Abstract
Stress is a complex experience that carries both aversive and motivating properties. Chronic stress causes an increase in the risk of depression, is well known to increase relapse of drug seeking behavior, and can adversely impact health. Several brain systems have been demonstrated to be critical in mediating the negative affect associated with stress, and recent evidence directly links the actions of the endogenous opioid neuropeptide dynorphin in modulating mood and increasing the rewarding effects of abused drugs. These results suggest that activation of the dynorphin/kappa opioid receptor (KOR) system is likely to play a major role in the pro-addictive effects of stress. This review explores the relationship between dynorphin and corticotropin releasing factor (CRF) in the induction of dysphoria, the potentiation of drug seeking, and stress-induced reinstatement. We also provide an overview of the signal transduction events responsible for CRF and dynorphin/KOR-dependent behaviors. Understanding the recent work linking activation of CRF and dynorphin/KOR systems and their specific roles in brain stress systems and behavioral models of addiction provides novel insight to neuropeptide systems that regulate affective state.
Keywords: Dynorphin, Kappa opioid receptor, Corticotropin-Releasing Factor, Corticotropin-releasing hormone, Addiction, Stress, Reward, Anxiety, Dysphoria, Aversion, Cocaine, p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, urocortin, reinstatement, preference, drug abuse, relapse
1.0 Introduction
Continuous exposure to stressful experience is well known to induce despair and escalate the risk of mood disorders and drug abuse (Gold and Chrousos, 2002; Volkow and Li 2004; Koob and Kreek, 2007). Two major families of stress neuropeptides that mediate these processes are the corticotropin-releasing factor (CRF, also called corticotropin-releasing hormone, CRH) peptides and the dynorphins (Bale et al., 2005; Nestler and Carlezon, 2006). The existence of a CRF-like substance in hypothalamus was originally proposed in 1955, and CRF was isolated and sequenced by Vale and colleagues (Vale et al., 1981). Contemporaneously, other groups including de Castiglione also identified CRF-related peptides sauvagine and urotensin alongside CRF in the early 1980s (Erspamer et al., 1980; Rivier et al., 1983). Urocortin was isolated and characterized in the mid 1990’s (Vaughan et al., 1995), and found to have a sequence that was related to CRF peptides. CRF was first thought only to be critical for the regulation of endocrine stress physiology by regulation of the hypothalamic pituitary axis (HPA) and subsequent adrenocorticosteroid release. Later in adrenalectomized animals, however, CRF and related neuropeptides were also demonstrated to maintain homeostasis in response to stressful stimuli in behavioral models suggesting that CRF may have other effects in addition to HPA activation (Velduis and De Wied, 1984), and CRF receptors are broadly distributed in brain (Bale and Vale, 2004).
At around the time that CRF was isolated, the endogenous opioid peptide dynorphin A was discovered by Goldstein and colleagues (Goldstein et al., 1979, 1981). Soon afterwards, other forms of dynorphin (α-neo-endorphin, dynorphin A(1-8), and dynorphin B) were also described (Seizinger et al., 1981; Weber et al., 1981; Cone et al., 1983). The effects of the dynorphins were initially characterized in the peripheral nervous system including the guinea pig ileum longitudinal muscle-myenteric plexus and mouse vas deferens tissue bath preparations where opioid receptors strongly inhibit electrically evoked smooth muscle contraction (Chavkin and Goldstein, 1981; Cox and Chavkin, 1982). Dynorphin was later found to mediate antinociceptive responses (Herman and Goldstein, 1985; Spampinato et al., 1985). Dynorphin has also been demonstrated to inhibit vasopressin release to cause diuresis and reduce blood pressure (Leander 1983; Grossman and Rees, 1983). In the central nervous system an extensive literature also described a role for dynorphin in the hippocampus (for review see Drake et al., 2007). In the hippocampus, dynorphin has been shown to inhibit glutamate release from mossy fiber terminals in the CA3 region and from perforant path afferents to the dentate gyrus and thus block LTP induction (Wagner et al., 1993), and elevation of dynorphin levels in hippocampus may contribute to aging-induced impairment of cognition and spatial learning (Jiang et al., 1989). Hippocampal dynorphin also reduces pilocarpine-induced seizure activity (Bausch et al., 1997), and elevated dynorphin in hippocampus is believed to be involved in human temporal lobe epilepsy (Houser, 1992). Other effects of dynorphin on brain function have been suggested by the actions of Salvinorin A, a highly selective and potent kappa opioid receptor (KOR) agonist found in salvia divinorum sage (used by natives in South America for thousands of years). Salvinorin A is strongly psychotomimetic and produces hallucinations in humans (Roth et al., 2002). In addition, dynorphin A levels are increased in the cerebrospinal fluid of some types of schizophrenic patients, and opioid antagonists reverse hallucinations in schizophrenia (Gunne et al., 1977; Heikkilä et al., 1990). These reports suggest an important role for dynorphin/KOR in cognition and perception.
Thus, dynorphin has been found to regulate neuronal excitability broadly in brain and can affect learning, cognition, seizures, nociception, and endocrine function. Recently, activation of the dynorphin/KOR system has also been shown to be necessary and sufficient for stress-induced behavioral responses in animal models of anxiety, depression, and drug seeking behaviors. This review will highlight the recently defined neurobehavioral interactions between CRF and dynorphin, and discuss recent evidence that links these two systems in the modulation of mood state.
2.0 Overview of the Pharmacology of CRF Receptors and Kappa Opioid Receptors
Since the initial discovery of CRF, a large family of CRF-related ligands and receptors have been discovered and pharmacologically characterized. The mammalian CRF family members include CRF, urocortin 1, urocortin 2, and urocortin 3 (Vale and Bale, 2004). These peptide ligands activate two different receptors in a variety of tissues. Both CRF receptors are class B, G-protein coupled receptors (GPCR) and to date, CRF1-R and CRF2-R receptor genes have been identified, with a near 70% amino acid sequence homology between the two (Dautzenberg and Hauger, 2002). CRF is more selective (about 10 fold) for CRF1 than CRF2 receptors, whereas urocortin 1 is thought to bind both receptors with equal affinity (Perrin et al., 1998). Both urocortin 2 and 3 are selective for the CRF2 over CRF1 receptor subtype (Bale and Vale, 2004). CRF1-R and CRF2-Rs have been demonstrated to activate the stimulatory G-protein, Gs, and to stimulate adenylyl cyclase, although evidence for promiscuous coupling to other G-proteins, including Gq/11 has been suggested in some systems (Dautzenberg et al., 2000; Dautzenberg and Hauger, 2002). More recent data also suggest that CRF action at CRF1 and CRF2 receptors can also initiate signaling through extracellular signal-regulated kinase (ERK 1/2) mitogen-activated protein kinase (MAPK) cascades (Brar et al., 2004).
