Abstract
Allogeneic hematopoietic cell transplantation (HCT) using reduced intensity conditioning (RIC) regimens is a potentially curative treatment for patients (patients) with myelofibrosis (MF), as we (1) and others have reported. Non-relapse mortality (NRM) from Graft vs. Host Disease (GVHD) and other complications has limited the success of this approach. As part of an ongoing prospective research study at City of Hope, a combination of tacrolimus/sirolimus +/− methotrexate (MTX) for GVHD prophylaxis has become the standard treatment for our allogeneic HCT patients. In this report, we present results for 23 consecutive patients, including extended follow up for 9 patients previously reported who received cyclosporine (CSA)/mycophenolate (MMF) +/− MTX, and the current series of 14 patients who received tacrolimus/sirolimus +/− MTX, and evaluate the impact of the GVHD prophylaxis regimen on the outcomes. Median follow up for alive patients was 29.0 months (9.5–97.0). The estimated 2 yr overall survival (OS) for the CSA/MMF cohort was 55.6 % (confidence interval 36.0, 71.3), and for the tacrolimus/sirolimus cohort it was 92.9% (63.3, 98.8) (p=0.047). The probability of grade III or IV acute GVHD was 60% for the CSA/MMF patients, and 10% for the tacrolimus/sirolimus group (p=0.0102). No significant differences were seen for grade II to IV acute GVHD in the two groups. We conclude that the combination of tacrolimus/sirolimus +/− MTX for GVHD prophylaxis in the setting of RIC HCT for MF appears to reduce the incidence of severe acute GVHD and NRM, and leads to improved OS compared to CSA/MMF +/− MTX.
Keywords: Myelofibrosis, reduced intensity conditioning, allogeneic transplantation, tacrolimus/sirolimus
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is the only known potentially curative treatment for patients with primary or secondary myelofibrosis (MF) (2–5). The applicability of this approach to the population of patients with MF has been limited by the availability of suitable donors, high rates of morbidity and non-relapse mortality (NRM) associated with fully myeloablative conditioning regimens, older age of the patients, and concerns about delayed engraftment or graft failure in patients with splenomegaly and fibrotic marrows. Increased success in identifying well matched unrelated donors (MUDs) for patients who lack a matched related donor has helped to expand the pool of patients to whom allogeneic HCT can be offered. More recently, we (1) and others have reported our experiences utilizing reduced intensity conditioning (RIC) regimens as an encouraging strategy to reduce early morbidity and TRM associated with allogeneic HCT and to extend the option of allogeneic HCT to older patients (3;4;6–9).
The incidence of severe acute and chronic GVHD leading to significant morbidity and early NRM continues to be a problem whether a myeloablative or RIC regimen is utilized. Most studies have used some combination of cyclosporine (CSA) or tacrolimus with methotrexate (MTX) and/or mycophenalate (MMF) for GVHD prophylaxis. Recently, a novel combination of tacrolimus and sirolimus has been tested in place of these more traditional immunosuppressive combinations. There are data supporting the efficacy of tacrolimus/sirolimus in preventing clinically significant acute GVHD(10–12). There are also concerns at some centers about increased toxicity from this combination in the form of higher rates of sinusoidal obstructive syndrome (SOS, previously known as veno-occlusive disease) and thrombotic microangiopathy (13–15).
In an attempt to reduce the risk of mortality related to GVHD, we modified our protocol to include tacrolimus/sirolimus +/− MTX for GVHD prophylaxis in place of CSA/MMF +/− MTX for patients undergoing RIC allogeneic HCT utilizing GCSF primed peripheral blood stem cells (PBSC) from either matched sibling donors or MUDs. We now present the results for this group of 14 patients, extend the follow up for the original 9 patients previously reported, and evaluate the impact of the GVHD prophylaxis regimen on outcomes.
Methods
Patients and GVHD Prophylaxis
Twenty three consecutively treated patients with myelofibrosis (MF) who received reduced intensity conditioning (RIC) regimens for allogeneic hematopoietic cell transplantation (HCT) were assessed as part of a formal treatment strategy approved by the Institutional Review Board of City of Hope (Duarte, CA). The first nine patients were treated between May 10, 2000 and February 11, 2004. The second group of 14 patients was treated between July 15, 2005 and November 22, 2007. The date of analysis was August 14, 2008.
