Abstract
Loss of Rb1 tumor suppressor gene function is involved in the genesis of most human cancers. Novel therapies targeting Rb1 have been slow to develop due to our incomplete understanding of its molecular mechanisms of action. Rb1 protein (pRb) binds a host of cellular genes and proteins, and these molecular interactions mediate its various functions. Given the potential complexity of these molecular interactions and the lack of established methods for pRb purification, it has been difficult to systematically identify gene and protein interactions relevant to tumor suppression in different tissues in vivo. To address this limitation, we have generated a dual affinity tagged Rb1 allele in the mouse. The tagged allele functions as wild type and the encoded protein can be purified by tandem affinity chromatography. This allele will facilitate identification and characterization of native pRb molecular interactions in any tissue accessible in the mouse.
Keywords: tumor suppressor, development, cell cycle, affinity purification
Mutational inactivation of the Rb1 gene is the rate limiting molecular alteration causing retinoblastoma. In addition, Rb1 is the central component of a molecular pathway that is deregulated in many other types of human cancer (Burkhart and Sage, 2008; Malumbres and Barbacid, 2001; Sherr, 2000). Consistent with an important role for Rb1 in a wide range of human malignancies, patients with mutant Rb1 that are successfully treated for retinoblastoma have significantly increased risk of mortality from second primary tumors in other organs (Acquaviva et al., 2006; Eng et al., 1993; Hansen et al., 1985). Given these observations, the Rb1 tumor suppressor pathway is an important molecular target for the diagnosis and treatment of cancer.
Rb1 protein functions by binding cellular proteins and regulating their function. When pRb is lost or inhibited, these cellular proteins are liberated from normal regulation and can contribute to neoplasia. The best-characterized pRb associated proteins are the E2F transcription factors (Attwooll et al., 2004; Bracken et al., 2004; Goodrich, 2006). E2Fs are sequence specific DNA binding transcription factors that regulate gene expression. When in complex with E2Fs at a promoter, Rb1 protein (pRb) recruits chromatin modifying corepressors to repress gene expression. Since E2Fs regulate genes required for the cell cycle, loss of Rb1 derepresses the expression of such genes thereby stimulating abnormal cell proliferation. Compound genetic deletion of E2F and Rb1 in mice reduces the excess cell proliferation and resulting tumorigenesis observed upon deletion of Rb1 alone (Lee et al., 2002; Yamasaki et al., 1998; Ziebold et al., 2003), verifying that E2Fs are downstream mediators of tumorigenesis upon loss of pRb.
While pRb/E2F interaction is clearly important for tumor suppression, pRb has been implicated to interact with more than 150 additional cellular proteins(Goodrich, 2006; Morris and Dyson, 2001). Currently identified pRb associated proteins have been discovered by candidate testing or by two-hybrid based protein interaction screening methods. Most of the currently identified pRb interaction partners are either transcription factors or play some role in transcriptional regulation. This observation has inspired efforts to identify direct gene targets of pRb mediated transcriptional regulation by genome wide chromatin immunoprecipitation based approaches(Wells et al., 2003). It is clear from these initial efforts that pRb potentially interacts with hundreds of genes. Thus pRb mediated tumor suppression can be understood, to a large extent, by the identity of the proteins and genes it interacts with in a particular tissue.
Despite intense scientific interest, there remain significant gaps in our understanding of how pRb suppresses tumorigenesis in a given tissue. Technical limitations have heretofore prevented systematic identification of pRb associated genes and proteins. While chromatin immunoprecipitation has been successfully used to identify pRb at predicted candidate genes, currently available antibodies have insufficient specificity for unbiased identification of pRb gene targets at the genome wide level(Stengel et al., 2009). Similarly, available pRb antibodies do not yield protein in sufficient yield or specificity for robust systematic identification of copurifying proteins. Since Rb1 protein exists simultaneously in different protein complexes, pRb does not fractionate in well-defined peaks using chromatography that separates based on biochemical or physical characteristics. Thus methods for the purification of native pRb from cells or tissues have not been established. Given the complexity of these molecular interactions, the genes and proteins pRb interacts with are likely to vary depending on biological context. Engineered cell culture based experimental systems, therefore, may miss pRb interactions important for function in a particular tissue in vivo. In an attempt to overcome these limitations, we have generated an affinity tagged Rb1 allele that allows purification of pRb by tandem affinity chromatography from any tissue available in the mouse.
