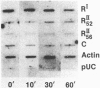
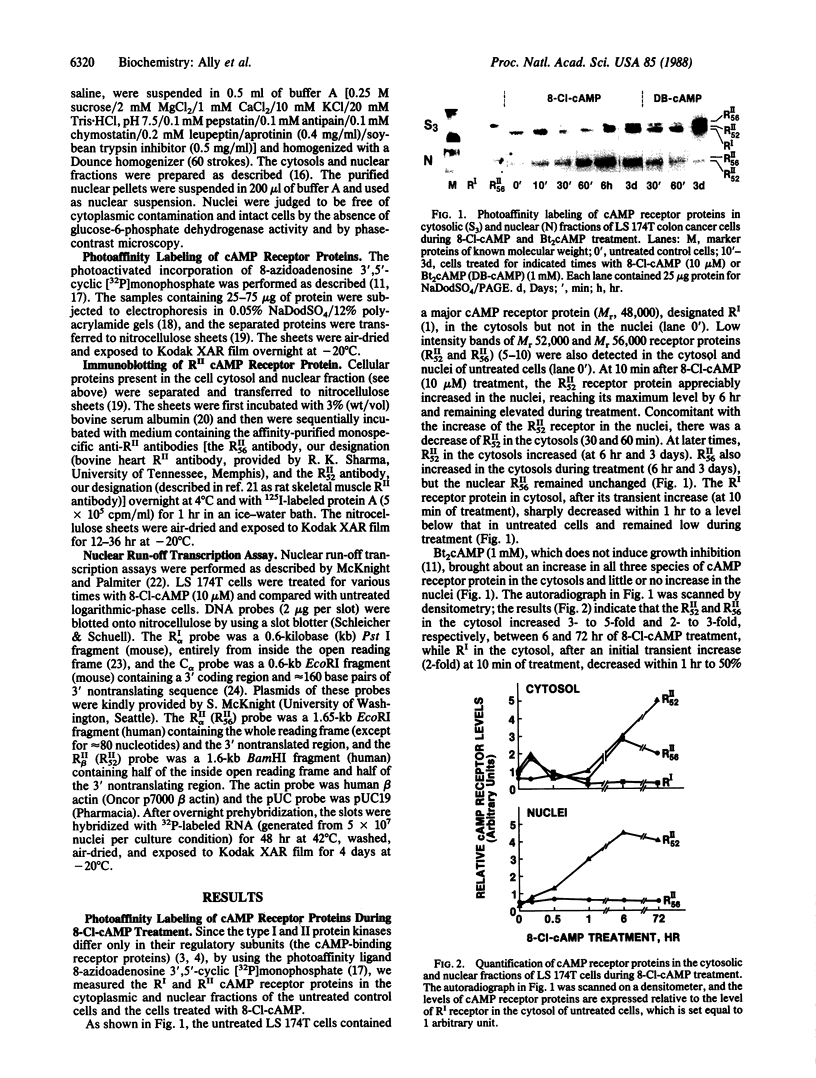
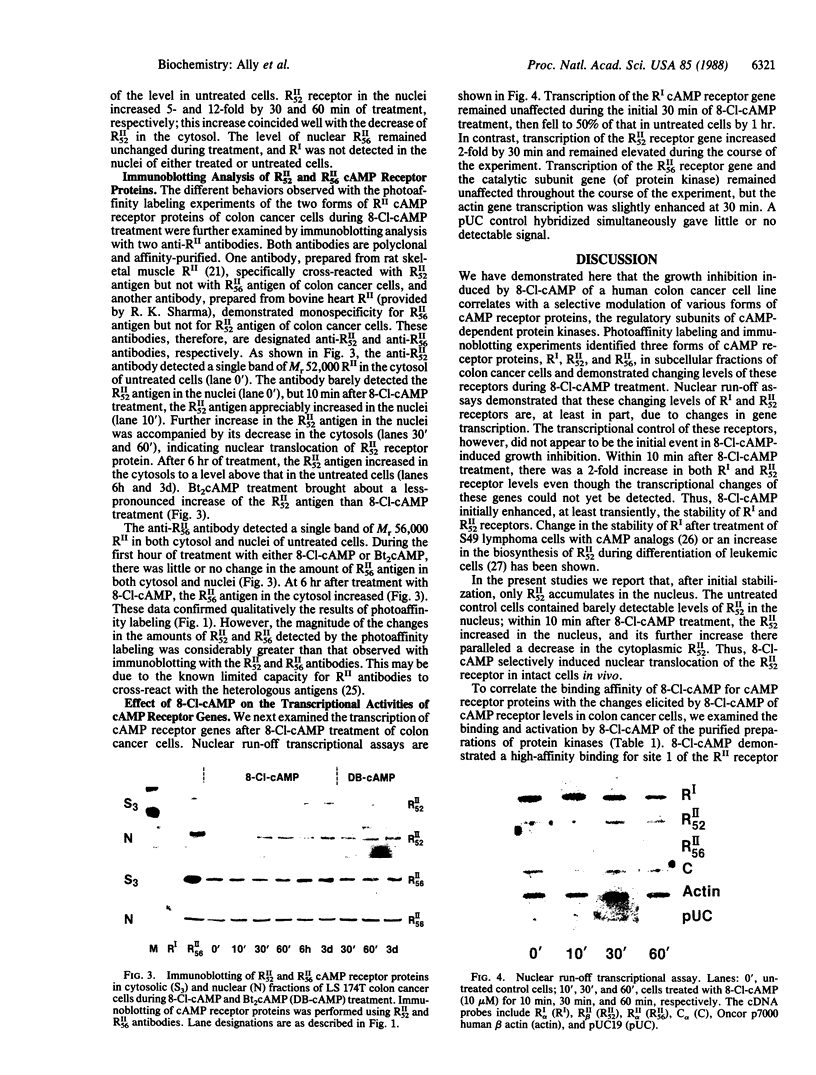
Abstract
Differential expression of type I and type II cAMP-dependent protein kinase isozymes has been linked to growth regulation and differentiation. We examined the expression of protein kinase isozymes in the LS 174T human colon cancer cell line during 8-chloroadenosine 3',5'-cyclic monophosphate (8-Cl-cAMP)-induced growth inhibition. Two species of RII (the regulatory subunit of protein kinase type II) with apparent Mr 52,000 (RII52) and Mr 56,000 (RII56) and a single species of RI (the regulatory subunit of protein kinase type I) with Mr 48,000 were identified in the cancer cells. RI and both forms of RII were covalently labeled with 8-azidoadenosine 3',5'-cyclic [32P]monophosphate, and two anti-RII antibodies that exclusively recognize either RII52 or RII56 resolved two forms of the RII receptors. 8-Cl-cAMP treatment induced a decrease of RI and an increase of both RII52 and RII56 in the cytosols of cancer cells and rapid translocation (within 10 min) of RII52 from the cytosol to nucleus. 8-Cl-cAMP caused transcriptional activation of the RII52 receptor gene and inactivation of the RI receptor gene. It also exhibited high-affinity site-1-selective binding to the purified preparations of both RII receptor proteins. Thus, differential regulation of various forms of cAMP receptor proteins is involved in 8-Cl-cAMP-induced regulation of cancer cell growth, and nuclear translocation of RII52 receptor protein appears to be an early event in such differential regulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cho-Chung Y. S., Bodwin J. S., Clair T. Cyclic AMP-binding proteins: inverse relationship with estrogen-receptors in hormone-dependent mammary tumor regression. Eur J Biochem. 1978 May;86(1):51–60. doi: 10.1111/j.1432-1033.1978.tb12283.x. [DOI] [PubMed] [Google Scholar]
- Clegg C. H., Correll L. A., Cadd G. G., McKnight G. S. Inhibition of intracellular cAMP-dependent protein kinase using mutant genes of the regulatory type I subunit. J Biol Chem. 1987 Sep 25;262(27):13111–13119. [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- DeBortoli M. E., Abou-Issa H., Haley B. E., Cho-Chung Y. S. Amplified expression of p21 ras protein in hormone-dependent mammary carcinomas of humans and rodents. Biochem Biophys Res Commun. 1985 Mar 15;127(2):699–706. doi: 10.1016/s0006-291x(85)80218-7. [DOI] [PubMed] [Google Scholar]