The first nine patients all received cyclosporine (CSA)/mycophenolate (MMF) as prophylaxis against graft vs. host disease (GVHD), whereas the second group of 14 patients received a combination of tacrolimus/sirolimus. There were 7 female donors and 16 males, with 3 cases of female donor to male recipients (Table 1). The median age was 58 yrs (range 39–69) with 12 females, 11 males. Eighteen patients had primary MF. Two of nine CSA/MMF patients developed MF secondary to a prior myeloproliferative disorder and 3/14 tacrolimus/sirolimus patients had secondary MF. The Philadelphia chromosome and the bcr-abl gene were excluded for all patients using standard methods. JAK2 kinase V617F mutation analysis was available pre-HCT only for 4/14 patients in the tacrolimus/sirolimus cohort. Two of these four patients were positive and two were negative. At the time of diagnosis, the risk scores for the 17 patients with primary MF using the new prognostic scoring system from the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) were: low for 3 patients; intermediate-1 for 7 patients; intermediate-2 for 3 patients; and high for 4 patients. (16) At the time of HCT, the Lille risk score was high for 10 patients, intermediate for 12, and low for 1. Fifteen of the 23 patients (8/9 in the CSA/MMF cohort; 7/14 in the tacrolimus/sirolimus cohort) had ≥ 1% blasts in the peripheral blood before HCT, but all had fewer than 5% blasts in their pre-HCT bone marrow samples. Twenty-two of the 23 patients were classified as MF-3, and one pt was classified as MF-2.(17) Cytogenetic data were available for 20 of the 23 patients in this study. Isolated del(20q), which is considered a favorable prognostic abnormalities along with isolated del(13q) (18) was found in only one subject. Ten of these 20 patients had normal cytogenetics, and nine had a variety of other clonal changes including +8 in one patient.
Table 1.
Summary of Patient Characteristics and Transplant Outcomes (n=23)
| Variable | Mean ± SD or N(%) |
Median (Range) |
|---|---|---|
| Patient Age at Transplant (Years) | 58 (39 – 69) | |
| Patient Gender | ||
| Female | 12 (52.2) | |
| Male | 11 (47.8) | |
| Donor Gender | ||
| Female | 7 (30.4) | |
| Male | 16 (69.6) | |
| Female Donor to Male Recipient Transplants | 2 (8.7) | |
| Donor Type | ||
| Sibling | 8 (34.8) | |
| Matched Unrelated | 15 (65.2) | |
| Stem Cell Source | ||
| Bone Marrow | 2 (8.7) | |
| Peripheral Blood | 21 (91.3) | |
| CD34 Cell Dose | 7.8 (1.1–20.0) | |
| Time from Diagnosis to Transplant (Months) | 21 (2.0–249.0) | |
| Lille Risk Score at HCT | ||
| Low | 1 (4.4) | |
| Intermediate | 12 (52.1) | |
| High | 10 (43.5) | |
| Time to Engraftment (Days) | ||
| Absolute Neutrophil Count ≥500 | 16.5 (8.0–26.0) | |
| Platelets ≥20 | 18.0 (10.0–110.0) | |
| Transfusion Dependent Post-HCT | ||
| Red Blood Cells | 21 (91.3) | |
| Platelets | 15 (65.2) | |
| GVHD Prophylaxis | ||
| CSA, MMF, +/−MTX | 9 (39.1) | |
| Tacrolimus/Sirolimus, +/−MTX | 14 (60.9) | |
| Acute GVHD | ||
| None | 7 (30.4) | |
| Grade I | 4 (17.4) | |
| II | 7 (30.4) | |
| III | 2 (8.7) | |
| IV | 3 (13.0) | |
| Time to Acute GVHD Onset (Days) | 32 (10–60) | |
| Chronic GVHD | ||
| No | 6 (30.0) | |
| Yes | 14 (70.0) | |
| Limited | 4 (28.6) | |
| Extensive | 10 (43.5) | |
| Died ≤ 100 Days | 3 | |
| Time to Chronic GVHD Onset (Days) | 197 (100–351) | |
| Constitutional Symptoms | ||
| Yes | 2 (8.7) | |
| Fevers only | 1 | |
| Night Sweats only | 1 | |
| No | 21 (91.3) | |
| Relapse post HCT | ||
| Yes | 1 (4.0) | |
| No | 22 (96.0) | |
| Follow-Up (Months) | ||
| All patients | 24.7 (0.7–97.6) | |
| Alive | 29.0 (9.5–97.6) | |
| Dead | 10 (0.7–21.6) | |
| Number of Death Events post HCT | ||
| Alive | 18 (78.3) | |
| Dead | 5 (21.7) | |
| Cause of Death (Transplant Related) |
||
| Yes | 5 (100.00) | |
| No | 0 (0.00) | |
| Cause of Death | ||
| Graft Failure, Sepsis | 1 | |
| GVHD, Sepsis | 1 | |
| GVHD, Sepsis, Graft failure | 1 | |
| Infection | 1 | |
| Respiratory Failure, Alveolar hemorrhage, Renal Failure |
1 | |
Eighteen of the 23 patients (9/9 of the patients in the CSA/MMF cohort; 9/14 of the patients in the tacrolimus/sirolimus cohort) had spenomegaly either at the time of diagnosis or during the course of their disease, ranging from 2–3 cm below the left costal margin to extending into the right pelvis. Nine of these 18 patients underwent splenectomy prior to HCT. The decision to proceed to splenectomy was made by each patient and physician team on a case by case basis. In general, splenectomy was recommended for patients with significant symptoms such as intractable pain or inability to eat, who were deemed to be suitable surgical candidates.