The affinity tagged Rb1 allele was created by targeted homologous recombination in mouse ES cells. The targeting vector contained 7.8 kilobase pairs (kbp) of Rb1 from intron 24 to exon 27 (Fig. 1A). A PGK-neo selection cassette flanked by loxP sites was inserted within intron 26. The dual affinity tag was inserted in-frame 5′ to the native stop codon in the 3′ homology arm. The tag was fused to the 3′ end of the gene to avoid potential effects that 5′ insertions may have on the normal activity of the Rb1 promoter. This was a concern because naturally occurring base changes in the Rb1 promoter are associated with low penetrance retinoblastoma(Harbour, 2001), suggesting promoter alterations are detrimental to normal Rb1 function. The tag was composed of a 10 amino acid (aa) spacer, a six histidine tag for purification by metal chelate chromatography, a 9 aa spacer, the tobacco etch virus protease cleavage site, a 9aa spacer, and the FLAG immunoaffinity tag (Fig. 1B). This tag was chosen based on its utility and low toxicity upon expression in mouse embryonic stem cells, relative to other tag combinations (J.F. Greenblatt, University of Toronto, personal communication). Two hundred-thirty five ES cell clones surviving drug selection were screened by Southern blotting. Three of these clones gave the expected fragment size for the correctly targeted allele using a 5′ flanking probe (Fig. 1C). Correct targeting was verified by DNA sequencing, and one correctly targeted ES cell clone was used to generate chimeric mice. The chimeric mice were crossed to C57BL/6J mice thus giving rise to a founder strain of mice carrying the tagged Rb1 allele on a mixed 129/SvJae X C57BL/6J genetic background.
Figure 1. Construction of a dual affinity tagged Rb1 allele in the mouse.
A) A schematic representation of the exon/intron structure of the targeted region of the Rb1 gene, the targeting vector, and the expected structure of the successfully targeted allele. Exons are numbered and shown as solid boxes. The asterisks and white space shows the position of the dual affinity tag and stop codon. The open boxes represent the neomycin selection cassette and the pBSK cloning vector. LoxP sites are represented by arrowheads. The position of the BglII (B) restriction enzyme sites and the expected sizes of fragments detected by Southern blotting using a 5′ flanking probe are indicated. B) The amino acid sequence of the dual affinity tag inserted 5′ of the native Rb1 stop codon. The histidine tag, tobacco etch virus protease cleavage site, and FLAG tag are indicated. Nine to ten amino acid spacers are included between each element and the Rb1 coding sequence. C) Southern blot analysis of BglII restricted genomic DNA from five ES cell clones transfected with the targeting vector. A 5′ flanking probe was used to probe the blot. Sizes of the bands are indicated in kilobase pairs (kbp) based on comparison to molecular weight markers run on the gel. Both the wild type (expected 18 kbp band) and the correctly targeted allele (expected 12 kbp band) are detected in three of the five clones shown.
The founder strain was subsequently mated to a Cre deleter strain to remove the neomycin selection cassette (Tallquist and Soriano, 2000). Cre mediated deletion of the selection cassette was confirmed by PCR (Fig. 2A). This recombined and tagged allele (Rb1T) is identical to the wild type allele except for the inclusion of the dual affinity tag and replacement of 10 bp of intron 26 sequences with a loxP site flanked by ClaI and SalI restriction sites. Mice carrying the Rb1T allele were then backcrossed ten generations with C57BL/6J in order to remove the Cre transgene and homogenize the genetic background. A PCR assay has been developed for routine genotyping of tail DNA to distinguish the wild type and Rb1T alleles (Fig. 2C).
Figure 2. PCR assays for genotyping the Rb1T allele.