The dynorphin-kappa opioid receptor (KOR) system has also been well characterized at the pharmacological level. KOR is a GPCR, widely expressed throughout the brain, spinal cord, and peripheral tissues. KOR is selectively activated by the potent endogenous opioid peptide dynorphin (Chavkin et al., 1982), and each of the dynorphin opioids have structural features conferring KOR selectivity (Chavkin and Goldstein, 1981). Many studies have shown that KOR mediates signal transduction via coupling to the inhibitory G-protein, Gi. This results in an inhibition of adenylyl cyclase through the alpha subunit of the G-protein. KOR activation also causes increased potassium channel conductance and decreased calcium conductance via the G-protein beta-gamma subunit (Dhawan et al., 1996). KOR modulation of these ion channels on neurons is inhibitory resulting in decreased cell firing and neurotransmitter release. Contemporary studies have also revealed a role for KOR in the activation of ERK, JNK, and p38 MAPK signal transduction cascades (Bruchas et al., 2006, 2007; Belsheva et al., 1998; Belsheva et al., 2005; McLennan et al., 2008). KOR-dependent activation of the ERK 1/2 signal transduction cascade has been extensively characterized by the Coscia group, who have demonstrated that KOR increases ERK phosphorylation via phosphoinositide 3-kinase, PKCzeta, and Ca2+ mobilization (Belsheva et al., 2005). KOR-dependent ERK activation was later shown by the same group to require the beta-gamma subunit of the G-protein, as well as the signal-scaffold protein arrestin for the prolonged phase of ERK phosphorylation (McLennan et al, 2008). The behavioral implications of KOR-induced ERK 1/2 activation are unknown, although recent work has demonstrated that repeated swim stress causes KOR-induced ERK 1/2 phosphorylation in the nucleus accumbens (Bruchas et al., 2008) suggesting that activation of this pathway may mediate some of the stress-induced behaviors controlled by KOR. KOR-dependent p38 activation has been demonstrated to require receptor phosphorylation by G-protein coupled receptor kinase 3 (GRK3), and arrestin3 recruitment (Bruchas et al., 2006). Furthermore, inhibition of p38 MAPK using the selective p38 inhibitor SB203580 has been shown to block kappa-opioid induced behavioral responses including conditioned place aversion (CPA) and stress-induced immobility (Bruchas et al., 2007). In both CRF and KOR receptor systems, recent biochemical studies using in vivo behavioral models have demonstrated how CRF and KOR activation ultimately leads to changes in behavioral output, and further characterization of the receptor signal transduction pathways and the behavioral consequences will be necessary.
3.0. Kappa Opioid System and Stress-induced Behaviors
Stress-induced opioid peptide release has been reported for all three opioid systems (e.g. β-endorphin, enkephalin and dynorphin), and this release has been demonstrated to be associated with stress-induced analgesic effects in rodent models. Forced swim stress (FSS) in mice has been demonstrated to increase stress-induced analgesic responses in a mu-opioid dependent manner (Rubinstein et al., 1996). In addition, delta-opioid receptor agonists reduce swim-stress induced immobility in the FSS test in rats (Broom et al., 2002). The involvement of dynorphin in stress-induced analgesia has also been demonstrated in both rats and mice (Takahashi et al., 1990, Watkins et al., 1992, McLaughlin et al., 2003). In recent reports exposure to forced swim stress (acutely or subchronically) or social defeat stress in mice resulted in an increase in tail withdrawal latency in the tail flick assay. This effect was blocked by the KOR antagonist norBNI and is absent in dynorphin knockout mice (McLaughlin et al., 2003; McLaughlin et al., 2006; Bruchas et al., 2007a). Furthermore, stress exposure has been shown to increase dynorphin levels (Nabeshima et al., 1992). Together, these reports demonstrate that stress can cause an increase in endogenous opioid peptide release, and dynorphin is one of the key opioid peptides released in this response.
Using an antibody directed at the specific phosphorylation site in KOR that mediates arrestin activation and receptor desensitization (McLaughlin et al., 2004), we were recently able to visualize sites of dynorphin action in the mouse brain following stress exposure, or CRF injection (Bruchas et al., 2007a; Land et al., 2008). This antibody is targeted to an epitope of KOR that is phosphorylated by G-protein coupled receptor kinase 3 at the serine 369 residue (McLaughlin et al., 2003; 2004) following dynorphin release and subsequent receptor binding. Using this approach, one is able to detect the brain structures and cell types where dynorphin acts to modulate the stress response. In two recent studies (Land et al., 2008; Bruchas et al., 2007a), we reported that both CRF and stress increase KORp-ir (phospho-KOR immunoreactivity) in several key mouse brain structures previously demonstrated to be involved in the stress response. The brain structures where stress-induced KORp labeling occurs include the dorsal raphe nucleus, basolateral amygdala, hippocampus, ventral pallidum, ventral tegmental area, nucleus accumbens, and bed nucleus of the stria terminalis (see Land et al., 2008 for summary). CRF and stress-induced KORp-ir in these brain structures is a key step in understanding potential sites of dynorphin action, and allows for discrete cell type and brain structure resolution. Following stress, KOR agonist-, or CRF-induced KORp-ir has been found to be colocalized with GAD positive (GABAergic), glial fibrillary acidic protein (GFAP, astrocytes) positive, tyrosine hydroxylase positive (dopaminergic), and tryptophan hydroxylase positive (serotonergic) cell types, suggesting that KOR has an important role in the modulation of signaling in several neuronal and related cell types. It is important to note, however, that the KORp antibodies cannot separately resolve the actions of the differently processed forms of dynorphin neuropeptides, nor can this receptor phospho-labeling technique be used for fine temporal resolution of dynorphin activity. The increase in immunoreactivity will only occur at sites where KOR and G-Protein Receptor Kinase (GRK3) co-localize and increase in KORp-ir is only evident if the phosphorylation persists prior to tissue fixation. The sensitivity (i.e. how much receptor activation or occupancy is required) and selectivity of antibodies (i.e. how well does the antibody distinguish phosphorylated from non-phosphorylated states of the receptors) should always be considered when interpreting anatomical data, and although KORp staining may not have been visualized, it is still plausible that KOR activation may have occurred in structures that did not show significant increases in KORp immunoreactivity.