Conditioning Regimen
The RIC regimen consisted of Fludarabine (Flu)/ melphalan (Mel) for 23 patients, including all 14 of the tacrolimus/sirolimus patients, and Flu/total body irradiation for one patient in the CSA/MMF cohort. That patient received Flu 30 mg/m2 per day intravenously (IV) for 3 days on days −3 to −1, followed by a single dose of total body irradiation of 200 cGy on day 0. The treatment schedule for the eight patients who received Flu/Mel and CSA/MMF was Flu 25 mg/m2 per day IV for 5 days given on days −6 to −2, Mel 140mg/m2 IV on day −1, CSA 1.5 mg/kg IV starting on day −1, and MMF at 15 mg/kg IV twice a day starting 2 hours after the end of the stem cell infusion on day 0. For the 14 patients who received Flu/Mel followed by tacrolimus/sirolimus, the schedule was slightly different. The Flu was administered at 25 mg/m2 per day IV for 5 days given on days −9 to −5, Mel 140mg/m2 IV on day −4, followed by tacrolimus 0.02 mg/kg/day by continuous IV infusion starting on day −3. Tacrolimus dose was adjusted to maintain a whole blood trough plasma concentration of 5–10 ng/ml when used in combination with sirolimus. Sirolimus was administered as an oral loading dose of 12 mg on day −3, followed by 4 mg orally daily. Sirolimus dose was adjusted to maintain a whole blood trough plasma concentration of 5–10 ng/ml. Six of the 7 recipients of MUD stem cells who received CSA/MMF prophylaxis, and 7/8 recipients of MUD stem cells who received tacrolimus/sirolimus were also given MTX 5 mg/m2 IV on days +1, +3, and +6.
HLA Matching and Stem Cell Source
Eight patients received stem cell products from HLA fully matched siblings (2/9 CSA/MMF patients; 6/14 tacrolimus/sirolimus patients) and 15 from matched unrelated donors (MUDs) (7/9 CSA/MMF patients; 8/14 tacrolimus/sirolimus patients). The MUDs were selected using polymerase chain reaction sequence-specific primer or polymerase chain reaction sequence-specific oligonucleotide probe techniques at HLA-A, -B, -C, -DR, and -DQ loci. The donors for 10 patients were 10/10 matched; micromismatched at HLA-DQB1 for one patient in the first group; micromismatched at HLA-B and -DRB1 for the second donor for the one patient with graft failure in the first group; micromismatched at HLA-A, -B, -C, and -DQB1 for one patient in the first group; micromismatched at HLA-B and major mismatched at HLA-C for one patient in the first group; micromismatched at HLA-B and -DR for one patient in the second group; and major mismatched at HLA-C for one patient in the second group.
The source of stem cells was GCSF primed peripheral blood for 21 patients, and unprimed bone marrow for 2 patients. The median cell dose was 7.8 × 10^6 CD34 cells/kg.
Supportive Care
All patients were supported during the HCT according to standard operating procedures including the management of infectious complications, and the use of low dose continuous infusion of heparin for prophylaxis against sinusoidal obstructive syndrome (SOS) (also called veno-occlusive disease).
Results
Engraftment
Neutrophil engraftment was defined as absolute neutrophil count (ANC) ≥ 500 × 103 /µl , achieved and sustained for 3 consecutive days with no subsequent decline. Platelet engraftment was defined as platelet count of 50,000/ µl with no transfusions for the past 7 days. Twenty-two patients achieved ANC engraftment by day +8 to 26 after HCT (median 16.5 days). One patient’s nadir was above an ANC of 500 during the transplant. One patient in the first group never engrafted despite three transplant events. He was very large (109 kg), had moderate splenomegaly, and the CD34 cell counts were low in all three stem cell products (0.7–2.7 ×106 /kg). Eighteen of the 23 patients achieved platelet engraftment by day +10 to 110 (median 18.0 days). Four patients never engrafted with platelets before they expired, and one patient’s nadir was above 50,000/ µl. (Table 1). There was a trend towards faster engraftment in splenectomized patients, but this was not statistically significant. (Data not shown).
Disease status after HCT
The results of chimerism studies using FISH or STR DNA analysis for the first 9 patients in this study were previously reported.(1) Only one of those 9 patients, had relapsed transiently and then achieved a complete remission again after withdrawal of immunosuppressive therapy as the only intervention. All 5 surviving patients from that group remain disease free at the time of this analysis.
For the tacrolimus/sirolimus cohort of patients, STR analyses showed 100% donor cells at the time of the latest assay in all 13 surviving patients. One of these 14 patients relapsed at day +59, but achieved a complete remission after withdrawal of immunosuppression and decitabine therapy.
The response of MF to HCT was assessed using the European Myelofibrosis Network criteria(19). For the 22 patients with MF-3 pre-HCT, post-HCT samples were available for review for 20 of the patients at varying time points ranging from 30 days to 3 years (median 138 days). The post-HCT MF score was MF-3 in 12/20, MF-2 in 6/20, and MF-1 in 2/20. One patient had MF-2 pre-HCT, and MF-0 at 392 days post-HCT. The cellularity of the latest bone marrow samples ranged from <5 to 90% (median 30%).