Representative ethidium bromide stained agarose gels resolving PCR fragments amplified from genomic DNA of mice with the indicated genotypes. The pixels have been inverted for clarity. Tag represents the modified allele retaining the neomycin selection cassette. T represents the final tagged allele lacking the neomycin selection cassette. The wild type allele is represented by +. A) A PCR assay designed to detect the cre recombined tagged allele lacking the neomycin selection cassette (255 bp) or the wild type allele (217 bp). B) A PCR assay designed to detect the presence of the selection cassette (430 bp). C) The PCR assay used for routine genotyping of mice containing the Rb1T (407 bp) and wild type alleles (282 bp).
Mouse embryonic development is exquisitely sensitive to deficiencies in Rb1 function. Mice completely or partially deficient for Rb1 die during midgestation with defects in a number of tissues including the brain, liver, placenta, and eye among others (Sun et al., 2006; Wikenheiser-Brokamp and Wikenheiser-Brokamp, 2006). Mice homozygous for the Rb1T allele are viable and do not exhibit phenotypes distinguishable from wild type mice. Rb1T/T mice are fertile, are not tumor prone, and live a typical life span. These observations indicate that modification of Rb1 to include the dual affinity tag has not compromised normal function of the protein.
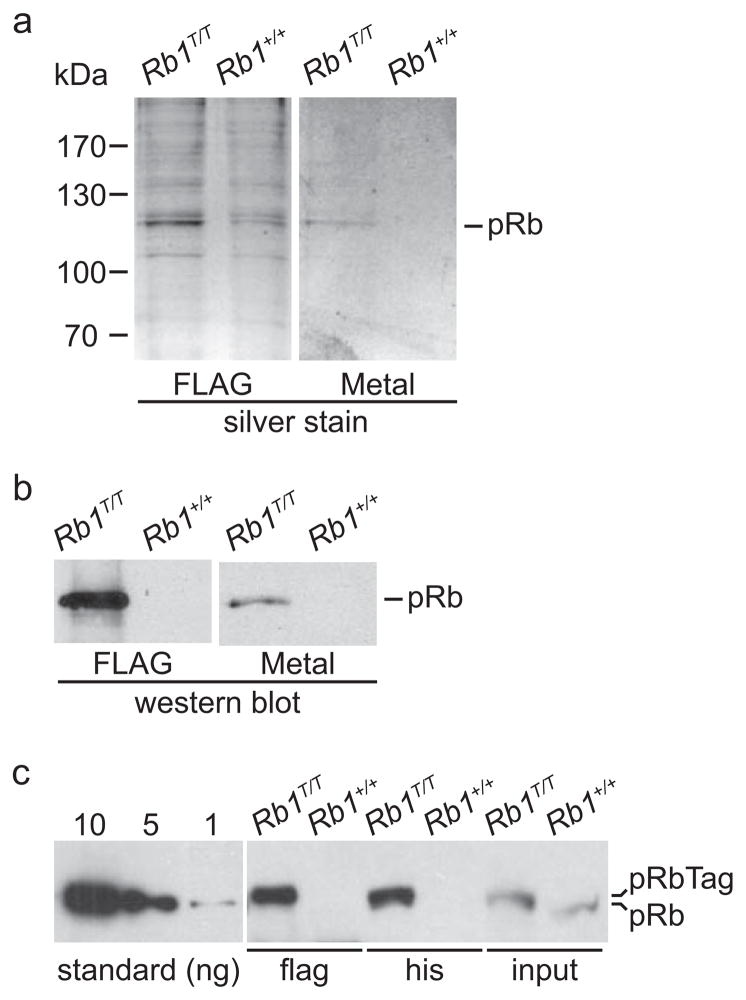
To test whether pRb can be isolated from Rb1T/T mice using tandem affinity purification (TAP), we prepared spleen nuclear extracts and purified them using FLAG immunoaffinity chromatography followed by metal chelate chromatography. Eluants from each column were analyzed by SDS-PAGE and compared to similarly fractionated extracts from wild type mice expressing untagged pRb. Both FLAG immunoaffinity and metal chelate affinity resins were able to purify silver stainable quantities of pRb from Rb1T/T mice, but not from wild type mice (Fig. 3A, B). In order to estimate the yield of tagged pRb from immunoaffinity and metal chelate chromatography, purified material was analyzed by semi-quantitative western blotting using purified pRb produced in a bacterial expression system as a standard(Connell-Crowley et al., 1997). We estimate that 15–20 ng of pRb was present per mg of spleen nuclear extract (Fig. 3C). The yield of pRb from FLAG immunoaffinity chromatography was about 4.5 ng per mg extract (~25–30% yield), while the yield from metal chelate chromatography was about 4 ng per mg extract (~20–25% yield). Purified extracts were also analyzed by two-dimensional gel electrophoresis, and a number of protein spots specifically co-purified with pRb from Rb1T/T mice (Fig. 4). We note that phosphatases inhibitors are not routinely used, thus the species of pRb observed on these gels is predominantly underphosphorylated likely due to the presence of phosphatases activity associated with tissue extracts. In sum, these results confirm that the Rb1 allele has been modified correctly to include the FLAG and 6XHis affinity tags. Further, this modification facilitates the purification of silver stainable quantities of pRb at reasonable yield from native mouse tissue by affinity chromatography.
