Abstract
Background and Aims:
The outcome of patients with hepatocellular carcinoma (HCC) remains poor because of late diagnosis. The aim of this study was to compare the accuracy of alpha fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) in the early diagnosis of HCC.
Methods:
Among 1031 patients randomized in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) Trial, a nested case-control study of 39 HCC cases (24 early stage) and 77 matched controls was conducted to compare the performance of AFP and DCP. Testing was performed on sera from 12 months prior (month −12) to the time of HCC diagnosis (month 0).
Results:
The sensitivity and specificity of DCP at month 0 was 74% and 86% at a cutoff of 40 mAU/mL and 43% and 100% at a cutoff of 150 mAU/mL. The sensitivity and specificity of AFP at month 0 was 61% and 81% at a cutoff of 20 ng/mL and 22% and 100% at a cutoff of 200 ng/mL. At month −12, the sensitivity and specificity at the low cutoff was 43% and 94% for DCP and 47% and 75% for AFP. Combining both markers increased the sensitivity to 91% at month 0 and 73% at month 12 but the specificity decreased to 74% and 71%. Diagnosis of early HCC was triggered by surveillance ultrasound in 14, doubling of AFP in 5 and combination of tests in 5 patients.
Conclusions:
Biomarkers are needed to complement ultrasound in the detection of early HCC but neither DCP nor AFP is optimal.
Keywords: hepatitis C, interferon, cirrhosis
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the third most common cause of cancer-related death worldwide 1. The incidence of HCC in the United States is increasing and is largely attributed to hepatitis C 2. While the survival of patients with most malignancies has improved over the last decade, 5-year survival of patients with HCC has remained less than 10% 3. The poor outcome of patients with HCC is related to the late detection with more than two-thirds of patients diagnosed at advanced stages of disease 4. Cirrhosis of any cause and chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) are the most common risk factors for HCC 5. Thus, surveillance of populations at-risk may detect tumors at an early stage when curative interventions can be implemented. Several studies reported a benefit of HCC surveillance on survival 6, 7 and guidelines from professional organizations recommend HCC surveillance for at-risk populations 5, 8.
A major problem with HCC surveillance is the lack of reliable biomarkers. Alpha fetoprotein (AFP) is the most widely used biomarker for HCC surveillance; however, not all HCCs secrete AFP. Furthermore, AFP may be elevated in patients with chronic liver disease in the absence of HCC. The low sensitivity and specificity of AFP in detecting early HCC led the American Association for the Study of Liver Diseases (AASLD) Practice Guidelines Committee to recommend that ultrasound alone (without AFP) be used for HCC surveillance 5. However, the interpretation of ultrasound is operator-dependent and can be difficult in persons who are obese or have underlying cirrhosis. Therefore, reliable biomarkers to complement ultrasound are needed.
Des-gamma-carboxy prothrombin (DCP) has been used widely in Japan for HCC diagnosis and surveillance 9-16. DCP is an abnormal prothrombin molecule that is generated as a result of an acquired defect in the posttranslational carboxylation of the prothrombin precursor in malignant cells; this prothrombin defect in malignant cells is similar to the deficit in vitamin K deficiency and has been called Prothrombin Induced by Vitamin K Absence (PIVKA) 17, 18. Experience with DCP in western countries, particularly the United States, is limited.
To date, most studies on HCC biomarkers have focused on the accuracy of these markers at the time of HCC diagnosis. Few studies have included cohorts of at-risk persons followed prospectively to determine the utility of these markers in detecting early stage HCC, before clinical manifestations 19. Of note, some studies included patients with early stage liver disease, who are at low risk of HCC, and, in most studies, the performance of the biomarkers was assessed by focusing on baseline values rather than on serial determinations over time 13,20-26. The Hepatitis C Antiviral Long-Term Treatment against Cirrhosis (HALT-C) Trial enrolled patients with chronic hepatitis C and bridging fibrosis or cirrhosis, who failed to respond or to achieve a sustained virologic response (SVR) to combination therapy with pegylated interferon and ribavirin. These patients were randomized to maintenance therapy with peginterferon alfa-2a 90 mcg/week or to no treatment for 3.5 years 27, 28. One of the goals of the HALT-C Trial was to identify and validate serum markers for the surveillance of HCC. In an earlier report on 1,145 patients enrolled in the Lead-in phase, we observed that baseline AFP levels were >20 ng/mL in 16.6% of patients and were significantly higher in patients with cirrhosis, women, and blacks 29. Furthermore, AFP levels decreased during peginterferon and ribavirin treatment. The aims of the current analysis were to (a) compare the impact of demographics and liver histology on baseline AFP and DCP values, (b) compare the effect of maintenance peginterferon on changes in AFP and DCP values during the randomized study, (c) determine the accuracy of AFP and DCP in the detection of HCC during a 12-month period before HCC diagnosis, and (d) determine whether an increase in AFP or a suspicious nodule on ultrasound occurred earlier in the HCC cases.
PATIENTS AND METHODS
HALT-C Trial design, HCC definition and surveillance
The design of the HALT-C Trial has been described previously27, 28. All patients were required to have an ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI) with no evidence of hepatic mass lesions suspicious for HCC and a serum AFP <200 ng/mL prior to enrollment (except for 3 patients who had AFP values of 206, 212, and 315 ng/mL). Liver biopsies were reviewed in conference by a panel of 12 hepatic pathologists, who used the Ishak scoring system to stage fibrosis (0-6) 30.