For the 14 patients whose spleens were intact at the time of HCT, the splenomegaly was reported as resolved in 7 at varying lengths of time post-HCT, with the latest time of 594 days after HCT. There were no data available for 6 patients, and study pt 4 died with splenomegaly still present at day +21 after his third HCT procedure. There was no difference in outcomes for the patients who underwent splenectomy before HCT versus those who did not. Only two patients were known to be positive for the JAK2 V617F mutation pre-HCT, and both of them were JAK2 negative at days +108 and 99 post-HCT, respectively. One patient was JAK2 negative at day +272 post-HCT, but there were no data to compare pre-HCT. Two patients were JAK2 negative pre-HCT and were not tested post-HCT.
GVHD and other regimen related toxicities
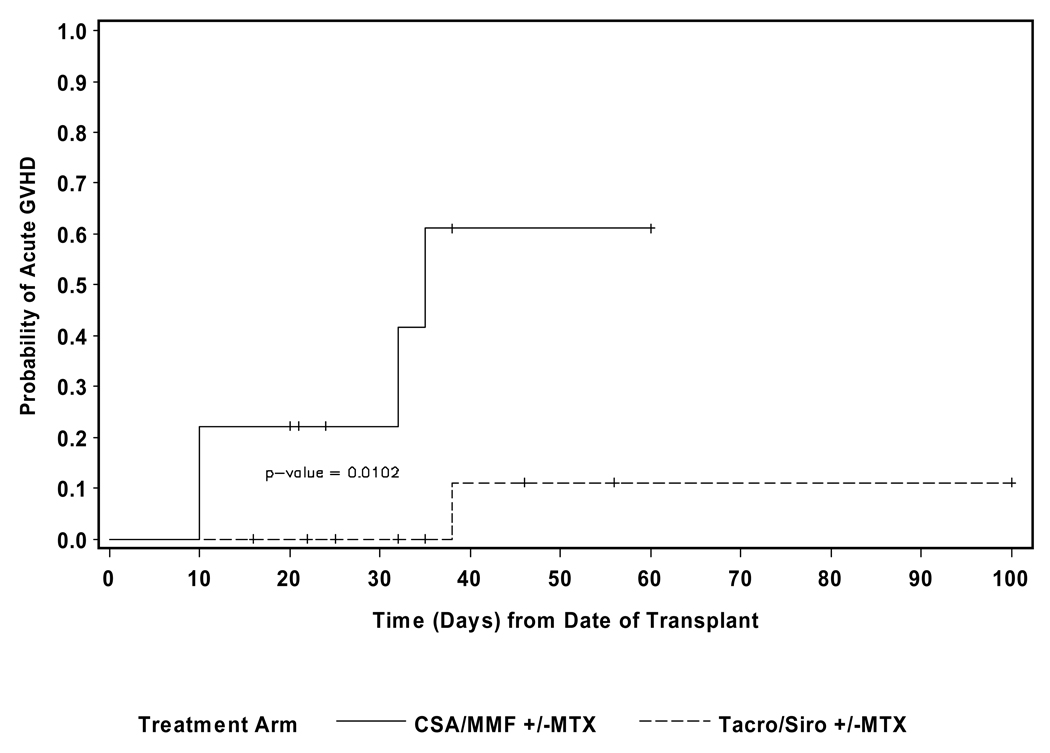
Acute GVHD developed in 16/23 of the patients. Maximum grade was grade I for 4/23, grade II for 7/23, grade III for 2/23, and grade IV for 3/23. The median time to onset of acute GVHD was 32 days (range 10–60). The probability of grade III or IV acute GVHD was 60% for the CSA/MMF patients, and 10% for the tacrolimus/sirolimus group (p=0.01). (See Figure 1) There was no significant difference in the incidence of grade II–IV acute GVHD between the two groups of patients. Neither the type of donor (sib versus MUD), the degree of HLA matching, nor the gender of the donor were significant predictors of grade III or IV acute GHVD (p=0.6, 0.73 and 1.0, respectively). Although the development of chronic GVHD was not found to be significantly associated with OS, five of the six evaluable patients in the CSA/MMF cohort developed chronic GVHD (4/5 extensive) compared to 9/14 patients (6/9 extensive) in the acrolimus/sirolimus group.
Figure 1.
Days to acute GVHD-Grade III or greater stratified by GVHD prophylaxis
None of the 23 patients developed clinically apparent SOS or thrombotic microangiopathy (TMA).