Figure 3. Purification of pRb from Rb1T/T mice.
A) Spleen nuclear extracts from mice of the indicated genotypes were purified using sequential FLAG immunoaffinity (FLAG) and metal affinity (Metal) resins. Material eluted from the indicated resin was analyzed by SDS-PAGE and the gels silver stained. The position of molecular weight standards is indicated. The position of the pRb band is also indicated. B) Aliquots of the purified extracts were analyzed for the presence of pRb by western blotting. C) Rb1 protein was purified from spleen nuclear extract from mice of the indicated genotypes by either FLAG immunoaffinity or histidine metal chelate chromatography and the material analyzed by western blotting. Input represents western analysis of 15% of the nuclear extract used for chromatography. The left panel shows western blot analysis of known quantities, in nanograms, of pRb purified from a bacterial expression system. Although separated, all samples were analyzed on the same western blot.
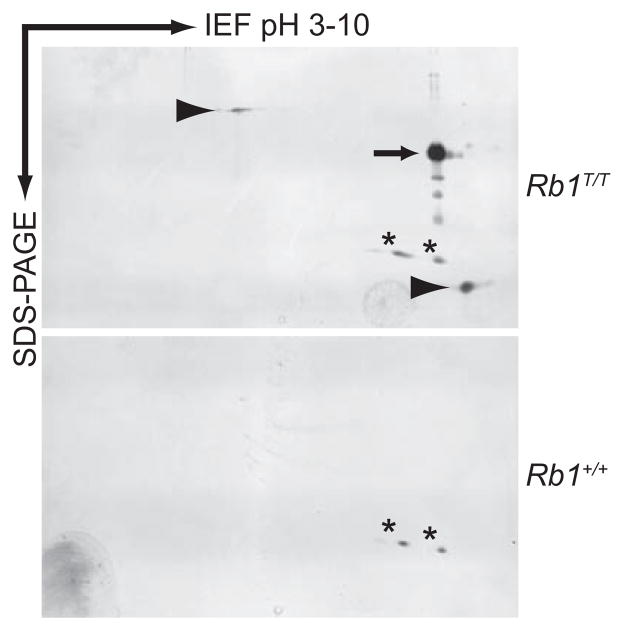
Figure 4. Two dimensional gel electrophoresis of tagged pRb purified from mouse spleen.
Material eluted from the final metal affinity resin in figure 3 was analyzed by 2D gel electrophoresis. Isoelectric focusing was used to separate proteins in the horizontal direction with the pH range indicated. SDS-PAGE was used to separate proteins in the vertical direction. The genotype of the mice from which the spleen extracts were derived is shown. The arrow shows the position of purified pRb. Arrowheads show the position of two protein spots that co-purify with pRb, but are not present in the background control sample from untagged mice. The asterisks show protein spots that are present in the untagged sample and represent non-specific background contamination.