Patients were seen every 3 months during the 3.5 years of the randomized trial. Blood samples were collected at each visit for subsequent research testing including assays for HCC biomarkers. AFP levels at enrollment and every 3 months were tested at the local clinical laboratories. DCP levels at enrollment and during follow-up were tested on all patients with an enzyme immunoassay (PIVKA II, Eisai Company, Tokyo, Japan) in a central laboratory at the University of Michigan. Ultrasound examinations were repeated 6 months after enrollment and again every 12 months. Patients with an elevated or rising AFP and those with new lesions on ultrasound were evaluated further by CT or MRI.
Two definitions of HCC were adopted as described previously, one for “definite” HCC and one for “presumed” HCC 31. Definite HCC was defined by histologic confirmation or a new mass lesion on imaging with AFP levels increasing to >1,000 ng/mL. Presumed HCC was defined as a new mass lesion on ultrasound in the absence of histology and AFP <1,000 ng/mL in conjunction with one of the following characteristics: a) 2 liver imaging studies showing a mass lesion with characteristics of HCC (arterial enhancement ± wash out), b) progressively enlarging lesion on ultrasound leading to death, or c) 1 additional imaging study showing a mass lesion with characteristics of HCC that either increased in size over time or was accompanied by AFP level >200 ng/mL and more than tripling of baseline value. All cases of HCC were adjudicated by an Outcomes Review Panel to ascertain that they met predefined diagnostic criteria and to determine the date when these criteria were first met. The radiology reports of all HCC cases were retrospectively reviewed by the HCC working group to determine the date when the last imaging showed no hepatic lesion, the date when imaging first revealed a suspicious nodule, and the tumor stage at diagnosis. Early stage HCC was defined as a single tumor nodule <3 cm in diameter with no evidence of vascular invasion or metastasis. For each case, the HCC working group also determined whether a doubling in AFP or a lesion detected on ultrasound occurred earlier. A doubling of AFP instead of an absolute cutoff value was chosen because all patients had 3-monthly AFP monitoring, 19% of patients had baseline AFP values exceeding 20 ng/mL, and because gender, race, cirrhosis and interferon therapy can affect AFP values.
Nested case-control study
A nested case-control study was used to compare the accuracy of AFP and DCP in the detection of HCC during a 12-month period before the diagnosis of HCC. For this study, all 39 HCC cases (33 definite – 32 histologically confirmed and 6 presumed) diagnosed between randomization and 3.8 years after randomization were included. For each case, 2 controls without HCC, matched for treatment assignment, presence of cirrhosis on baseline biopsy and length of follow-up, were selected. Blood samples at the time of HCC diagnosis and months −3, −6, −9, and −12 before the diagnosis of HCC from HCC cases and at corresponding time points from the matched controls were tested for AFP and DCP. Time 0 for the controls corresponded to the study visit closest to the time of HCC diagnosis in the matched case. To ensure that the controls did not have subclinical HCC at time 0, only patients who had more than 12 months follow-up beyond time 0 with no evidence of HCC were included in the matching process.
One control was subsequently excluded because of high DCP values related to coumadin use, leaving 77 controls. DCP values were not available to investigators and therefore, played no prospective role in diagnosis of HCC in contrast to AFP and ultrasound.
Statistical analyses
Statistical analyses were performed at the Data Coordinating Center with SAS release 9.1 (SAS Institute, Cary, NC). Because of the wide range in AFP and DCP values, log10 AFP and log10 DCP were used for all analyses unless specified otherwise. Comparisons of baseline AFP and DCP values according to fibrosis strata, gender, race/ethnicity and age were performed with t-test and Wilcoxon rank sum test. The effect of maintenance peginterferon treatment on AFP and DCP values was analyzed by comparing changes in these values between year 3.5 after randomization and time of randomization in the two treatment groups. For this analysis, control patients who subsequently developed HCC (beyond year 3.8) and those receiving coumadin were excluded.
For the nested case-control study, baseline characteristics of HCC cases and matched controls were compared with conditional logistic regression for matched pairs. The selected controls were also compared to the remaining non-HCC patients with either a chi-square or a t-test. Trends for both AFP and DCP values during specific times in the 12 months before the diagnosis of HCC were compared. For patients with missing DCP values due to insufficient stored samples, the mean values before and after the missing values (+/− 3 months) were used. Changes in AFP and DCP over the 12 months prior to HCC diagnosis were compared with mixed linear models. For analyses comparing the accuracy of AFP and DCP in differentiating HCC cases from controls, area under the receiver operator characteristic curve (AUROC) was calculated and only patients with available values for both markers at the same time point were included. Controls were excluded if data were not available for their case. Unmatched logistic regression was used to calculate sensitivity, specificity, and receiver operator characteristic curves. A 2-sided significance level of 5% was used for all analyses.
RESULTS
Baseline AFP and DCP values of all randomized patients
Of the 1,050 patients randomized, baseline AFP and/or DCP values were not available in 19. Thus, 1,031 (98.2%) patients were included in this analysis. Baseline characteristics of these patients (39 HCC cases, 77 matched controls and 915 other non-HCC patients) are shown in Table 1. The mean age of these 1,031 patients was 50 years, 29% were women, and 18% were black. Cirrhosis was present on baseline biopsies in 41% of patients. Half (49%) of the patients were randomized to maintenance peginterferon. The results of the HALT-C Trial showed that low dose peginterferon had no effect on outcomes including the development of HCC 28, 31; therefore, patients in the two treatment groups were combined for this analysis.