Survival
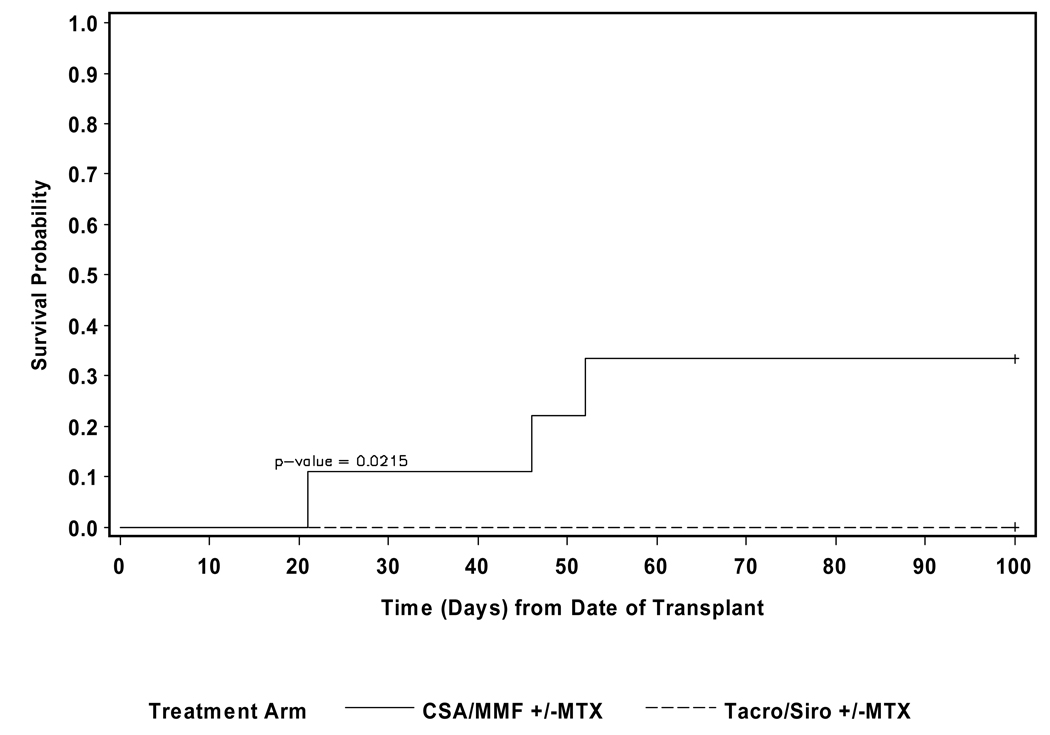
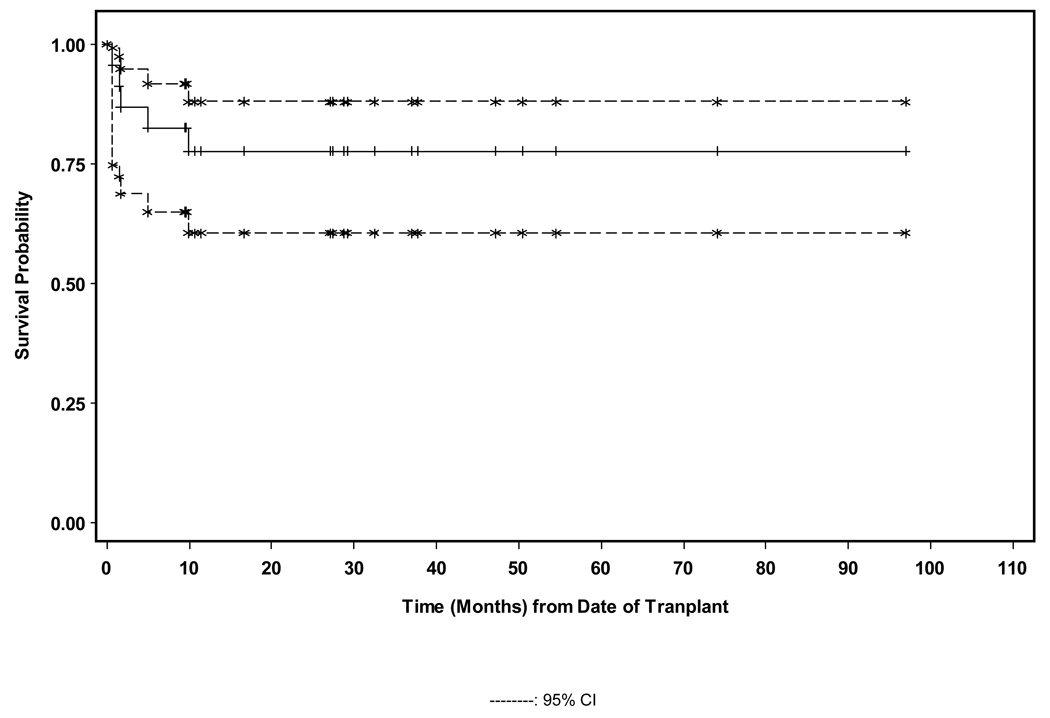
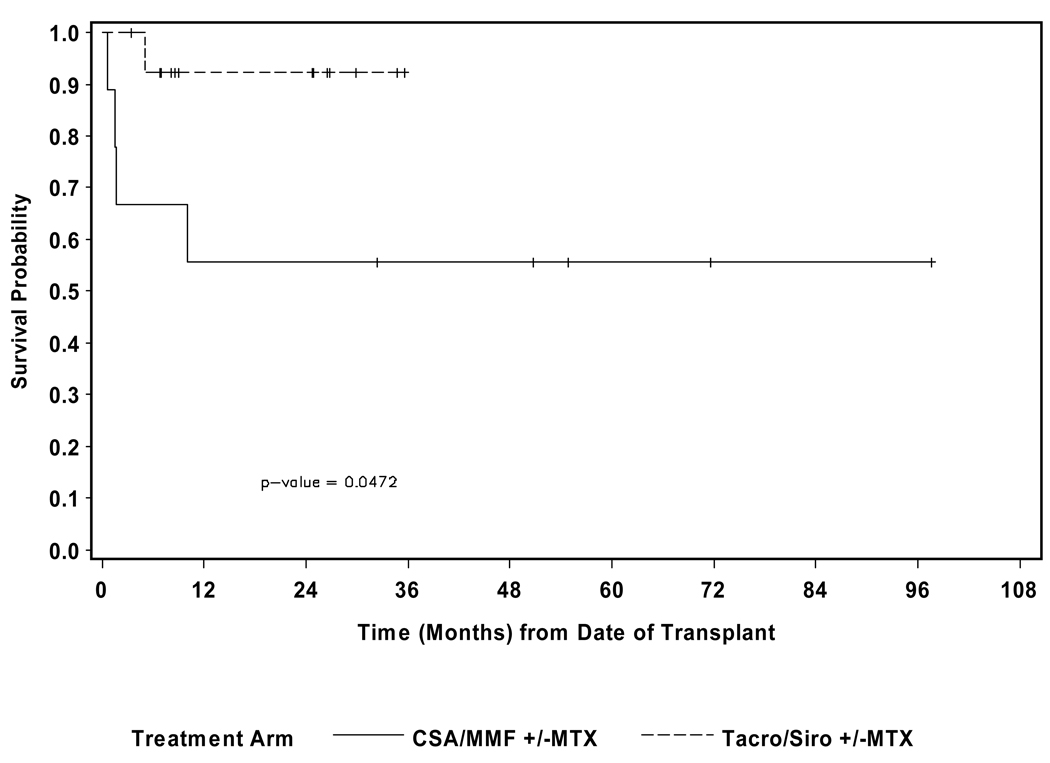
The median follow-up for alive patients was 29.0 months (9.5–97.0). The 100-day treatment related mortality (TRM) was 33.3% for the CSA/MMF patients and 0 for the tacrolimus/sirolimus group (p=0.02). (See Figure 2) Five patients died- 3/9 of the CSA/MMF group from GVHD +/− infections and 1/9 from graft failure with sepsis; 1/14 of the tacrolimus/sirolimus group died from GVHD and multi-organ failure. No patients died from relapsed MF. The estimated 2 yr overall survival (OS) for the CSA/MMF cohort was 55.6% (confidence intervals 36.0, 71.3), and for the tacrolimus/sirolimus cohort it was 92.9% (63.3, 98.8) (p=0.047). (See Figures 3A and 3B) There was no significant different in overall survival between patients who underwent splenectomy pre-HCT versus those who did not.
Figure 2.
Non-relapse mortality-100 days stratified by GVHD prophylaxis
Figure 3.
Figure 3A. Overall survival.
Figure 3B. Overall survival stratified by GVHD prophylaxis
Discussion
Allogeneic HCT is the only potentially curative therapy for patients with MF. The introduction of RIC regimens has allowed investigators to extend this modality to older patients. It is not clear whether RIC offers an advantage over fully myeloablative patients who are younger(6;8), or whether HCT is the optimal therapy over chemotherapy especially for younger patients(20). We have reported on our initial experience with a group of 9 patients treated with RIC and CSA/MMF +/− MTX for GVHD prophylaxis(1). The main cause of NRM in that group was GVHD and related complications. In an effort to improve on those outcomes, we changed the GVHD prophylaxis regimen to tacrolimus/sirolimus +/− MTX. There have been encouraging reports about the efficacy of this regimen in preventing GVHD(11;12;14). There have also been concerns about higher risks of developing SOS and TMA with this and related combinations(13;15). Sirolimus in particular has been implicated. Some contributing factors to these complications include the use of busulfan or fully myeloablative conditioning regimens(21).
