scholar]
- 16.Wang XY, Huynh SC, Burlutsky G, et al. Reproducibility and effect of magnification on optical coherence tomography measurements in children. Am J Ophthalmol. 2007;143(3):484–488. doi: 10.1016/j.ajo.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 17.Leung CK, Cheung CY, Winreb RN, et al. Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.07-1326. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Forooghian F, Cukras C, Beyerle CB, et al. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-2113. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauhan DS, Marshall J. The interpretation of optical coherence tomography images of the retina. Invest Ophthalmol Vis Sci. 1999;40(10):2332–2342. [PubMed] [Google Scholar]
- 20.Srinivasan VJ, Monson BK, Wojtkowski M, et al. Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49(4):1571–1579. doi: 10.1167/iovs.07-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadda SR, Wu Z, Walsh AC, et al. Errors in retinal thickness measurements obtained by optical coherence tomography. Ophthalmology. 2006;113(2):285–293. doi: 10.1016/j.ophtha.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Campbell RJ, Coupland SG, Buhrmann RR, Kertes PJ. Effect of eccentric and inconsistent fixation on retinal optical coherence tomography measures. Arch Ophthalmol. 2007;125:624–627. doi: 10.1001/archopht.125.5.624. [DOI] [PubMed] [Google Scholar]
- 23.Bressler NM, Edwards AR, Antoszyk AN, et al. Diabetic Retinopathy Clinical Research Network. Retinal thickness on Stratus optical coherence tomography in people with diabetes and minimal or no diabetic retinopathy. Am J Ophthalmol. 2008;145(5):894–901. doi: 10.1016/j.ajo.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alamouti B, Funk J. Retinal thickness decreases with age: an OCT study. Br J Ophthalmol. 2003;87:899–901. doi: 10.1136/bjo.87.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelty PJ, Payne JF, Trivedi RH, et al. Macular thickness assessment in healthy eyes based on ethnicity using Stratus OCT optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49(6):2668–2672. doi: 10.1167/iovs.07-1000. [DOI] [PubMed] [Google Scholar]