In addition to the anatomical and biochemical evidence, the connection between stress-induced dynorphin release and behavioral responses has been investigated. For instance, KOR antagonism, KOR gene knockout, or prodynorphin gene disruption blocks swim-stress induced immobility, a popular rodent model for evaluating the potential efficacy of anti-depressant-like compounds (Newton et al., 2002; Shirayama et al., 2004; McLaughlin et al., 2003; Mague et al., 2003). Furthermore, the over-expression of a cAMP response element binding protein (CREB), which drives prodynorphin gene induction, produced an increase in immobility in the FSS, and this effect was sensitive to KOR antagonism (Pliakas et al., 2001). Social defeat stress, a paradigm developed in both rats and mice by Miczek et al. (2008), has been used to determine a role for the dynorphin/KOR system in defeat behavior following inescapable attacks by an aggressor animal. Mice lacking a functional dynorphin gene, or pretreated with the KOR antagonist norBNI displayed less total time in defeat postures than their control counterparts (McLaughlin et al., 2006). This report implicates the dynorphin/KOR system in both the psychological and physical properties of the stress response.
Recent work directly examined the dynorphin/KOR stress experience in mice using cue associations (Land et al., 2008). These experiments were designed to quantify the motivational effects of stress and the role of KOR in modulating those associated behaviors. Mice trained to associate an odorant cue with forced swim stress, later show avoidance to that odorant when presented in the distal arm of a T-maze in a following trial. This avoidance behavior was blocked by dynorphin gene deletion or KOR antagonism (norBNI pretreatment). In addition, footshock stress in a shuttle box assay was measured. Like the odorant aversion paradigm, animals made strong associations with the context where they were shocked and avoided that context later. Mice pretreated with norBNI, or lacking dynorphin, however appeared to be insensitive to the aversive effects of these stressors and spend equal time on shock and non-shock sides of the box. Together, these data suggest that dynorphin/KOR activity has a principal role in the aversive stress experience. (See Table 1 for a summary of current literature).
Table 1. Studies Reporting Stress-ralated Behaviors mediated by the Dynorphin-Kappa Opioid Receptors System.
| Stimulus | Behavior | Species | Reference |
|---|---|---|---|
| Stress | Swim-stress immobility | Mouse/Rat | Pliakas et al., 2001; McLauglin et al., 2003; Shiriyama et al., 2004; Mague et al. 2003 |
| Social Defeat Stress | Mouse | McLaughlin et al., 2006 | |
| Potentiation of Cocaine CPP | Mouse | McLaughlin et al., 2003; McLaughlin et al., 2006 | |
| Stress-induced Reinstatement | Mouse/Rat | Beardsley et al., 2005; Redila and Chavkin, 2008; Carey et al., 2007 | |
| Monkey | Valdez et al., 2007 | ||
| Novel Object Recognition | Mouse | Carey et al., 2009 | |
| Stress-induced Avoidance | Mouse | Land et al., 2008 | |
| KOR | Conditioned Place Aversion | Mouse/Rat | Bals-Kubik et al. 1993; Land et al., 2008; Contarino and Papeleo et al., 2005; Kim et al., 2004; Shippenberg and Herz 1986; Bruchas et al., 2007 |
| Potentiation of Cocaine CPP | Mouse | McLaughlin et al., 2003; McLaughlin et al., 2006 | |
| Intracranial Self Stimulation | Rat | Tomasiewicz et al., 2008 | |
| Anxiety-like behavior | Mouse/Rat | Knoll et al., 2007; Wittman et al., 2009 | |
| Reinstatement to Drug Seeking | Mouse/Monkey | Redila and Chavkin, 2008; Valdez et al., 2007 | |
| Locomotor Activity | Rat | Heidbreder et al., 1993 | |
| CRF-R | Conditioned Place Aversion | Mouse | Land et al., 2008 |
| Anxiety-like Behavior | Mouse | Wittman et al., 2009, Beliki-Gorzo et al., 2008 |
4.0 Dynorphin/KOR and Drug Seeking
Building on this prior work and early evidence that implicated a role for the KOR in producing dysphoric responses in humans (Pfeiffer et al., 1986), studies using neurochemical and electrophysiological methods demonstrated that KOR activation in mesolimbic structures including the ventral tegmental area and nucleus accumbens decreased dopamine transmission (Werling et al, 1998; Margolis et al., 2003). Co-administration of KOR agonists with cocaine also inhibited the induction of cocaine conditioned place preference (Shippenberg et al., 1996; Shippenberg et al., 2007) in rat models. Using microdialysis, these reports were fundamental in establishing the concept that KOR can modulate monoaminergic systems and allowed for quantification of KOR-mediated suppression of dopamine release. Given that dopamine is thought to be a key neurotransmitter that regulates reward and modulates drug seeking (Wise, 2008), these reports led to the hypothesis that the dynorphin/KOR system might play a role in drug abuse behaviors.
Early theories in establishing a role for the dynorphin/KOR system in drug abuse behavior centered around the idea that because administration of KOR agonists immediately before conditioning decreased cocaine reward and dynorphin mRNA was increased following repeated cocaine injection; it was proposed that the dynorphin/KOR system must therefore act as a “brake” to oppose reward processing. In contrast, our group has presented evidence for a different model of dynorphin/KOR role in drug abuse behavior in which stress induced release of dynorphin mediates the increased rewarding effects of cocaine (McLaughlin et al., 2003; Redila and Chavkin, 2008). In the process of investigating a role for the dynorphin/KOR system in stress-responses, McLaughlin et al (2003) determined that prior activation of KOR via agonist (60 minutes before cocaine conditioning) or via stress-induced dynorphin release caused a potentiation (doubling) of cocaine conditioned place preference score in the stressed group, that was blocked by KOR antagonism or dynorphin gene disruption (McLaughlin et al., 2003). These results initially seemed to support the ‘brake’ hypothesis whereby stress induces dynorphin release subsequently causing KOR phosphorylation, receptor desensitization and internalization of KOR. According to this concept, the inactivation of KOR by repeated exposure to endogenous dynorphin action would remove the ‘brake’ caused by dynorphin, and the absence of dynorphin action would increase the rewarding effects of cocaine. Although plausible, further experiments gave results that were inconsistent with this concept; specifically, neither KOR antagonism nor dynorphin gene disruption alone potentiate cocaine conditioned place preference (McLaughlin et al., 2003). KOR inactivation would be expected to produce the same release of the ‘brake’ as KOR desensitization. This data suggests that the removal of the brake is unlikely to be the primary mechanism mediating potentiation of cocaine reward. The alternative explanation of these data is that prior stress exposure causes a dysphoric state encoded by dynorphin release and subsequent KOR activation and that the resulting dysphoria enhances the rewarding valence of cocaine (Figure 1, and Section 7.0). Furthermore, the time dependence of KOR activation on different signaling networks to alter behavior should be noted along with potential differences between cocaine, other psychostimulants, and opiates on the kinetic parameters effecting CPP potentiation. It will be important to parallel the cocaine CPP studies with other drugs including nicotine, amphetamine, and morphine since it has been reported that stress effects on CPP to opiates and psychostimulants can vary (Der-Avakian et al., 2007). However, in modulating pro-addictive behavior dynorphin/KOR has also recently been implicated in dependence-induced ethanol self administration as well as acquisition of nicotine self-administration in both rats and mice (Walker and Koob, 2008; Galeote et al., 2009) suggesting a conserved role for KOR in other drug abuse related behaviors. Further study with other drugs of abuse besides cocaine will be critical in our understanding of how KOR activation mediates behavioral effects.