All of our patients had varying degrees of splenomegaly, and nine of them had undergone splenectomy pre-HCT. There was no apparent impact of the presence or absence of the spleen on outcomes post-HCT, including engraftment, GVHD, and survival, as has been reported previously(22).
It is hypothesized that the main therapeutic benefit of RIC HCT is the development of graft vs. MF effect post-HCT as an alloreactive immunologic reaction. The fact that our surviving patients are all free of disease is an affirmation of that postulate. The post-HCT experiences of two of our patients, one in the first cohort, and one in the second cohort, speak more clearly to the benefits of the graft vs. MF effect. Both patients relapsed after HCT, and were salvaged to a long lasting remission by the removal of immunosuppression alone in the first of these two patients, and by the combination of withdrawal of immunosuppression and the addition of the hypomethylating agent, decitabine, in the second patient.
The discovery of the JAK2 V617F mutation in patients with a variety of myeloproliferative disorders including MF has enhanced our understanding of the pathogenesis of these diseases, and has opened the door to new targeted therapy.(23) There does not appear to be an influence on outcomes after HCT based on JAK2 mutation status (24), but PCR assays for JAK2 mutation offer a sensitive technique for monitoring minimal residual disease, and for directing post-HCT therapy for early relapse or persistent disease(25–29). We had limited data about JAK2 mutation status in our patients pre-HCT. In our two informative patients, the PCR for JAK2 mutation was positive pre-HCT, and became negative post-HCT, as an additional indicator of the curative impact of this approach.