- Døskeland S. O. Evidence that rabbit muscle protein kinase has two kinetically distinct binding sites for adenosine 3' ; 5'-cyclic monophosphate. Biochem Biophys Res Commun. 1978 Jul 28;83(2):542–549. doi: 10.1016/0006-291x(78)91024-0. [DOI] [PubMed] [Google Scholar]
- Døskeland S. O., Ogreid D. Characterization of the interchain and intrachain interactions between the binding sites of the free regulatory moiety of protein kinase I. J Biol Chem. 1984 Feb 25;259(4):2291–2301. [PubMed] [Google Scholar]
- Døskeland S. O., Ogreid D., Ekanger R., Sturm P. A., Miller J. P., Suva R. H. Mapping of the two intrachain cyclic nucleotide binding sites of adenosine cyclic 3',5'-phosphate dependent protein kinase I. Biochemistry. 1983 Mar 1;22(5):1094–1101. doi: 10.1021/bi00274a016. [DOI] [PubMed] [Google Scholar]
- Ekanger R., Sand T. E., Ogreid D., Christoffersen T., Døskeland S. O. The separate estimation of cAMP intracellularly bound to the regulatory subunits of protein kinase I and II in glucagon-stimulated rat hepatocytes. J Biol Chem. 1985 Mar 25;260(6):3393–3401. [PubMed] [Google Scholar]
- Hofmann F., Beavo J. A., Bechtel P. J., Krebs E. G. Comparison of adenosine 3':5'-monophosphate-dependent protein kinases from rabbit skeletal and bovine heart muscle. J Biol Chem. 1975 Oct 10;250(19):7795–7801. [PubMed] [Google Scholar]
- Jahnsen T., Hedin L., Kidd V. J., Beattie W. G., Lohmann S. M., Walter U., Durica J., Schulz T. Z., Schiltz E., Browner M. Molecular cloning, cDNA structure, and regulation of the regulatory subunit of type II cAMP-dependent protein kinase from rat ovarian granulosa cells. J Biol Chem. 1986 Sep 15;261(26):12352–12361. [PubMed] [Google Scholar]
- Kapoor C. L., Beavo J. A., Steiner A. L. Radioimmunoassay of the regulatory subunit of type I cAMP-dependent protein kinase. J Biol Chem. 1979 Dec 25;254(24):12427–12432. [PubMed] [Google Scholar]
- Kapoor C. L., Cho-Chung Y. S. Compartmentalization of regulatory subunits of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in MCF-7 human breast cancer cells. Cancer Res. 1983 Jan;43(1):295–302. [PubMed] [Google Scholar]
- Katsaros D., Tortora G., Tagliaferri P., Clair T., Ally S., Neckers L., Robins R. K., Cho-Chung Y. S. Site-selective cyclic AMP analogs provide a new approach in the control of cancer cell growth. FEBS Lett. 1987 Oct 19;223(1):97–103. doi: 10.1016/0014-5793(87)80517-3. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Ogreid D., Døskeland S. O., Miller J. P. Evidence that cyclic nucleotides activating rabbit muscle protein kinase I interact with both types of cAMP binding sites associated with the enzyme. J Biol Chem. 1983 Jan 25;258(2):1041–1049. [PubMed] [Google Scholar]