Figure 1. Theoretical Framework of Activation of Dynorphin/KOR mediated Hedonic Processes before and after stress.
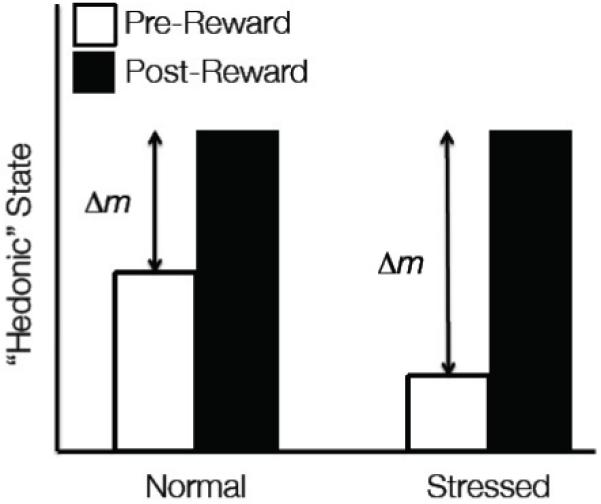
The hedonic response (mood state) in a normal animal carries a total potential reward value Δm (for change in mood) after rewarding drug. The stressed animal has a larger Δm because the activation of dynorphin/KOR causes an increase in dysphoria, shifting the moods state negatively and thus increasing the amplitude of Δm. The reward (drug of abuse) now has as a larger potential positive valence so that the animal experiences more rewarding effects of the drug.
This role of dynorphin/KOR in drug abuse behavior was further expanded to investigate stress-induced reinstatement to drug seeking. Using three separate animal behavioral models (mice, rats, and squirrel monkeys), it has been found that stress-induced dynorphin release and direct stimulation of kappa-receptors with selective agonist can cause a dynorphin/KOR-dependent reinstatement of extinguished cocaine CPP or drug self-administration (Beardsley et al., 2005; Redila and Chavkin, 2008, Valdez et al., 2007, Carey et al., 2007). Of note are the findings that KOR antagonism and dynorphin gene disruption only block stress-induced reinstatement but not cocaine prime-induced reinstatement, corroborating the critical role of the dynorphin/KOR system in the stress response. While these studies are compelling, more work on KOR in rat reinstatement paradigms is needed as in one study (Valdez et al., 2007) KOR-agonists caused reinstatement yet these effects were not blocked by the selective KOR antagonist norBNI. The reasons for this are unclear but may be due to the pharmacology of the KOR agonists used (enadoline and spiradoline) and the collateral agonist-like long-lasting pharmacology of KOR antagonists (Bruchas et al., 2007b). Additionally, norBNI seems to have increased priming induced reinstatement in one report (Beardsley et al., 2005) however this norBNI effect on prime-induced reinstatement was not observed by our group (Redila and Chavkin, 2008).
Consistent with the concept that dynorphin/KOR modulate pro-addictive behaviors is recent data showing dynorphin/KOR-dependent maintains drug seeking in cocaine and ethanol dependence. These studies found that blockade of dynorphin/KOR receptors selectively decreases ethanol and cocaine dependence (Walker and Koob, 2008; Carlezon et al., 1998). Together these reports reveal a critical role for dynorphin/KOR activity in stress-induced reinstatement and acute withdrawal symptoms (Summarized in Table 1).
5.0. CRF and Drug Seeking
One of the primary mechanisms for relapse to drug seeking in humans is stress exposure (Kreek et al., 2009). In addition, the neuronal systems involved in stress-regulation undergo changes and alterations in response to the presence of the drug of abuse, which can later affect the persistence to drug taking by the individual (Koob and LeMoal, 2008). For instance, the CRF-regulated hypothalamic-pituitary axis and the central brain stress circuits lose equilibrium following chronic drug exposure. Due to these factors, several animal models have investigated the role of the CRF system in regulating stress-induced reinstatement and withdrawal behaviors (for a complete review see Shalev et al., 2009; Papaleo et al., 2007). Acute withdrawal releases CRF, adrenocorticotropic hormone (ACTH), and corticosterone for all most major classes of abused drugs (Kreek and Koob, 1998). In ethanol-dependent rats CRF receptor antagonists block both drug and alcohol self-administration in substance-dependent animals (Funk et al., 2006; Greenwell et al., 2007) supporting a role for CRF systems in drug dependence.
CRF has also been implicated in stress-induced relapse to drug taking. It is believed that stress/CRF may modulate a state change distinguished by anxiety-like and dysphoria-like negative mood states. (Koob, 2008; Shaham et al., 2000). In animal models of drug abuse, CRF and adrenergic mechanisms have been shown to mediate stress-induced relapse (Erb et al., 2001; Erb and Stewart, 1998; Le et al., 2000). The CRF antagonist D-Phe-CRF, infused locally into the bed nucleus of the stria terminalis (BNST) is able to reduce footshock-induced but not cocaine-induced reinstatement (Erb et al., 2001). Local infusion of CRF into the ventral tegmental area causes reinstatement (Wang et al., 2005a), More specifically, CRF1-R activity has been suggested to be involved in reinstatement to drug-seeking behavior and aversion-induced by acute withdrawal (Shaham et al., 1998; Contarino and Papaleo, 2005). Recent circuit analysis using slice electrophysiology has revealed that the pharmacological role of CRF1-R and CRF2-R in excitatory synaptic transmission is altered following cocaine exposure, further emphasizing a dynamic process whereby drug taking can alter stress/CRF physiology (Liu et al., 2005). In total, these reports define a clear role of CRF in drug seeking behavior, and have led our group and others (Koob et al., 2008) to hypothesize that the similarities between CRF and dynorphin/KOR in these models suggests a plausible direct connection between the two systems.