In this current report, we extend the follow up of the original series of 9 patients treated with RIC for MF, and we contrast the results of this group to those of a second series of 14 patients who received the same conditioning regimen and stem cell source, but who received tacrolimus/sirolimus +/− MTX instead of CSA/MMF +/− MTX. The main findings were that the risk of developing Grade III–IV acute GVHD was significantly lower in the second series of patients. This reduction in GVHD risk translated into a significant reduction in 100-day NRM to 0%, and a significant improvement in OS for the tacrolimus/sirolimus group at 92.9%. The incidence of chronic GVHD remains a significant issue even with the use of the tacrolimus/sirolimus combination. These results compare very favorably to recent reports using either RIC or myeloablative conditioning regimens with OS ranging from 39 to 100%(2;3;3–5;7–9).
Acknowledgements
This study was supported by grants CA30206 and CA33572 from the National Institutes of Health. We thank the dedicated nurses of the City of Hope Bone Marrow Unit for their excellent care of our patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement: None of the authors has anything to disclose.
Reference List
- 1.Snyder DS, Palmer J, Stein AS, et al. Allogeneic Hematopoietic Cell Transplantation following Reduced Intensity Conditioning for Treatment of Myelofibrosis. Biology of Blood and Marrow Transplantation. 2006;12(11):1161–1168. doi: 10.1016/j.bbmt.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Patriarca F, Bacigalupo A, Sperotto A, et al. Allogeneic hematopoietic stem cell transplantation in myelofibrosis: the 20-year experience of the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Haematologica. 2008;93(10):1514–1522. doi: 10.3324/haematol.12828. [DOI] [PubMed] [Google Scholar]
- 3.Kerbauy DMB, Gooley TA, Sale GE, et al. Hematopoietic Cell Transplantation as Curative Therapy for Idiopathic Myelofibrosis, Advanced Polycythemia Vera, and Essential Thrombocythemia. Biology of Blood and Marrow Transplantation. 2007;13(3):355–365. doi: 10.1016/j.bbmt.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Papageorgiou SG, Castleton A, Bloor A, Kottaridis PD. Allogeneic stem cell transplantation as treatment for myelofibrosis. Bone Marrow Transplant. 2006;38(11):721–727. doi: 10.1038/sj.bmt.1705516. [DOI] [PubMed] [Google Scholar]
- 5.Rondelli D, Barosi G, Bacigalupo A, et al. Allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in intermediate or high risk patients with myelofibrosis with myeloid metaplasia. Blood. 2005;105(10):4115–4119. doi: 10.1182/blood-2004-11-4299. [DOI] [PubMed] [Google Scholar]
- 6.Merup M, Lazarevic V, Nahi H, et al. Different outcome of allogeneic transplantation in myelofibrosis using conventional or reduced-intensity conditioning regimens. Brit J Haematol. 2006;135(3):367–373. doi: 10.1111/j.1365-2141.2006.06302.x. [DOI] [PubMed] [Google Scholar]
- 7.George B, Kerridge I, Gottlieb D, et al. A reduced intensity conditioning protocol associated with excellent survival in patients with myelofibrosis. Bone Marrow Transplant. 2008 doi: 10.1038/bmt.2008.219. [DOI] [PubMed] [Google Scholar]
- 8.Gupta V, Kroger N, Aschan J, et al. A retrospective comparison of conventional intensity conditioning and reduced-intensity conditioning for allogeneic hematopoietic cell transplantation in myelofibrosis. Bone Marrow Transplant. 2009 doi: 10.1038/bmt.2009.10. [DOI] [PubMed] [Google Scholar]
- 9.Rondelli D. Allogeneic hematopoietic stem cell transplantation for myelofibrosis. Haematologica. 2008;93(10):1449–1450. doi: 10.3324/haematol.13801. [DOI] [PubMed] [Google Scholar]
- 10.Cutler C, Kim HT, Hochberg E, et al. Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biology of Blood and Marrow Transplantation. 2004;10(5):328–336. doi: 10.1016/j.bbmt.2003.12.305. [DOI] [PubMed] [Google Scholar]