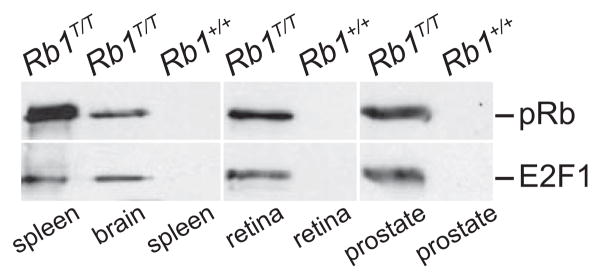
In order to test the general applicability of these methods for purification from other tissues, we prepared brain, retinal, and prostate extracts from both Rb1T/T and wild type mice. Extracts purified by tandem affinity chromatography were analyzed for the presence of pRb by western blotting. Consistent with the data from spleen extracts, pRb was detected in the purified fractions from each of the additional Rb1T/T tissue extracts (Fig. 5). Rb1 protein was not detected in fractions purified from untagged, wild type mice. We also probed these western blots for E2F1 protein, a well characterized pRb binding partner. In each sample where pRb was detected, co-purifying E2F1 was also detected (Fig. 5). Thus using a standard set of extraction and affinity purification conditions, pRb can be purified from a number of different mouse tissues by using the Rb1T allele. Further, these conditions are sufficient to maintain at least some pRb protein interactions known to occur within the cell.
Figure 5. E2F1 co-purifies with tagged pRb from several mouse tissues.
Spleen, brain, retina, or prostate tissue extracts from mice of the indicated genotypes were purified by tandem affinity chromatography as in figure 3, and the purified material analyzed for the presence of pRb and E2F1 protein by western blot analysis. Tagged pRb can be successfully purified from all four of these tissues, and the purification conditions are sufficient to maintain a known pRb interaction with E2F1.
We describe the construction and initial characterization of a mouse strain in which the Rb1 gene has been modified to encode a dual affinity tag at the 3′ end of its coding sequence. Mice homozygous for the modified allele do not exhibit phenotypes distinguishable from wild type mice. Thus appending the 49 amino acid dual affinity tag to the carboxy terminus does not compromise normal pRb function. Further, the tag is accessible permitting purification of pRb by tandem FLAG immunoaffinity and 6XHis metal affinity chromatography under conditions that maintain normal protein interactions. Given these observations, it is likely that appending alternative tags to the carboxy terminus of pRb, and possibly other pocket family proteins, will also be feasible.
Tandem affinity purification has been a powerful tool for identifying protein interaction networks (Koch et al., 2007; Krogan et al., 2006). Typically this is performed by mass spectrometry based identification of proteins that copurify with the tagged protein. Similar analysis can be done using the mouse strain described here to systematically identify pRb interaction partners in different biological contexts. For example, the proteins that interact with pRb in normal and tumor tissue can be compared to identify interactions that are lost or gained during tumorigenesis. These changes identify candidate interactions that mediate tumor suppression in the given tissue. Similarly, the purified pRb is ideally suited for analysis of post translational modifications by mass spectrometry. Changes in such modifications can be monitored during tumorigenesis to assess how they regulate pRb tumor suppressor activity. TAP has also been adapted for use in chromatin immunoprecipition genomic location analysis (Lambert et al., 2009). Advantages of TAP over immunoprecipitation for this type of analysis include superior sensitivity, increased specificity, and standardized methodology not subject to bias introduced by use of different antibodies. Given these advantages, the TAP tagged Rb1 allele described here has the potential to overcome the limitations of currently available antibodies for genomic location analysis of pRb in native mouse tissue.
Most studies using TAP for analysis of higher eukaryotes have relied on ectopic expression of tagged fusion proteins in cells cultured in vitro (Gregan et al., 2007). The general approach we describe here extends this methodology in a number of important ways. One, the tagged fusion protein is expressed from the native gene. This obviates potential limitations associated with non-physiological levels of ectopic expression. Two, phenotypic analysis of mice can be used to rigorously verify the normal function of the fusion protein. Three, use of mice homozygous for the tagged allele eliminates potential complications associated with competition between the tagged fusion protein and the untagged native protein (Forler, 2003). Four, the tagged allele can easily be bred into different mouse models, tumor models for example, to assess how molecular interactions change in disease. Finally, protein can be purified from any native tissue available in the mouse. The dual affinity tagged allele described here should thus facilitate characterization of the molecular interactions that underlie Rb1 function in a variety of biological contexts.