6.0. The CRF-Dynorphin/KOR Connection
Early reports established a potential serial relationship of CRF-induced dynorphin release between the two systems, in vivo (Nikolarakis et al., 1986; Song and Takemori, 1992). Using spinal cord preparations coupled with radioimmunoassay these two reports were the first to show that CRF induces dynorphin release. Recent evidence has demonstrated that dynorphin/KOR signaling occurs downstream of CRF release because the KOR-antagonist norBNI blocks CRF-induced conditioned place aversion, and CRF-induced anxiety-like behaviors (Land et al., 2008; Bruchas et al., in review). Land et al. (2008) also reported that blockade of CRF2-Rs, with the selective CRF2-R antagonist, antisauvagine-30 (ASV-30) does not prevent kappa-agonist-induced conditioned place aversion. Furthermore, Contarino and Papaleo (2005) demonstrated that in CRF1-R (-/-) animals, conditioned place aversion to KOR agonist is still intact. Whereas urocortin 3, the selective CRF2-R agonist, induced CPA that was blocked by pretreatment with norBNI (Land et al., 2008), and CRF2-R knockout animals do not show CRF-induced CPA (unpublished observations). In another report, CRF1-R-dependent anxiety-like behavior was determined to be sensitive to norBNI, and KORp-ir in the amygdala was evaluated via a CRF1-R-dependent mechanism following CRF injection. Together these reports have cemented the relationship between both the CRF1 and CRF2-receptor systems and the dynorphin/KOR systems in the modulation of anxiety-like and dysphoria-like behavioral events. Additionally, these data support the unidirectional nature of CRF-to-dynorphin/KOR activation in the brain and argue that stress stimulates CRF/Urocortin 3, which then functions to increase dynorphin release and subsequent activation of the kappa-opioid system in specific stress circuits.
Also worthy of consideration is the possibility that dynorphin/KOR initiates CRF release which then mediates stress-induced behavioral responses. Recent work has shown that KOR agonist-induced reinstatement to cocaine seeking in primates is blocked by selective CRF1-R antagonists (Valdez et al., 2007). This report is interesting given that it suggests an opposing directional nature of the CRF-KOR connection, distinct from those in the early work by Song and Takemori (1992), and recent work by Land et al. (2008). The reasons for this discrepancy are unclear, but could be due to several factors including the concept of a KOR-to-CRF pathway in the Valdez study being attributed to the inherent changes induced by cocaine exposure. As previously mentioned (Liu et al., 2005), cocaine is well known to alter stress neurocircuitry in a number of model systems.
Since CRF and KOR induce HPA activation it is also important to consider the role of glucocorticoids in dynorphin/KOR-mediated behavior (Iyengar et al. 1986; Howell and Muglia, 2006). Corticosterone has been implicated in stress-induced increases in drug self administration (Piazza et al. 1990; Shalev et al., 2003) but other studies find that corticosterone is not involved or indirectly plays a part in these behaviors (Shaham et al., 1997; Erb et al. 1998). Some data suggest that Dynorphin/KOR activity may be linked to corticosterone levels because they found that corticosterone increases at a faster rate following stress in mice lacking dynorphin (Belikei-Gorzo et al., 2008), yet this group found no differences in absolute corticosterone levels following stress or non-stressed conditions between wild type and dynorphin knockout animals. Furthermore, stress-induced corticosterone levels were not sensitive to dynorphin gene disruption or KOR antagonism (McLaughlin et al., 2006) in another report. In behavioral assays corticosterone itself does not induce a conditioned place aversion (Dietz et al., 2007; Cador et al., 1992), supporting the idea that stress-activated neuroendocrine systems act in different ways in the central and peripheral nervous system. Corticosterone itself is well known to mediate central effects of stress (Howell and Muglia, 2006), however it is unlikely to be mediating the aversive properties of the stress. Further study to determine if CRF and KOR can regulate one another in a feedback loop reminiscent of many other neuroendocrine peptide systems would be useful to resolve these ideas.
The behavioral reports describing interaction between CRF and dynorphin neuropeptide circuits are integral in our understanding of how the stress response is coded. The implication that the primary stress neuropeptide CRF causes dynorphin release to fine-tune mood-like states in the behaving animal is of particular interest to a number of lines of research, including drug addiction and depression-related research fields.
7.0 Dynorphin/KOR and the Allostatic Model of Addiction
One of the leading views in understanding the conceptual framework for how the mammalian brain becomes addicted includes the notion that the brain strives to maintain equilibrium (Koob and LeMoal, 2008). This concept is defined as allostasis, or stability through reorganization (McEwen, 1998). Allostatic processes are believed to be responsible for altering homeostatic processes via changing baseline conditions to such levels that a disease pathology arises. Examples of this include the development of drug craving in response to abstinence from a rewarding drug of abuse. Over time the individual develops a compensatory transformation in their valence for a rewarding drug. Due to this alteration the individual no longer seeks drug for its intrinsic rewarding properties, but rather for the goal of reaching a new baseline hedonic value. (See Figure 1 for summary schematic). This continued pursuit of drug becomes the key driving force or motivation to maintain drug seeking behavior over a period of time.
One key player in this allostatic process following drug exposure is the dynorphin/KOR system. Drugs of abuse have been demonstrated to activate the transcription factor cAMP-response element binding protein (CREB); which several studies have linked to anhedonia-like effects, increased immobility in forced swim stress tests (a model for antidepressant-like activity) and aversive responses (Carlezon et al., 2005). These effects of CREB have been associated with the modulatory increases in dynorphin and subsequent dysphoria-like behaviors due to KOR activation (Carlezon et al., 2005). Furthermore, recent work has also demonstrated that stress-induced dynorphin/KOR-dependent p38 mitogen-activated protein kinase activity is necessary for swim-stress immobility and conditioned place aversion (Bruchas et al., 2007). Stress and drugs of abuse cross-modulate dynorphin-dependent molecular pathways, indicating that stress-induced dynorphin release and KOR activation represent a major class of neuropeptide systems involved in the allostatic remodeling that occurs in depression and substance abuse.