- Ogreid D., Ekanger R., Suva R. H., Miller J. P., Sturm P., Corbin J. D., Døskeland S. O. Activation of protein kinase isozymes by cyclic nucleotide analogs used singly or in combination. Principles for optimizing the isozyme specificity of analog combinations. Eur J Biochem. 1985 Jul 1;150(1):219–227. doi: 10.1111/j.1432-1033.1985.tb09010.x. [DOI] [PubMed] [Google Scholar]
- Pomerantz A. H., Rudolph S. A., Haley B. E., Greengard P. Photoaffinity labeling of a protein kinase from bovine brain with 8-azidoadenosine 3',5'-monophosphate. Biochemistry. 1975 Aug 26;14(17):3858–3862. doi: 10.1021/bi00688a019. [DOI] [PubMed] [Google Scholar]
- Rannels S. R., Corbin J. D. Two different intrachain cAMP binding sites of cAMP-dependent protein kinases. J Biol Chem. 1980 Aug 10;255(15):7085–7088. [PubMed] [Google Scholar]
- Richards J. S., Rolfes A. I. Hormonal regulation of cyclic AMP binding to specific receptor proteins in rat ovarian follicles. Characterization by photoaffinity labeling. J Biol Chem. 1980 Jun 10;255(11):5481–5489. [PubMed] [Google Scholar]
- Robinson-Steiner A. M., Beebe S. J., Rannels S. R., Corbin J. D. Microheterogeneity of type II cAMP-dependent protein kinase in various mammalian species and tissues. J Biol Chem. 1984 Aug 25;259(16):10596–10605. [PubMed] [Google Scholar]
- Robinson-Steiner A. M., Corbin J. D. Probable involvement of both intrachain cAMP binding sites in activation of protein kinase. J Biol Chem. 1983 Jan 25;258(2):1032–1040. [PubMed] [Google Scholar]
- Schwartz D. A., Rubin C. S. Identification and differential expression of two forms of regulatory subunits (RII) of cAMP-dependent protein kinase II in Friend erythroleukemic cells. Differentiation and 8-bromo-cAMP elicit a large and selective increase in the rate of biosynthesis of only one type of RII. J Biol Chem. 1985 May 25;260(10):6296–6303. [PubMed] [Google Scholar]
- Steinberg R. A., Agard D. A. Turnover of regulatory subunit of cyclic AMP-dependent protein kinase in S49 mouse lymphoma cells. Regulation by catalytic subunit and analogs of cyclic AMP. J Biol Chem. 1981 Nov 10;256(21):10731–10734. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. D., McKnight G. S. Expression of cDNAs for two isoforms of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1987 Nov 5;262(31):15202–15207. [PubMed] [Google Scholar]