Table 1.
Baseline Characteristics of HCC cases and Controls
| HCC cases | Matched controls |
Other non-HCC patients |
|
|---|---|---|---|
| No. of patients | 39 | 77 | 915 |
| Demographics | |||
| Age, years | 51.9 ± 5.6 | 51.6 ± 8.3 | 50.0 ± 7.1 |
| Gender, % female | 18 | 25 | 30 |
| Race/ethnicity, % | |||
| Non-Hispanic White | 62 | 60 | 73 |
| Hispanic | 5 | 6 | 8 |
| Black | 28 | 32 | 16 |
| Others | 5 | 1 | 2 |
| Metabolic factors | |||
| BMI, kg/m2 | 27.8 ± 4.3 | 29.4 ± 4.7 | 30.0 ± 5.6 |
| Diabetes, % | 18 | 38 | 23 |
| Viral factors | |||
| HCV genotype 1, % | 90 | 96 | 93 |
| HCV RNA, log10 IU/mL | 6.37 ± 0.54 | 6.44 ± 0.6 | 6.44 ± 0.52 |
| Labs | |||
| Hemoglobin, g/dL | 14.8 ± 1.6 | 14.8 ± 1.4 | 15.0 ± 1.4 |
| WBC × 1000/mm3^ | 4.9 ± 1.4 | 5.8 ± 1.9 | 5.8 ± 1.9 |
| Platelet × 1000/mm3* | 123 ± 51 | 155 ± 54 | 167 ± 67 |
| 104 (58-288) | 148 (51-331) | 162 (39-426) | |
| Albumin, g/dL^ | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.9 ± 0.4 |
| AST, U/L | 123 ± 80 | 98 ± 68 | 85 ± 56 |
| ALT, U/L | 141 ±116 | 113 ± 74 | 105 ± 76 |
| Alkaline phosphatase, U/L^ | 131 ±62 | 106 ± 49 | 98 ± 44 |
| 119 (50-341) | 92 (48-308) | 89 (20-478) | |
| Total bilirubin, mg/dL | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.8 ± 0.4 |
| INR* | 1.08 ± 0.08 | 1.02 ± 0.08 | 1.04 ± 0.12 |
| HCC markers | |||
| AFP, ng/mL | 30.7 ± 36.9 | 20.4 ± 33.3 | 16.7 ± 28.8 |
| Log10 AFP, ng/mL^ | 1.25 ± 0.44 | 1.02 ± 0.47 | 0.97 ± 0.42 |
| DCP, mAU/mL | 55.8 ± 54.9 | 27.9 ± 13.0 | 41.3 ± 103.9 |
| Log10 DCP, mAU/mL* | 1.6 ± 0.34 | 1.40 ± 0.19 | 1.47 ± 0.26 |
| Cirrhosis on biopsy, % | 56 | 57 | 39 |
| Esophageal varices, %* | 53 | 24 | 25 |
| Maintenance IFN, % | 49 | 49 | 49 |
p <0.01
p <0.05 for comparisons between HCC cases and matched controls
Data provided are percentages or means ± SD
Median (range) are provided for variables that are not normally distributed
Impact of demographics and liver histology on baseline AFP and DCP values
Both AFP (p<0.0001) and DCP (p<0.0001) values were significantly higher in patients with cirrhosis (Ishak fibrosis 5-6) than those with bridging fibrosis (Ishak 3-4). Mean ± SD AFP values were 23.6 ± 36.4 and 13.3 ± 22.7 ng/mL (p<0.0001) while mean ± SD DCP values were 48.8 ± 114.7 and 35.2 ± 85.3 mAU/mL (p<0.0001) for patients with cirrhosis and those with bridging fibrosis, respectively. Whereas AFP values were higher in women than men: 19.6 ± 29.7 vs. 16.7 ± 29.5 ng/mL (p=0.0004), DCP values were higher in men than women: 44.5 ± 115.9 vs. 31.8 ± 25.8 mAU/mL (p=0.002). AFP values were significantly higher in blacks than Caucasians: 23.1 ± 29.4 vs. 15.6 ± 28.7 ng/mL (p<0.0001) while DCP values were significantly higher in Caucasians than blacks: 43.9 ± 114.6 vs. 30.2 ± 21.9 mAU/mL (p=0.0005). Age had no effect on baseline AFP or DCP values.
Impact of peginterferon on AFP and DCP values in patients with no HCC
During the randomized phase, mean DCP values gradually increased among both treated and untreated patients, but to a similar degree. The mean DCP values increased from 36.6 mAU/mL at the time of randomization to 53.1 mAU/mL at the end of the 3.5 year randomized study in the treated patients and from 35.4 to 50.8 mAU/mL in the untreated patients (p=0.65 for differences of means at the end of the randomized study). In contrast, mean AFP values remained lower among the patients randomized to receive peginterferon although the changes in both groups were minor. The mean AFP values decreased from 17.1 ng/mL at the time of randomization to 15.7 ng/mL at the end of the randomized study in the treated patients but increased from 17.1 ng/mL to 19.0 ng/mL in the untreated patients (p=0.046 for differences of means at the end of the randomized study).