Stress-induced excitation of the mesolimbic reward circuit through CRF activity increases the potential for relapse to drug seeking. In addition, dynorphin/KOR signaling is required for stress-induced reinstatement (Beardsley et al., 2005; Redila and Chavkin, 2008, Valdez et al., 2007, Carey et al., 2007). Together the CRF and KOR-dependent reinstatement to drug seeking suggests that these systems work in concert to modulate the valence associated with a drug of abuse or rewarding substance, because activation of these systems compels the animal to restart lever pressing for drug or motivates an animal to spend more time in a drug paired environment as measured by place preference (Beardsley et al., 2005; Redila and Chavkin, 2008; Carey et al., 2007). Additional evidence for an increase in negative affective state (via stress, CRF, and dynorphin/KOR activation) increasing the positive valence of rewarding drugs stems from work showing that stress potentiates cocaine conditioned place preference in a dynorphin/KOR-dependent manner (McLaughlin et al., 2003, 2006a, 2006b). In these experiments it was demonstrated that animals pretreated with norBNI, or lacking dynorphin still show normal conditioned responses to cocaine through place preference. However, stressed animals or animals pretreated with KOR agonists prior to the conditioning session double (potentiate) their place preference score in a dynorphin/KOR-dependent fashion. These results coupled with data showing that stimulation of KOR through stress or injection with a selective agonist can cause reinstatement to drug seeking suggest that the stress experience changes the potential rewarding value of a previously associated rewarding drug. The mechanism for this is likely due to the animal having a greater total negative valence to overcome due to the aversive qualities of the stress experience (Figure 1). Dynorphin as a mediator of dysphoria-like behavior implicates activity of this system as one of the primary mediators of the anti-reward system following drug withdrawal, drug craving, and relapse to drug seeking (See Figure 1 for conceptualization). It is important to note, however, that dynorphin/KOR activation may also be involved in the differential temporal aspects of reward behavior to drugs of abuse. For instance, in a recent study the direct effects of KOR manipulation of reward were investigated using the intracranial self-stimulation (ICSS) approach. They found that U69,593, the selective KOR agonist blocked the facilitating properties of cocaine on ICSS threshold responses at the 15 minute time point (Tomasiewwicz et al. 2008) in conjunction to the 15 minute prior injection of U50, 488 causing a block of CPP in other studies (McLaughlin et al., 2006b); however at the 60 minute time point following U50,488 injection the animals show potentiation of cocaine place preference. Since place preference paradigms require an associative learning component that could be modified by stress it will be important to distinguish KOR-dependent short-term and long term (chronic) behavioral responses in future investigations. Together, these studies suggest that understanding the mechanisms, brain structures, and cell types by which dynorphin/KOR mediates dysphoria-like behaviors may provide new insights and understanding of drug addiction.
8.0. Future Investigations Arising
The mounting evidence demonstrating that stress induces dynorphin release and KOR activation, as a consequence of activation by the CRF system is only the beginning of a long series of investigations required to dissect the mechanisms governing these effects. In the Bruchas et al. (2007a) report and Land et al., (2008) study, it was demonstrated that stress and CRF cause KOR activation in several key brain structures associated with reward, stress, and pathologies such as addiction and depression. The process by which KOR acts in these structures to modulate affect is a critical question. KOR action in the mesolimbic dopamine circuit is well characterized, however other monoamines and neuropeptide systems are also likely to be involved in KOR-mediated behaviors. This includes a circuit analysis to identify key sites of dynorphin release and KOR activation in the brain and the cell types where KOR mediates its dysphoria-like behavioral effects. Brain areas of obvious interest include the central sites of dopamine action, but are not limited to the ventral tegmental area, nucleus accumbens, and frontal cortical structures. However, it is important to consider alternative brain areas that we identified using the KORp-ir anatomical approach regarding the KOR system. Areas of particular interest include the basolateral amygdala, hypothalamus, locus coeruleus, and dorsal raphe nucleus.
The reasons for examining these other systems and brain structures as they related to stress-induced behaviors comes from the anatomical and physiological evidence for a function of KOR outside the context of the mesolimbic dopamine circuit. For example, dynorphin and hypocretin are colocalized in the hypothalamus (Chou et al., 2001). In the hypothalamus dynorphin has been demonstrated to inhibit postsynatic hypocretin/orexin neurons in the hypothalamus via hyperpolarization and increased G-protein gated inwardly rectifying K+ channel current (Li and van den Pol 2006). Like the dynorphin/KOR system the hypocretin/orexin system has also been implicated in stress-induced reinstatement of drug-seeking (Aston-Jones et al., 2009) suggesting a potential interplay between these two systems in the mediation of stress-induced behaviors. Further analysis of these two neuropeptides in concert is required in order to better understand the behavioral consequences of this dynorphin-mediated hyopcretin regulatory activity. Very recent anatomical and physiological evidence has shown that dynorphin/KOR activity at noradrenergic sites, acting presynaptically in the locus coeruleus (LC), may inhibit afferent signaling to the LC (Kreibich et al., 2008; Reyes et al. 2009). Future studies examining the behavioral mechanisms for these physiological effects in the LC is needed. Furthermore, KOR is expressed in the dorsal raphe (Land et al. 2008) and has been shown to modulate serotonin systems by reducing dorsal raphe and nucleus accumbens serotonin (5-HT) efflux (Werling et al., 1989; Tao and Auerbach, 2002; Tao and Auerbach, 2005; Tao et al., 2007). The consequences of KOR modulation of norepinephrine and 5-HT is unknown and behavioral data linking these two systems is limited.
Additional investigations of the molecular and physiological properties of stress-induced KOR activation will require biochemical analysis of the downstream signal transduction cascades and electrophysiology to investigate the biophysical properties of KOR activation following stress. It is well established that opioid receptors can modulate potassium and calcium channels via activation of the beta/gamma subunit of the G-protein; however in a temporal sense only recent studies have examined the long term effects of KOR activation on signal transduction. Some of this work has described an arrestin-dependent p38 MAPK activation that is required for KOR-mediated aversive behavior (Bruchas et al., 2007), although further study is needed to understand the substrate of this KOR signaling partner.
Recent evidence in GPCR drug discovery and specifically opioid receptor pharmacology suggest that receptor-selective agonists can vary in their ability to induce MAPK activation (Bruchas et al., 2006; 2007b) or cause internalization and down regulation (Wang et al., 2005b). Taking advantage of these findings may provide conformation-selective KOR partial agonists that produce analgesic effects by stimulation of KOR, but that do not cause dysphoria, do not activate p38, and do not result in receptor internalization. In addition, the findings that implicate KOR in depression/dysphoria-like behavioral responses along with drug addiction intimate the need for the further development of KOR antagonist compounds for the potential alleviation of mood-related disorders. In human studies, KOR antagonists have been demonstrated to be effective in the treatment of opioid dependence (Rothman et al., 2000).