Methods
Generation of the affinity tagged Rb1 allele
A BAC clone spanning the 3′ end of the Rb1 gene was isolated from a 129/SV mouse strain genomic library and used as a template to PCR amplify the homology arms for construction of the targeting vector. A 917 bp fragment including intron 26 and the termination codon in exon 27 was modified by PCR to insert the dual affinity tag and then subcloned into a previously isolated 3′ homology arm spanning exon 27. A 5.2 kbp 5′ homology arm spanning exons 24 through 26 was subcloned into the NotI and EcoRI sites of the 3′ homology arm vector. The final targeting vector was created by inserting a floxed PGKneo selection cassette into intron 26 by subcloning using the SalI and SmaI sites of the homology arm vector. All Rb1 coding sequence in the targeting vector, including the dual affinity tag, was verified by DNA sequencing. The targeting construct was electroporated into a 129/SV derived embryonic stem cell line and G418 resistant colonies screened by Southern blotting of BglII restricted genomic DNA using a 5′ flanking probe. Correctly targeted ES cells were used to construct germ line transmitting chimeras by blastocyst injection. Transmission of the targeted alleles was confirmed by Southern blotting and PCR analysis of genomic DNA extracted from tail biopsies. The Cre deleter mouse strain used to remove the neomycin selection cassette was obtained from Jackson Laboratory (Bar Harbor, Maine).
PCR genotyping
Routine genotyping of genomic DNA extracted from tail biopsy specimens was performed by PCR assays. All PCR reactions were performed in Taq buffer (Fermentas Inc., Hanover, MD). PCR was initiated with a 3 minute denaturation cycle at 94°C, followed by 30 cycles of 30 seconds denaturation, 30 seconds annealing at 61°C, and 30 seconds extension at 72°C. After a final 10 minute extension at 72C, PCR products were resolved by agarose gel electrophoresis and stained with ethidium bromide. Primers mRbHisPCR15 (5′-TACATGTATGCAGTATGATTTGATGGC-3′) and mRbHisPCR16 (5′-GGAGAGTAAGGAAGTTTAACAAGCC-3′) generate a 255bp band from the tagged allele lacking the neo selection cassette (Rb1T) and a 217 bp band from the wild type allele. Primers mRbHisPCR15 and mRbHisPCR17 (5′TGCTACTTCCATTTGTCACGTC-3′) generate a 430 bp fragment from the neo cassette containing tagged Rb1 allele (Rb1tag). Primers mRbHisPCR1 (5′-CTGCCCCTGGTCCACTTACT-3′) and mRbHisPCR2 (5′-TGGCTTACGAATCACCCA-3′) amplify a 407 bp band from the dual affinity tagged Rb1 allele lacking the neo cassette (Rb1T) and a 282 bp fragment from the wild type allele.
Protein purification and analysis
Nuclear extracts were prepared by homogenizing tissue in 10mM HEPES(pH7.9), 10mM KCl, 0.1mM EDTA, 0.1mM EGTA, 0.5mM 4-(2-Aminoethyl) benzenesulfonyl fluoride (AEBSF), 2 μg/ml aprotinin, 2 μg/ml leupeptin. Cells were allowed to swell on ice for 15 minutes at which time they were extracted by addition of NP-40 to a final concentration of 0.62%. The extract was vortexed and clarified by centrifugation at 1,500g for 2 minutes. The nuclear pellet was resuspended in complete digestion buffer (Nuclear complex Co-IP kit, Active Motif-Carlsbad, CA), sonicated, and digested with enzymatic shearing cocktail (Nuclear complex Co-IP kit, Active Motif-Carlsbad, CA) for 10 minutes at 37°C. The reaction was stopped by addition of EDTA on ice. The extract was clarified by centrifugation at 16,000g for 1 minute at 4°C.
