Abstract
Background
Ewing sarcoma (ES) is a malignant tumor of bone or soft tissue. One of the few risk factors for developing ES is race, with a higher incidence in populations of European rather than African or Asian ancestry. The goal of this study was to evaluate racial and ethnic differences in presentation and overall survival (OS) among patients diagnosed with ES before age 40.
Methods
Data from the SEER database identified1715 patients with ES <40 years of age from 1973–2005. Racial and ethnic group differences were compared using chi square tests. OS was estimated by Kaplan-Meier analysis and compared using log-rank tests and Cox models.
Results
Black patients had significantly more soft-tissue tumors compared to white non-Hispanic patients (p < 0.0001). Asian and white Hispanic patients had an intermediate frequency of soft tissue tumors that also differed from white non-Hispanic patients (p < 0.0001). White Hispanic patients presented with a higher proportion of larger tumors compared to white non-Hispanic patients (p = 0.042). Black patients were older than white non-Hispanic patients (p = 0.012). Sex, frequency of pelvic tumors, and metastatic status did not differ by ethnicity or race. OS was different according to race and ethnicity. Even after controlling for known confounders, OS was significantly worse for black, Asian, and white Hispanic patients compared to white non-Hispanic patients (p=0.0031, p= 0.0182 and p=0.0051, respectively).
Conclusion
Ethnic and racial differences in characteristics and outcomes for patients with ES exist. Understanding the etiology of these differences requires further study.
Keywords: Ewing sarcoma family of tumors, prognosis, soft-tissue, race, ethnicity, SEER
Introduction
Ewing sarcoma (ES) is a highly malignant small round cell tumor of bone or soft tissue and is the second most frequent primary malignant bone tumor in children and adolescents.1 ES shows an irregular geographic pattern of incidence and overall survival (OS).2, 3 Whites are much more frequently affected while there are low rates in East Asian and African populations.4–7 ES has been poorly characterized in Hispanic populations.
For several adult malignancies, differences in clinical presentation and outcome by race and ethnicity have been previously demonstrated.8–11 In pediatric cancer the impact of race and ethnicity on outcome is less clear.12, 13 Despite the recognition that the incidence of ES differs by race, disparities in clinical presentation and outcome have not been well studied for children and young adults with this disease. Given the stark differences in incidence of this disease in black and Asian populations, we hypothesized that disease characteristics and outcomes might differ in these populations compared to white non-Hispanic patients. We performed a secondary data analysis using the SEER database to evaluate racial and ethnic differences in clinical presentation and OS among patients diagnosed with Ewing sarcoma before the age of 40 in the United States.
Methods
Patient Population
The US National Cancer Institute's SEER database contains 1715 patients < 40 years of age between 1973–2005. Our analysis included 17 SEER registries representing data from multiple institutions from diverse geographic parts of the United States.14 The SEER Program currently collects and publishes cancer incidence and survival data from 17 population-based cancer registries that cover one quarter (26%) of the US population. In the SEER Program registries, data on patient demographics, primary tumor site, tumor morphology and stage at diagnosis, first course of treatment, and follow-up for vital status are routinely assembled.
On January 1, 1973 SEER began collecting data on cancer cases. Currently, the following population-based cancer registries are part of the SEER program: Alaska Native Tumor Registry, Arizona Indians, Los Angeles, San Francisco-Oakland, San Jose-Monterey, Greater California, Connecticut, Detroit, Atlanta, Rural Georgia, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, Seattle-Puget Sound and Utah. Data for 2005 include the standard set based on July 1 populations and a set that has been adjusted for the population shifts due to hurricanes Katrina and Rita. Inclusion criteria for geographic areas to be selected for the SEER Program are based on their ability to operate and maintain a high quality population-based cancer reporting system and their epidemiologically significant population subgroups. The population covered by SEER is comparable to the general US population with a trend to be somewhat more urban and to have a higher proportion of foreign-born persons than the general US population.15
All patients with histologically confirmed ES, peripheral primitive neuroectodermal tumors, and Askin tumors of the bone, soft tissue and organs were eligible for the study. Patients were identified using the corresponding ICD-O-2 and ICD-O-3 codes for these diagnoses. Patients ≥ 40 years of age at the time of diagnosis (n = 239) were excluded from the analysis.
SEER classifies race into 28 mutually exclusive groups by using information from the medical record. Race was classified as white, black, or Asian if there was concordant evidence in the SEER registries coding “Race/ethnicity” and “Race recode (W, B, AI, API)”. Hispanic ethnicity was determined by using stated ethnicity in the medical record, national origin on the death certificate, life history and/or spoken language, place of birth, and surname. Classification of Hispanic patients was based on concordant evidence in the SEER variables “NHIA derived Hispanic origin” and “Spanish surname or origin”. In case of discordance between these variables (n = 2), missing values (n = 15), or no more evidence of Hispanic ethnicity than Spanish surname (n = 19), data were declared as unknown and excluded from the analysis. For this analysis, patients were grouped into 4 different groups as white non-Hispanic, white Hispanic, Asian non-Hispanic (Asian) and black non-Hispanic (black) based on their race and ethnicity classification. Native American patients (n = 14), Asian Hispanic patients (n = 1) and black Hispanic patients (n = 1) were excluded due to small patient numbers.