Case-control study on the accuracy of AFP and DCP in the diagnosis of HCC
The baseline characteristics of the 39 HCC cases and 77 controls are shown in Table 1. Twenty-four (61.5%) HCC cases had early tumors at the time of diagnosis. The cases and controls were well matched for age, gender, and race/ethnicity. HCC cases had more advanced liver disease and higher baseline DCP (p=0.002) and AFP values than the controls (p =0.02).
Changes in AFP and DCP values between month -12 and month 0
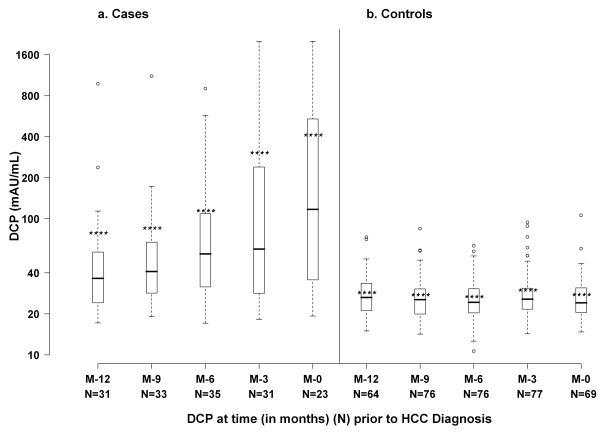
Figure 1 displays AFP values in the HCC cases and controls at month −12, −9, −6, −3 and 0 (time of HCC diagnosis). Mean ± SD AFP values increased from 37.0 ± 51.3 ng/mL at month −12 to 157.6 ± 296.6 ng/mL at month 0 in the HCC cases (p<0.0001) but remained unchanged in the controls: 17.4 ± 25.8 and 23.0 ± 34.2 ng/mL, respectively. Similarly, mean ± SD DCP values increased from 79.1 ± 172.5 mAU/mL at month −12 to 413.4 ± 597.7 mAU/mL at month 0 (p<0.0001) in the HCC cases while DCP values remained stable in the controls: 28.8 ± 11.4 and 27.9 ± 13.1 mAU/mL, respectively (Figure 2).
Figure 1.
Box plot of AFP values in HCC cases and matched controls
Figure 2.
Box plot of DCP values in HCC cases and matched controls
Sensitivity and specificity of AFP and DCP and combination of both markers in differentiating HCC cases from controls at fixed cutoff values
Table 2 shows the sensitivity and specificity of AFP alone, DCP alone, and the combination of both markers in differentiating all HCC cases from controls at various time points from month −12 to month 0. For each marker, two fixed cutoff values were chosen based on previously published literature: 20 and 200 ng/mL for AFP and 40 and 150 mAU/mL for DCP 32, 33. At the lower cutoff value, the sensitivity of DCP increased from 43% at month −12 to 74% at month 0 while the sensitivity of AFP increased minimally, from 47% to 61%. This same trend was observed at the higher cutoff values for both DCP and AFP. When the two markers were combined, the sensitivity was enhanced compared to that of either marker alone suggesting that DCP and AFP are complementary. As an example, at month 0, the sensitivity increased from 74% for DCP alone and 61% for AFP alone to 91% when either DCP >40 mAU/mL or AFP >20 ng/mL was considered.
Table 2.
Sensitivity and Specificity of DCP alone, AFP alone, and the combination of both markers in differentiating HCC cases from controls at two fixed cutoff values
| Months from HCC diagnosis |
Sensitivity | Specificity | Sensitivity | Specificity |
|---|---|---|---|---|
| DCP (mAU/mL) | >=40 | >=150 | ||
| 0 | 74% | 86% | 43% | 100% |
| −3 | 65% | 84% | 39% | 100% |
| −6 | 63% | 88% | 11% | 100% |
| −9 | 52% | 88% | 6% | 100% |
| −12 | 43% | 94% | 3% | 100% |
| AFP (ng/mL) | >=20 | >=200 | ||
| 0 | 61% | 81% | 22% | 100% |
| −3 | 58% | 80% | 13% | 98% |
| −6 | 57% | 76% | 3% | 100% |
| −9 | 45% | 77% | 6% | 100% |
| −12 | 47% | 75% | 3% | 100% |
| DCP and/or AFP | DCP >=40 or AFP >=20 | DCP >=40 and AFP >=20 | ||
| 0 | 91% | 74% | 43% | 93% |
| −3 | 87% | 69% | 35% | 95% |
| −6 | 86% | 69% | 34% | 96% |
| −9 | 82% | 67% | 15% | 97% |
| −12 | 73% | 71% | 17% | 98% |
When the analysis was limited to early stage HCC cases and their controls, the sensitivity and specificity of both DCP and AFP at all time points were similar to the results for all HCC cases and controls.
Accuracy of AFP, DCP and the combination of both markers in differentiating HCC cases from controls
Table 3 shows the AUROC of AFP, DCP and the combination AFP and DCP in differentiating HCC cases from controls at various time points between month −12 and month 0. The AUROC for DCP was higher than that for AFP at all time points except at month −3 when both markers were comparable but none of the differences were significant. The combination of both markers in which either AFP or DCP was above a specified cutoff yielded a higher AUROC at all time points and the combination was significantly better than AFP alone at all time points and significantly better than DCP alone at month −9. Figure 3 illustrates the ROC curve at the time of HCC diagnosis; the AUROC was 0.82 for DCP, 0.79 for AFP, and 0.92 for the combination of the two markers.
Table 3.