The role of CREB in modulating dynorphin is also being widely pursued by several groups. Recent work has determined that CREB regulates stress-induced reinstatement, depression, and sensitivity to reward and aversion (Kreibich and Blendy, 2004; Blendy 2006; Dinieri et al., 2009), as well as the relationship of dopamine to KOR activation. Building on the additional evidence showing that KOR is linked to the modulation of other monoaminergic systems including serotonin and norepinephrine (Tao and Auerbach, 2002; Werling et al., 1989; Kreibich et al., 2008; Reyes et al., 2009) will also provide new insight into how KOR regulates mood state. In any case, the dynorphin/KOR system is widely expressed in the mammalian brain on numerous cell types and in several brain structures many of which have yet to be investigated at the molecular and systems levels. Dynorphin has been a noted as one of the most highly conserved neuropeptides throughout primate evolution (Rockman et al., 2005) so it is clear that more investigation and study of the dynorphin/KOR system is warranted.
9.0. Summary
A chief tenant of addiction is the development of negative emotional responses during drug abstinence. The neuropharmacology underlying these processes is only now being evaluated, but is believed to be due to a reduction in reward circuits and an increase in anti-reward systems including dynorphin/KOR. Two major neuropeptide systems that mediate these effects are CRF and dynorphin. Recent work has demonstrated a direct causal connection between stress-induced CRF release initiating dynorphin release and KOR activation, therefore implicating these two systems as critical mediators of the stress-induced relapse to drug seeking. The vulnerability associated with addiction, and reinstatement to drug seeking is likely to involve dynorphin/KOR regulation of anti-reward processes. The culmination of this work suggests that blockade of KOR receptors with selective antagonists may be a useful and powerful therapeutic treatment for protecting individuals from depression, and relapse to drug abuse.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Classification Terms: Neuropeptides, Special Issue
References
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56:112–21. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005;48:1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–95. [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcheva MM, Vogel Z, Ignatova E, Avidor-Reiss T, Zippel R, Levy R, Young EC, Barg J, Coscia CJ. Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gbetagamma subunits. J Neurochem. 1998;70:635–45. doi: 10.1046/j.1471-4159.1998.70020635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmüller D, Zimmer A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33:425–36. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology. 2004;145:1718–29. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26:744–55. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007a;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007b;282:29803–298.11. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Ahmed SH, Koob GF, Le Moal M, Stinus L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992;597:304–9. doi: 10.1016/0006-8993(92)91487-y. [DOI] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RI, Weber E, Barchas JD, Goldstein A. Regional distribution of dynorphin and neo-endorphin peptides in rat brain, spinal cord, and pituitary. J Neurosci. 1983;11:2146–2152. doi: 10.1523/JNEUROSCI.03-11-02146.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci U S A. 2005;102:18649–54. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–7. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Higelin J, Teichert U. Functional characterization of corticotropin-releasing factor type 1 receptor endogenously expressed in human embryonic kidney 293 cells. Eur J Pharmacol. 2000;390:51–59. doi: 10.1016/s0014-2999(99)00915-2. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Rozeske RR, Bland ST, Watkins LR, Maier SF. The effects of a single session of inescapable tailshock on the subsequent locomotor response to brief footshock and cocaine administration in rats. Psychopharmacology (Berl) 2007;191:899–907. doi: 10.1007/s00213-006-0677-8. [DOI] [PubMed] [Google Scholar]
- Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–92. [PubMed] [Google Scholar]
- Dietz D, Wang H, Kabbaj M. Corticosterone fails to produce conditioned place preference or conditioned place aversion in rats. Behav Brain Res. 2007;181:287–91. doi: 10.1016/j.bbr.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinieri JA, Nemeth C,L, Parsegian A, Carle T, Gurevich VV, Gurevich E, Neve RL, Nestler EJ, Carlezon WA., Jr. Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. J Neurosci. 2009;29:1855–9. doi: 10.1523/JNEUROSCI.5104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–5. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–36. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeote L, Berrendero F, Bura SA, Zimmer A, Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int J Neuropsychopharmacol. 2009;12:615–25. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci U S A. 1979;76:6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–43. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A, Rees LH. The neuroendocrinology of opioid peptides. Br Med Bull. 1983;39:83–88. doi: 10.1093/oxfordjournals.bmb.a071796. [DOI] [PubMed] [Google Scholar]
- Gunne LM, Lindström L, Terenius L. Naloxone-induced reversal of schizophrenic hallucinations. J Neural Transm. 1977;40:13–19. doi: 10.1007/BF01250276. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–8. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- Heikkilä L, Rimón R, Terenius L. Dynorphin A and Substance P in the cerebrospinal fluid of schizophrenic patients. Psychiatry Res. 1990;34:229–236. doi: 10.1016/0165-1781(90)90001-l. [DOI] [PubMed] [Google Scholar]
- Herman BH, Goldstein A. Antinociception and paralysis induced by intrathecal dynorphin A. J Pharmacol Exp Ther. 1985;232:27–32. [PubMed] [Google Scholar]
- Houser CR. Morphological changes in the dentate gyrus in human temporal lobe epilepsy. Epilepsy Res Suppl. 1992;7:223–234. [PubMed] [Google Scholar]
- Howell MP, Muglia LJ. Effects of genetically altered brain glucocorticoid receptor action on behavior and adrenal axis regulation in mice. Front Neuroendocrinol. 2006;27:275–84. doi: 10.1016/j.yfrne.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Kim HS, Wood PL. Kappa opiate agonists modulate the hypothalamic-pituitary-adrenocortical axis in the rat. J Pharmacol Exp Ther. 1986;238:429–36. [PubMed] [Google Scholar]