FLAG immunoaffinity purification was performed using anti-FLAG M2 affinity resin (Sigma-St. Louis, MO). Nuclear extract was added to the resin and samples rotated overnight at 4°C. The bound resin was washed with co-IP buffer (50 mM Tris-Cl [pH7.9], 50 mM NaCl, 0.1 mM EDTA, 1% glycerol, 0.2% NP-40, add 0.5 mM AEBSF) and purified material eluted with 150 μg/ml 3X FLAG peptide (Sigma-St. Louis, MO) in co-IP buffer for 30 minutes at 4°C. While TEV protease digestion can be used to elute bound material from the affinity matrix, 3X FLAG peptide elution was routinely used due to superior elution efficiency. Metal affinity purification was performed using Talon Metal Affinity resin according to manufacturer’s recommendations (Clontech-Mountain View, CA). The purified extract was prepared for two dimensional gel electrophoresis using the 2D CleanUp kit as recommended (BioRad-Herculues, CA).
The concentrated sample was resolved by isoelectric focusing and SDS-PAGE using a Protean IEF cell system using precast gels (BioRad-Hercules, CA). SDS-PAGE gels were stained using the Silver Staining Kit as directed (GE Healthcare Biosciences-Uppsala). Western blotting and staining for Rb1 and E2F1 proteins was performed as previously described (Sun et al., 2006).
Acknowledgments
This work was supported by grant CA70292 from the National Institutes of Health (DWG). We thank Jack Greenblatt (University of Toronto) for helpful discussions regarding the design of the dual affinity tag. We acknowledge Yanqing Wang for assistance with mouse husbandry and Meenalakshmi Chinnam for helpful comments on the manuscript. Aimee Stablewski of the RPCI Gene Targeting Core, supported by NIH Cancer Center Support Grant CA016056, provided helpful advice and assistance in the construction of the mice.
References
- Acquaviva A, Ciccolallo L, Rondelli R, Balistreri A, Ancarola R, Cozza R, Hadjistilianou D, Francesco SD, Toti P, Pastore G, Haupt R, Carli M, Santoro N, Cataldo AD, Fiorillo A, Indolfi P, Nucci P, Sandri A, Porta F, Porcaro AB, Tamaro P, Morgese G. Mortality from second tumour among long-term survivors of retinoblastoma: a retrospective analysis of the Italian retinoblastoma registry. Oncogene. 2006;25:5350–5357. doi: 10.1038/sj.onc.1209786. [DOI] [PubMed] [Google Scholar]
- Attwooll C, Denchi EL, Helin K. The E2F family: specific functions and overlapping interests. EMBO J. 2004;23:4709–4716. doi: 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer. 2008;8:671–682. doi: 10.1038/nrc2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell-Crowley L, Harper JW, Goodrich DW. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng C, Li FP, Abramson DH, Ellsworth RM, Wong FL, Goldman MB, Seddon J, Tarbell N, Boice JD., Jr Mortality From Second Tumors Among Long-Term Survivors of Retinoblastoma. J Natl Cancer Inst. 1993;85:1121–1128. doi: 10.1093/jnci/85.14.1121. [DOI] [PubMed] [Google Scholar]
- Forler DK. An efficient protein complex purification method for functional proteomics in higher eukaryotes. Nat Biotechnol. 2003;21:89–92. doi: 10.1038/nbt773. [DOI] [PubMed] [Google Scholar]
- Goodrich DW. The retinoblastoma tumor-suppressor gene, the exception that proves the rule. Oncogene. 2006;25:5233–5243. doi: 10.1038/sj.onc.1209616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregan J, Riedel CG, Petronczki M, Cipak L, Rumpf C, Poser I, Buchholz F, Mechtler K, Nasmyth K, Gregan J, Riedel CG, Petronczki M, Cipak L, Rumpf C, Poser I, Buchholz F, Mechtler K, Nasmyth K. Tandem affinity purification of functional TAP-tagged proteins from human cells. Nature Protocols. 2007;2:1145–1151. doi: 10.1038/nprot.2007.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MF, Koufos A, Gallie BL, Phillips RA, Fodstad O, Brogger A, Gedde-Dahl T, Cavenee WK. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985;82:6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbour JW. Molecular basis of low-penetrance retinoblastoma. Arch Ophthalmol. 2001;119:1699–1704. doi: 10.1001/archopht.119.11.1699. [DOI] [PubMed] [Google Scholar]