AUROC for DCP >40 mAU/mL, AFP >20 ng/mL or the combination of the 2 markers (either marker elevated) in differentiating HCC cases from controls
| Months from HCC diagnosis |
AUROC (95% CI) | ||
|---|---|---|---|
| DCP | AFP | DCP / AFP | |
| All HCC cases and controls* | |||
| 0 | 0.82 (0.68-0.95) | 0.79 (0.68-0.90) | 0.92 (0.84-0.998) |
| −3 | 0.78 (0.67-0.90) | 0.80 (0.70-0.89) | 0.87 (0.79-0.95) |
| −6 | 0.83 (0.74-0.92) | 0.72 (0.61-0.82) | 0.88 (0.80-0.95) |
| −9 | 0.79 (0.70-0.89) | 0.70 (0.59-0.80) | 0.84 (0.75-0.92) |
| −12 | 0.69 (0.56-0.82) | 0.61 (0.48-0.75) | 0.75 (0.63-0.86) |
P < 0.01 for AFP compared to the combination of AFP or DCP at months 0, −6, and −9 and p < 0.05 at months −3 and −12. P < 0.05 for DCP compared to AFP and DCP combined at month −9.
Figure 3.

ROC curves of AFP alone, DCP alone, and combination of both markers (either marker increase) in differentiating HCC cases from controls at the time of HCC diagnosis
Specificity of AFP alone and DCP alone for various levels of sensitivity
Table 4 shows the specificity of AFP alone and DCP alone at pre-specified sensitivities from 50% to 100% and the corresponding cutoff values, determined from the ROC curves, for these markers. As the predefined sensitivity increased, the specificity decreased. At a predefined sensitivity of 90%, the specificity of DCP ranged from 21% to 47% and of AFP from 15% to 57% at various time points between month −12 and month 0; the cutoff values for these two markers at month 0 were 21.8 mAU/mL and 8.2 ng/mL, respectively.
Table 4.
Specificity of DCP alone or AFP alone in differentiating HCC cases from controls at various levels of predefined sensitivity
| DCP (mAU/mL) | AFP (ng/mL) | ||||
|---|---|---|---|---|---|
| Months from HCC diagnosis |
Sensitivity | Cutoff values |
Specificity | Cutoff values |
Specificity |
| 50% | |||||
| 0 | 117.0 | 100% | 29.0 | 86% | |
| −3 | 61.0 | 93% | 33.0 | 85% | |
| −6 | 55.9 | 97% | 35.5 | 84% | |
| −9 | 42.0 | 88% | 15.0 | 73% | |
| −12 | 35.7 | 87% | 11.9 | 67% | |
| 75% | |||||
| 0 | 29.1 | 63% | 14.0 | 74% | |
| −3 | 26.5 | 52% | 11.3 | 67% | |
| −6 | 31.6 | 78% | 9.1 | 51% | |
| −9 | 28.4 | 67% | 8.3 | 52% | |
| −12 | 24.1 | 44% | 6.0 | 35% | |
| 90% | |||||
| 0 | 21.8 | 23% | 8.2 | 47% | |
| −3 | 21.0 | 25% | 8.5 | 57% | |
| −6 | 24.0 | 47% | 6.7 | 38% | |
| −9 | 23.4 | 39% | 6.7 | 39% | |
| −12 | 19.4 | 21% | 3.9 | 15% | |
| 100% | |||||
| 0 | 19.3 | 12% | 5.9 | 35% | |
| −3 | 18 | 7% | 3 | 15% | |
| −6 | 17.1 | 9% | 2.9 | 7% | |
| −9 | 19.1 | 19% | 1.9 | 3% | |
| −12 | 17.1 | 13% | 1.7 | 2% | |
As a corollary, at a predefined specificity of 90%, the sensitivity of DCP and AFP at month 0 was 70% and 30%, and the cutoff values were 44.5 mAU/mL and 87.2 ng/mL, respectively.
AFP versus ultrasound as the first indication of HCC
Among the 24 cases of early HCC, a suspicious nodule on ultrasound was detected earlier in 14 (58.4%) cases. In 5 (20.8%) cases, AFP values increased from 13 to 111, 5 to 42, 58 to 134, 46 to 119, and 4 to 30 ng/mL 37, 54, 68, 102, and 273 days, respectively before a suspicious nodule was detected on imaging, and 4 of these 5 patients had negative ultrasounds on the same day or up to 98 days after the doubling of AFP. In the remaining 5 cases, a suspicious nodule was detected on MRI or CT before AFP doubling in 4 patients and simultaneous with AFP doubling in 1 patient. In these 5 patients, ultrasounds performed 113, 207, 255, 333, and 550 days earlier did not reveal any suspicious nodule.
When all 39 HCC cases were analyzed, a suspicious nodule(s) on ultrasound was detected earlier in 19 cases, a doubling of AFP occurred earlier in 6 cases, and a suspicious nodule(s) on ultrasound and doubling of AFP occurred simultaneously in 3 cases. In the remaining 11 cases, CT or MRI detected a suspicious nodule(s) before AFP doubling in 8 cases and simultaneous with AFP doubling in 3 cases while ultrasounds performed 32-550 (median 212) days earlier did not reveal any suspicious nodule.