- Jiang HK, Owyang VV, Hong JS, Gallagher M. Elevated dynorphin in the hippocampal formation of aged rats: relation to cognitive impairment on a spatial learning task. Proc Natl Acad Sci USA. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Pollak KA, Hjelmstad GO, Fields HL. A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. Proc Natl Acad Sci U S A. 2004;101:5664–5669. doi: 10.1073/pnas.0401373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr. Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–45. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Schlussman SD, Reed B, Zhang Y, Nielsen DA, Levran O, Zhou Y, Butelman ER. Bidirectional translation research: Progress in understanding addictive diseases. Neuropharmacolgy. 2009;56:32–43. doi: 10.1016/j.neuropharm.2008.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP reponse element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ, Valentino RJ. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–24. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Leander JD. Further study of kappa opioids on increased urination. J Pharmaol Exp Ther. 1983;227:35–41. [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577–83. doi: 10.1523/JNEUROSCI.4196-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr. Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–94. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006a;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006b;31:787–94. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–83. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Myers LC, Zarek PE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Chavkin C. Prolonged kappa opioid receptor phosphorylation mediated by G-protein receptor kinase underlies sustained analgesic tolerance. J Biol Chem. 2004;279:1810–1818. doi: 10.1074/jbc.M305796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Xu M, Mackie K, Chavkin C. Phosphorylation of a carboxyl-terminal serine within the kappa-opioid receptor produces desensitization and internalization. J Biol Chem. 2003;278:34631–40. doi: 10.1074/jbc.M304022200. [DOI] [PubMed] [Google Scholar]
- McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J Neurochem. 2008;107:1753–1765. doi: 10.1111/j.1471-4159.2008.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–28. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima T, Katoh A, Wada M, Kameyama T. Stress-induced changes in brain Met-enkephalin, Leu-enkephalin and dynorphin concentrations. Life Sci. 1992;51:211–217. doi: 10.1016/0024-3205(92)90077-3. [DOI] [PubMed] [Google Scholar]
- Nestler E,J, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic beta-endorphin and dynorphin release by corticotropin-releasing factor (in vitro) Brain Res. 1986;399:152–5. doi: 10.1016/0006-8993(86)90610-4. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Kitchener P, Contarino A. Disruption of the CRF/CRF1 receptor stress system exacerbates the somatic signs of opiate withdrawal. Neuron. 2007;53:577–89. doi: 10.1016/j.neuron.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Sutton S, Bain DL, Berggren WT, Vale WW. The first extracellular domain of corticotropin releasing factor-R1 contains major binding determinants for urocortin and astressin. Endocrinology. 1998;139:566–570. doi: 10.1210/endo.139.2.5757. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminière JM, Maccari S, Mormède P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7400. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Chavkin C, van Bockstaele EJ. Subcellular targeting of kappa-opioid receptors in the rat nucleus locus coeruleus. J Comp Neurol. 2009;512:419–431. doi: 10.1002/cne.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C, Rivier J, Lederis K, Vale W. In vitro and in vivo ACTH-releasing activity of ovine CRF, sauvagine and urotensin 1. Regul Pept. 1983;5:139–43. doi: 10.1016/0167-0115(83)90121-0. [DOI] [PubMed] [Google Scholar]
- Rockman MV, Hahn MW, Soranzo N, Zimprich F, Goldstein DB, Wray GA. Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS Biol. 2005;3:e387. doi: 10.1371/journal.pbio.0030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99:11934–9. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Gorelick DA, Heishman SJ, Eichmiller PR, Hill BH, Norbeck J, Liberto JG. An open-label study of a functional opioid kappa antagonist in the treatment of opioid dependence. J Subst Abuse Treat. 2000;18:277–281. doi: 10.1016/s0740-5472(99)00074-4. [DOI] [PubMed] [Google Scholar]
- Rubinstein M, Mogil JS, Japón M, Chan EC, Allen RG, Low MJ. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1996;93:3995–4000. doi: 10.1073/pnas.93.9.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizinger BR, Hollt V, Herz A. Evidence for the occurrence of the opioid octapeptide dynorphin-(1-8) in the neurointermediate pituitary of rats. Biochem Biophys Res Common. 1981;102:197–205. doi: 10.1016/0006-291x(81)91507-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–90. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2009 doi: 10.1016/j.brainres.2009.07.028. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2003;168:170–6. doi: 10.1007/s00213-002-1200-5. 2003. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–66. [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Heidbreder C. Kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–54. [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–21. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–68. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Song ZH, Takemori AE. Stimulation by corticotropin-releasing factor of the release of immunoreactive dynorphin A from mouse spinal cords in vitro. Eur J Pharmacol. 1992;222:27–32. doi: 10.1016/0014-2999(92)90458-g. [DOI] [PubMed] [Google Scholar]
- Spampinato S, Candeletti S. Characterization of dynorphin A-induced antinociception at spinal level. Eur J Pharmacol. 1985;110:21–30. doi: 10.1016/0014-2999(85)90024-x. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Shiosaka S, Traub RJ, Ruda MA. Ultrastructural demonstration of synaptic connections between calcitonin gene-related peptide immunoreactive axons and dynorphin A(1-8) immunoreactive dorsal horn neurons in a rat model of peripheral inflammation and hyperalgesia. Peptides. 1990;11:1233–7. doi: 10.1016/0196-9781(90)90157-z. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:549–56. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. mu-Opioids disinhibit and kappa-opioids inhibit serotonin efflux in the dorsal raphe nucleus. Brain Res. 2005;1049:70–79. doi: 10.1016/j.brainres.2005.04.076. [DOI] [PubMed] [Google Scholar]
- Tao R, Ma Z, Thakkar MM, McCarley RW, Auerbach SB. Nociceptin/orphanin FQ decreases serotonin efflux in the rat brain but in contrast to a kappa-opioid has no antagonistic effect on mu-opioid-induced increases in serotonin efflux. Neuroscience. 2007;147:106–116. doi: 10.1016/j.neuroscience.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr. The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Rüedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–33. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin M,H, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Veldhuis HD, De Wied D. Differential behavioral actions of corticotropin-releasing factor (CRF) Pharmacol Biochem Behav. 1984;21:707–13. doi: 10.1016/s0091-3057(84)80007-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005a;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005b;312:220–30. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Maier SF. Kappa opiate receptors mediate tail-shock induced antinociception at spinal levels. Brain Res. 1992;582:1–9. doi: 10.1016/0006-8993(92)90310-6. [DOI] [PubMed] [Google Scholar]
- Weber E, Roth KA, Barchas JD. Colocalization of alpha-neo-dynorphin and dynorphin immunoreactivity in hypothalamic neurons. Biochem Biophys Res Commun. 1981;103:951–958. doi: 10.1016/0006-291x(81)90902-5. [DOI] [PubMed] [Google Scholar]
- Werling LL, Frattali A, Portoghese PS, Takemori AE, Cox BM. Kappa receptor regulation of dopamine release from striatum and cortex of rats and guinea pigs. J Pharmacol Exp Ther. 1998;246:282–286. [PubMed] [Google Scholar]
- Werling LL, McMahon PN, Portoghese PS, Takemori AE, Cox BM. Selective opioid antagonist effects on opioid-induced inhibition of release of norepinephrine in guinea pig cortex. Neuropharmacology. 1989;28:103–7. doi: 10.1016/0028-3908(89)90044-0. [DOI] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–85. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–20. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]