- 11.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109(7):3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alyea EP, Li S, Kim HT, et al. Sirolimus, Tacrolimus, and Low-Dose Methotrexate as Graft-versus-Host Disease Prophylaxis in Related and Unrelated Donor Reduced-Intensity Conditioning Allogeneic Peripheral Blood Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2008;14(8):920–926. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler C, Stevenson K, Kim HT, et al. Sirolimus is associated with veno-occlusive disease of the liver after myeloablative allogeneic stem cell transplantation. Blood. 2008;112(12):4425–4431. doi: 10.1182/blood-2008-07-169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furlong T, Kiem HP, Appelbaum FR, et al. Sirolimus in Combination with Cyclosporine or Tacrolimus Plus Methotrexate for Prevention of Graft-versus-Host Disease following Hematopoietic Cell Transplantation from Unrelated Donors. Biology of Blood and Marrow Transplantation. 2008;14(5):531–537. doi: 10.1016/j.bbmt.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platzbecker U, von Bonin M, Goekkurt E, et al. Graft-versus-Host disease Prophylaxis with Everolimus and Tacrolimus Is Associated with a High Incidence of Sinusoidal Obstruction Syndrome and Microangiopathy: Results of the EVTAC Trial. Biology of Blood and Marrow Transplantation. 2009;15(1):101–108. doi: 10.1016/j.bbmt.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Cervantes F, Dupriez B, Pereira A, et al. A new prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 17.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–1132. [PubMed] [Google Scholar]
- 18.Hussein K, Van Dyke DL, Tefferi A. Conventional cytogenetics in myelofibrosis: literature review and discussion. Eur J Haematol. 2009 doi: 10.1111/j.1600-0609.2009.01224.x. DOI 10.1111/j.1600-0609.2009.01224.x. [DOI] [PubMed] [Google Scholar]
- 19.Barosi G, Bordessoule D, Briere J, et al. Response criteria for myelofibrosis with myeloid metaplasia: results of an initiative of the European Myelofibrosis Network (EUMNET) Blood. 2005;106(8):2849–2853. doi: 10.1182/blood-2005-04-1520. [DOI] [PubMed] [Google Scholar]
- 20.Jabbour E, Verstovsek S. Treatment of myelofibrosis in younger patients: To transplant or not? American Journal of Hematology. 2009;84:131–132. doi: 10.1002/ajh.21368. [DOI] [PubMed] [Google Scholar]
- 21.Wolff D, Andree H, Hilgendorf I, Casper J, Freund M, Junghanss C. Sirolimus in Combination with Tacrolimus in Allogeneic Stem Cell Transplantation--Timing and Conditioning Regimen May Be Crucial. Biology of Blood and Marrow Transplantation. 2008;14(8):942–943. doi: 10.1016/j.bbmt.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Ciurea SO, Sadegi B, Wilbur A, et al. Effects of extensive splenomegaly in patients with myelofibrosis undergoing a reduced intensity allogeneic stem cell transplantation. Brit J Haematol. 2008;141(1):80–83. doi: 10.1111/j.1365-2141.2008.07010.x. [DOI] [PubMed] [Google Scholar]
- 23.Tefferi A. Essential thrombocythemia,polycythemia vera, and myelofibrosis: Current management and the prospect of targeted therapy. American Journal of Hematology. 2008;83:491–497. doi: 10.1002/ajh.21183. [DOI] [PubMed] [Google Scholar]
- 24.Ditschkowski M, Elmaagacli AH, Trenschel R, Steckel NK, Koldehoff M, Beelen DW. No Influence of V617F Mutation in JAK2 on Outcome after Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) for Myelofibrosis. Biology of Blood and Marrow Transplantation. 2006;12(12):1350–1351. doi: 10.1016/j.bbmt.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Fiorini A, Reddiconto G, Farina G, et al. Eradication of JAK2 V617F mutation after allogeneic transplantation in a patient with myelofibrosis with myeloid metaplasia. Leukemia. 2006;20(12):2198–2199. doi: 10.1038/sj.leu.2404430. [DOI] [PubMed] [Google Scholar]
- 26.Kroger N, Badbaran A, Holler E, et al. Monitoring of the JAK2-V617F mutation by highly sensitive quantitative real-time PCR after allogeneic stem cell transplantation in patients with myelofibrosis. Blood. 2007;109(3):1316–1321. doi: 10.1182/blood-2006-08-039909. [DOI] [PubMed] [Google Scholar]
- 27.Steckel NK, Koldehoff M, Ditschkowski M, Beelen DW, Elmaagacli AH. Use of the activating gene mutation of the tyrosine kinase (VAL617Phe) JAK2 as a minimal residual disease marker in patients with myelofibrosis and myeloid metaplasia after allogeneic stem cell transplantation. Transplantation. 2007;83(11):1518–1520. doi: 10.1097/01.tp.0000263393.65764.f4. [DOI] [PubMed] [Google Scholar]
- 28.Kroger N, Alchalby H, Klyuchnikov E, et al. JAK2-V617F-triggered preemptive and salvage adoptive immunotherapy with donor-lymphocyte infusion in patients with myelofibrosis after allogeneic stem cell transplantation. Blood. 2009;113(8):1866, 186a. doi: 10.1182/blood-2008-11-190975. [DOI] [PubMed] [Google Scholar]
- 29.Benjamini O, Koren-Michowitz M, Amariglio N, Kroger N, Nagler A, Shimoni A. Relapse of postpolycythemia myelofibrosis after allogeneic stem cell transplantation in a polycythemic phase: successful treatment with donor lymphocyte infusion directed by quantitative PCR test for V617F-JAK2 mutation. Leukemia. 2008;22(10):1961–1963. doi: 10.1038/leu.2008.215. [DOI] [PubMed] [Google Scholar]