DISCUSSION
This analysis took advantage of the large cohort of at-risk patients followed prospectively in the HALT-C Trial to compare the accuracy of AFP and DCP in the early detection of HCC and to determine factors that might affect the performance of these markers. The availability of samples before the diagnosis of HCC allowed for the comparison of the accuracy of AFP and DCP in differentiating HCC cases from matched controls before clinical diagnosis, an important feature in HCC surveillance. DCP had greater accuracy than AFP at all time points between month −12 and time of diagnosis but the differences were not statistically significant.
Using a cutoff value of 40 mAU/mL, DCP testing alone had a sensitivity of 74% and a specificity of 86% in differentiating HCC cases and controls at the time of diagnosis. In comparison, using a cutoff value of 20 ng/mL, AFP testing alone had a sensitivity of 61% and a specificity of 81%. When the higher cutoff values frequently used in clinical practice were applied, the sensitivities of the two markers were only 43% and 22%, at the time of diagnosis and decreased to 3% at month −12 for both markers. An important goal in cancer surveillance is the detection of preclinical tumors. Therefore, optimizing sensitivity is critical. At the lower cutoff values of DCP and AFP, sensitivities of these markers at month −12 were better: 43% and 47%, respectively (Table 2). Improvement of sensitivities to 90% could be achieved by further lowering of DCP and AFP cutoff values; (Table 4) however, the improved sensitivity would come at the expense of a decrease in specificity to 21% to 47% for DCP and 15% to 57% for AFP, which will lead to many unnecessary and expensive tests as well as patient and physician anxiety.
The low sensitivity and specificity of AFP led to the recommendation against its use in HCC surveillance unless ultrasonography is not available 5. Ultrasound, however is operator-dependent, and differentiation between regenerative or dysplastic nodules and HCC in a cirrhotic liver can be difficult. In this study, the diagnosis of 17 (10 of 24 early HCC) of 39 HCC cases was first triggered by an increase in AFP and/or suspicious nodule on CT or MRI. Although the HALT-C HCC surveillance protocol called for annual rather than every 6 month ultrasounds, this is consistent with the AASLD guidelines 5. We acknowledge that some patients did not adhere to the surveillance protocol, but 8 patients had ultrasounds within 6 months of HCC diagnosis and 6 patients had ultrasounds within 12 months of diagnosis that did not reveal any suspicious nodule. Admittedly, tumor nodules may have been present on ultrasounds in some of these patients and were missed by inexperienced radiologists. Nevertheless, these results reflect the real world experience where ultrasounds are frequently performed in community hospitals and not at academic liver centers. Therefore, new biomarkers with better sensitivity and specificity than AFP to complement ultrasound are needed.
Several case control studies have shown sensitivities of DCP of 28% to 89% and specificities of 87% to 96% in the diagnosis of HCC 10-12, 33, 34. In some studies DCP was more sensitive than AFP 12, 33, 34 while in other studies AFP was more sensitive 10, 11. A recent Japanese study of 1377 HCC patients and 355 non-HCC controls with chronic hepatitis or cirrhosis showed that the accuracy of DCP was inferior to AFP particularly for small tumors 15.
Various factors may influence the performance of HCC biomarkers including patient demographics, cause of underlying liver disease, presence of cirrhosis, tumor stage and tumor biology 35-38. Thus, Nguyen et al have reported that AFP was less sensitive in diagnosing HCC among African Americans with hepatitis C related cirrhosis compared to non-African Americans 38. In an earlier report of the HALT-C Trial data, baseline AFP values were noted to be significantly higher in patients with cirrhosis, women, and blacks 29. Increased AFP values in patients with cirrhosis versus those with earlier stage liver disease had been reported in other studies and attributed to increased liver injury and hepatocyte turnover. This is supported by the observation of a decline in AFP values during pegylated interferon and ribavirin treatment and a correlation between decrease in AFP and decrease in ALT values during treatment 29. Analysis of the baseline DCP and AFP values of 1031 patients included in the current analysis showed that DCP values were also significantly higher in patients with cirrhosis but lower in women and blacks. The finding that patient demographics influenced both DCP and AFP values but in opposite directions was surprising and merits further investigation. This observation might impact the accuracy of these biomarkers in the detection of HCC and underscores the importance of recall policies based on changes in biomarker values from baseline rather than absolute cutoff values in HCC surveillance. Data from the randomized phase of the HALT-C Trial confirmed that AFP values decreased during interferon treatment. The effect however, was minimal possibly related to the use of a lower dose of peginterferon. In contrast, DCP values were not affected by interferon treatment. The performance of cancer biomarkers may also be influenced by tumor stage; 24 of the 39 HCC cases in this analysis had early stage tumors. It is reassuring that the accuracy of DCP and AFP in detecting early stage tumors was similar to that of all tumors.
Neither DCP alone nor AFP alone was optimal in the detection of HCC but the combination of both markers enhanced the sensitivity indicating that these two markers are complementary. At a DCP cutoff value of >40 mAU/mL or AFP value of >20 ng/mL, sensitivity increased from 61% and 74% for each marker alone to 91% for both markers combined at month 0 and from 43% and 47% to 73% at month −12. Several other studies also showed that DCP and AFP are complementary 14, 16, 39 which is consistent with the fact that production of DCP and AFP in HCC occurs through different pathways and with our finding that gender and race had opposite effects on these two markers.
This study has several strengths including a homogenous underlying cause for liver disease in all patients, well-matched cases and controls, prospective follow-up of at least 12 months to rule out undiagnosed HCC among the controls, and predefined criteria for diagnosis of HCC with further review of each case by a panel of investigators. A unique aspect of our analysis was the availability of samples dating back 12 months before the diagnosis of HCC allowing the assessment of the performance of DCP and AFP in differentiating preclinical HCC cases from controls.