- Koch HB, Zhang R, Verdoodt B, Bailey A, Zhang CD, Yates JR, 3rd, Menssen A, Hermeking H, Koch HB, Zhang R, Verdoodt B, Bailey A, Zhang C-D, Yates JR, 3rd, Menssen A, Hermeking H. Large-scale identification of c-MYC-associated proteins using a combined TAP/MudPIT approach. Cell Cycle. 2007;6:205–217. doi: 10.4161/cc.6.2.3742. [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, Ignatchenko A, Li J, Pu S, Datta N, Tikuisis AP, Punna T, Peregrin-Alvarez JM, Shales M, Zhang X, Davey M, Robinson MD, Paccanaro A, Bray JE, Sheung A, Beattie B, Richards DP, Canadien V, Lalev A, Mena F, Wong P, Starostine A, Canete MM, Vlasblom J, Wu S, Orsi C, Collins SR, Chandran S, Haw R, Rilstone JJ, Gandi K, Thompson NJ, Musso G, St Onge P, Ghanny S, Lam MH, Butland G, Altaf-Ul AM, Kanaya S, Shilatifard A, O’Shea E, Weissman JS, Ingles CJ, Hughes TR, Parkinson J, Gerstein M, Wodak SJ, Emili A, Greenblatt JF. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- Lambert JP, Mitchell L, Rudner A, Baetz K, Figeys D, Lambert J-P, Mitchell L, Rudner A, Baetz K, Figeys D. A novel proteomics approach for the discovery of chromatin-associated protein networks. Mol Cell Proteomics. 2009;8:870–882. doi: 10.1074/mcp.M800447-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EY, Cam H, Ziebold U, Rayman JB, Lees JA, Dynlacht BD. E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell. 2002;2:463–472. doi: 10.1016/s1535-6108(02)00207-6. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Dyson NJ. Retinoblastoma protein partners. Adv Cancer Res. 2001;82:1–54. doi: 10.1016/s0065-230x(01)82001-7. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- Stengel KR, Thangavel C, Solomon DA, Angus SP, Zheng Y, Knudsen ES. Retinoblastoma/p107/p130 Pocket Proteins: Protein Dynamics and Interactions with Target Gene Promoters. J Biol Chem. 2009;284:19265–19271. doi: 10.1074/jbc.M808740200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Chang Y, Schweers B, Dyer MA, Zhang X, Hayward SW, Goodrich DW. An E2F binding-deficient Rb1 protein partially rescues developmental defects associated with Rb1 nullizygosity. Mol Cell Biol. 2006;26:1527–1537. doi: 10.1128/MCB.26.4.1527-1537.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Wells J, Yan PS, Cechvala M, Huang T, Farnham PJ. Identification of novel pRb binding sites using CpG microarrays suggests that E2F recruits pRb to specific genomic sites during S phase. Oncogene. 2003;22:1445–1460. doi: 10.1038/sj.onc.1206264. [DOI] [PubMed] [Google Scholar]
- Wikenheiser-Brokamp KA, Wikenheiser-Brokamp KA. Retinoblastoma family proteins: insights gained through genetic manipulation of mice. Cell Mol Life Sci. 2006;63:767–780. doi: 10.1007/s00018-005-5487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki L, Bronson R, Williams BO, Dyson NJ, Harlow E, Jacks T. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1(+/−)mice. Nat Genet. 1998;18:360–364. doi: 10.1038/ng0498-360. [DOI] [PubMed] [Google Scholar]
- Ziebold U, Lee EY, Bronson RT, Lees JA. E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol Cell Biol. 2003;23:6542–6552. doi: 10.1128/MCB.23.18.6542-6552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]