The most important limitations in the current analysis were the small number of HCC cases and the incomplete availability of samples at all of the time points from month −12 to month 0. Also, because study sites were aware of AFP but not DCP results, a confounding bias was introduced that may have influenced investigators to order additional testing based on AFP but not DCP results, and hence the accuracy of AFP vs. DCP for HCC diagnosis. Furthermore, only 61.5% of patients presented with early stage HCC. Non-compliance with the HCC surveillance protocol and variations in interpretation of ultrasound may have contributed to the delay in diagnosis in some cases but there is a clear need for better screening strategies. The differential effects of interferon treatment on AFP and DCP values may have confounded comparisons of the accuracy of these two markers but the impact is likely to be small. Finally, our results cannot be generalized to patients with liver disease not caused by HCV or to patients that are not Caucasians or Blacks.
In conclusion, this case-control study demonstrated that DCP was not superior to AFP in the early detection of HCC in patients with advanced hepatitis C and that neither AFP alone, DCP alone nor the combination of AFP and DCP was sufficiently accurate to be used for HCC surveillance. The sensitivity of these markers was maximal at the time of HCC diagnosis and considerably lower at earlier time points. Nevertheless, increasing AFP triggered the evaluation that led to the diagnosis of some cases of early HCC. This study demonstrated that DCP and AFP are complementary; therefore, prospective studies should be conducted to determine if combining both markers will improve the detection of early HCC and to establish the optimal cutoff values that should be used for patient recall and further testing. In addition, the influence of patient demographics and interferon treatment on DCP and AFP values should be confirmed and their impact on the performance of these markers in HCC surveillance determined. Until better serum markers are available, ultrasonography remains the preferred tool for HCC surveillance but reliable biomarkers to complement ultrasound may improve the detection of early HCC in clinical practice where interpretation of ultrasound is variable.
ACKNOWLEDGMENTS
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Funding for testing of des-gamma-carboxy prothrombin was supplied by Eisai Co., Ltd., through a Materials Cooperative Research and Development Agreement (M-CRADA) with the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, Cara C. Gooch
University of Colorado School of Medicine, Denver, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01) Gregory T. Everson, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Robert J. Fontana, MD, Joel K. Greenson, MD, Grace L. Su, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS, Mita Ghosh, BS.
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: Marc G. Ghany, MD, T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Chihiro Morishima, MD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Anne M. Stoddard, ScD, Linda Massey, Teresa M. Curto, MSW, MPH
Armed Forces Institute of Pathology, Washington, DC: Zachary D. Goodman, MD
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Abbreviations
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- AFP
alpha fetoprotein
- DCP
des-gamma-carboxy prothrombin
- PIVKA
prothrombin induced by vitamin K absence
- HALT-C
hepatitis C antiviral long-term treatment against cirrhosis
- CT
computed tomography
- MRI
magnetic resonance imaging
- AUROC
area under receiver operating characteristic curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
Financial Disclosures
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: A.S. Lok is a consultant; R.K. Sterling is a consultant, receives research support, and is on the speaker's bureau; J.C. Hoefs is on the speaker's bureau; T.R. Morgan is consultant, on the speaker's bureau and receives research support; A.M. Di Bisceglie is a consultant, on the speaker's bureau, and receives research support; W.M. Lee receives research support; and H.L. Bonkovsky receives research support. Financial relationships of the authors with Eisai Co., Ltd., are as follows: A.S. Lok receives research support. Authors with no financial relationships related to this project are: J.E. Everhart, E.C. Wright, H.-Y. Kim, and J.L. Dienstag.
REFERENCES
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Everhart JE, Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases . Primary Liver Cancer. In: Everhart JE, editor. Burden of Digestive Diseases in the United States. US Government Printing Office; Washington, DC: 2008. pp. 49–52. (NIH Publication No. 09-6443). 2008. [Google Scholar]
- 4.Stravitz RT, Heuman DM, Chand N, Sterling RK, Shiffman ML, Luketic VA, Sanyal AJ, Habib A, Mihas AA, Giles HC, Maluf DG, Cotterell AH, Posner MP, Fisher RA. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121:119–26. doi: 10.1016/j.amjmed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 6.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–22. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 7.McMahon BJ, Bulkow L, Harpster A, Snowball M, Lanier A, Sacco F, Dunaway E, Williams J. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B: a 16-year population-based study. Hepatology. 2000;32:842–6. doi: 10.1053/jhep.2000.17914. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J, HCC EPoEo Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. Journal of Hepatology. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 9.Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M, Saida Y, Takayama T, Yamaoka Y. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37–51. doi: 10.1111/j.1872-034X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- 10.Aoyagi Y, Oguro M, Yanagi M, Mita Y, Suda T, Suzuki Y, Hata K, Ichii K, Asakura H. Clinical significance of simultaneous determinations of alpha-fetoprotein and des-gamma-carboxy prothrombin in monitoring recurrence in patients with hepatocellular carcinoma. Cancer. 1996;77:1781–6. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1781::AID-CNCR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 11.Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, Ohnishi K. Serum des-gamma-carboxy prothrombin levels determined by a new generation of sensitive immunoassays in patients with small-sized hepatocellular carcinoma. American Journal of Gastroenterology. 1999;94:650–4. doi: 10.1111/j.1572-0241.1999.00930.x. [DOI] [PubMed] [Google Scholar]
- 12.Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des-gamma-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82:1643–8. doi: 10.1002/(sici)1097-0142(19980501)82:9<1643::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, Takahashi T, Kasukawa R. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036–40. doi: 10.1111/j.1572-0241.2000.01978.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamamura K, Shiratori Y, Shiina S, Imamura M, Obi S, Sato S, Yoshida H, Omata M. Unique clinical characteristics of patients with hepatocellular carcinoma who present with high plasma des-gamma-carboxy prothrombin and low serum alpha-fetoprotein. Cancer. 2000;88:1557–64. doi: 10.1002/(sici)1097-0142(20000401)88:7<1557::aid-cncr9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura S, Nouso K, Sakaguchi K, Ito YM, Ohashi Y, Kobayashi Y, Toshikuni N, Tanaka H, Miyake Y, Matsumoto E, Shiratori Y. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038–43. doi: 10.1111/j.1572-0241.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 16.Okuda H, Obata H, Nakanishi T, Furukawa R, Hashimoto E. Production of abnormal prothrombin (des-gamma-carboxy prothrombin) by hepatocellular carcinoma. A clinical and experimental study. J Hepatol. 1987;4:357–63. doi: 10.1016/s0168-8278(87)80546-9. [DOI] [PubMed] [Google Scholar]
- 17.Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427–31. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 18.Stenflo J, Fernlund P, Egan W, Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci U S A. 1974;71:2730–3. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrero JA. Screening tests for hepatocellular carcinoma. Clin Liver Dis. 2005;9:235–51. doi: 10.1016/j.cld.2004.12.006. vi. [DOI] [PubMed] [Google Scholar]
- 20.Ikoma J, Kaito M, Ishihara T, Nakagawa N, Kamei A, Fujita N, Iwasa M, Tamaki S, Watanabe S, Adachi Y. Early diagnosis of hepatocellular carcinoma using a sensitive assay for serum des-gamma-carboxy prothrombin: a prospective study. Hepatogastroenterology. 2002;49:235–8. [PubMed] [Google Scholar]
- 21.Pateron D, Ganne N, Trinchet JC, Aurousseau MH, Mal F, Meicler C, Coderc E, Reboullet P, Beaugrand M. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. Journal of Hepatology. 1994;20:65–71. doi: 10.1016/s0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 22.Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61–6. [PubMed] [Google Scholar]
- 23.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–9. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16:553–9. doi: 10.1046/j.1440-1746.2001.02470.x. [DOI] [PubMed] [Google Scholar]
- 25.Chalasani N, Horlander JC, Sr., Said A, Hoen H, Kopecky KK, Stockberger SM, Jr., Manam R, Kwo PY, Lumeng L. Screening for hepatocellular carcinoma in patients with advanced cirrhosis. Am J Gastroenterol. 1999;94:2988–93. doi: 10.1111/j.1572-0241.1999.01448.x. [DOI] [PubMed] [Google Scholar]
- 26.Izuno K, Fujiyama S, Yamasaki K, Sato M, Sato T. Early detection of hepatocellular carcinoma associated with cirrhosis by combined assay of des-gamma-carboxy prothrombin and alpha-fetoprotein: a prospective study. Hepatogastroenterology. 1995;42:387–93. [PubMed] [Google Scholar]
- 27.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, Everson GT, Di Bisceglie AM, Morgan TR, Ghany MG, Morishima C, Wright EC, Everhart JE. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–92. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008;359:2429–41. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, Wright EC, Everson GT, Lindsay KL, Lok AS, Lee WM, Morgan TR, Ghany MG, Gretch DR. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434–41. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 30.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 31.Lok AS, Seeff LB, Morgan TR, di Bisceglie AM, Sterling RK, Curto TM, Everson GT, Lindsay KL, Lee WM, Bonkovsky HL, Dienstag JL, Ghany MG, Morishima C, Goodman ZD. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–48. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 33.Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114–21. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 34.Lamerz R, Runge M, Stieber P, Meissner E. Use of serum PIVKA-II (DCP) determination for differentiation between benign and malignant liver diseases. Anticancer Res. 1999;19:2489–93. [PubMed] [Google Scholar]
- 35.Toyoda H, Kumada T, Osaki Y, Oka H, Urano F, Kudo M, Matsunaga T. Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin Gastroenterol Hepatol. 2006;4:1528–36. doi: 10.1016/j.cgh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3:79–87. doi: 10.3233/cbm-2007-3202. [DOI] [PubMed] [Google Scholar]
- 37.Yano Y, Yamashita F, Kuwaki K, Fukumori K, Kato O, Yamamoto H, Ando E, Tanaka M, Sata M. Clinical features of hepatitis C virus-related hepatocellular carcinoma and their association with alpha-fetoprotein and protein induced by vitamin K absence or antagonist-II. Liver Int. 2006;26:789–95. doi: 10.1111/j.1478-3231.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–7. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 39.Beale G, Chattopadhyay D, Gray J, Stewart S, Hudson M, Day C, Trerotoli P, Giannelli G, Manas D, Reeves H. AFP, PIVKAII, GP3, SCCA-1 and follisatin as surveillance biomarkers for hepatocellular cancer in non-alcoholic and alcoholic fatty liver disease. BMC Cancer. 2008;8:200. doi: 10.1186/1471-2407-8-200. [DOI] [PMC free article] [PubMed] [Google Scholar]