We examined the variables age (< 20 years at diagnosis vs. ≥ 20 years at diagnosis), sex, tumor size (≤ 5 cm vs. > 5 cm), tumor site (soft tissue vs. bone), pelvic site, stage (metastatic vs. localized), and year of diagnosis (in sequential 5-year blocks) to evaluate racial and ethnic differences in clinical presentation. On the basis of SEER historical staging system, disease stage was categorized as localized/regional or distant. In sensitivity analyses, age was also evaluated as a continuous variable and year of diagnosis was evaluated according to specific calendar year-intervals that corresponded to the sequential national phase 3 clinical trials open to US patients with ES. Analyses with these variables coded in this manner yielded similar results to the presented analyses.
Data on treatment received were also collected, with radiation therapy dichotomized as not given or given if performed at any time point during treatment (including radioactive implants and radioisotopes). Similarly, surgery was dichotomized as not used (except for diagnostic biopsy) or used as a component of local control. Data on the use of limb-sparing surgery were not reliably available. Adequate data to control for socioeconomic, environmental factors and access to health care were not available. Variables from the SEER database such as county or SEER registry were deemed inadequate to control for these factors.
Statistical Methods
Selected patient and tumor characteristics which appeared to differ between groups were evaluated statistically using chi square tests with the white non-Hispanic group, the group with the largest sample size, as reference. OS was estimated by Kaplan-Meier survival curves and group differences were compared using the log-rank test, again using the white non-Hispanic group as the reference. OS was expressed as Kaplan-Meier estimate with 95% confidence interval (CI). OS time was calculated as the number of completed months between the date of diagnosis and whichever occurred first: the date of death; the date last known to be alive; or December 31, 2005. The median follow-up time for the analyzed cohort was 92 months.
Cox proportional hazard models were used to assess the effect of race and ethnicity on OS while controlling for known confounders. The proportional hazards assumption was tested using time-varying covariates and confirmed with log-log survivor function plots. For combined race and ethnicity models, the proportional hazards assumption could not be confirmed when metastatic status was included as a variable. Subsequent models were stratified by metastatic status and these models met the proportional hazards assumption. For sensitivity models that evaluated ethnicity separately from race, the proportional hazards assumption was only confirmed after stratifying for metastatic status and year of diagnosis.
The SEER database was accessed using SEER*Stat, version 6.4.4. All statistical analyses were performed using SAS, version 9 and STATA, version 10.
Results
Patient Characteristics
A total of 1715 patients with ES diagnosed before age 40 years were reported to SEER between 1973 and 2005. After excluding 41 patients with unknown race/ethnicity, 14 Native American patients, 1 Asian Hispanic patient, and 1 black Hispanic patient, our study population included 1658 patients. Of these, 78.6% were white non-Hispanic, 14.0% white Hispanic, 4.9% Asian, and 2.5% black. The clinical characteristics of the study population according to race and ethnicity are shown in Table 1.
Table 1.
Prevalence of patient and tumor characteristics by race and ethnicity.
| White Non-Hispanic n = 1303 | White Hispanic n = 232 | Asian Non-Hispanic n = 81 | Black Non-Hispanic n = 42 | |
|---|---|---|---|---|
| Age < 20 Years | 68.4% (891/1303) | 69.4% (161/232) | 63.0% (51/81) | 50.0%* (21/42) |
|
| ||||
| Male | 60.0% (782/1303) | 59.1% (137/232) | 63.0% (51/81) | 61.9% (26/42) |
|
| ||||
| Primary Site | ||||
| Bone | 79.7% (1038/1302) | 62.6% (144/230) | 61.3% (49/80) | 54.8% (23/42) |
| Soft tissue | 20.3% (264/1302) | 37.4%* (86/230) | 38.8%* (31/80) | 45.2%* (19/42) |
|
| ||||
| Primary Site | ||||
| Pelvis | 23.5% (306/1302) | 22.6% (52/230) | 20.0% (16/80) | 11.9% (5/42) |
| Non-pelvis | 76.5% (996/1302) | 77.4% (178/230) | 80.0% (64/80) | 88.1% (37/42) |
|
| ||||
| Tumor Size | ||||
| ≤ 5 cm | 27.8% (144/518) | 19.1% (25/131) | 31.4% (16/51) | 28.0% (7/25) |
| > 5 cm | 72.2% (374/518) | 80.9%* (106/131) | 68.6% (35/51) | 72.0% (18/25) |
|
| ||||
| Distant Metastases at Diagnosis | 29.8% (348/1168) | 35.0% (72/206) | 30.7% (23/75) | 27.8% (10/36) |
p < 0.05 compared to reference group of white non-Hispanic patients.
Significant differences were noted according to age, primary tumor site (soft tissue vs. bone), and tumor size, while there was no difference in regard to sex, pelvic site, or metastatic status at diagnosis (Table 1). A lower proportion of black patients were diagnosed less than age 20 years compared to white non-Hispanics (p = 0.012). A greater proportion of black patients presented with soft tissue tumors rather than bone tumors compared to white non-Hispanic patients (p < 0.0001). Asian and white Hispanic patients had an intermediate frequency of soft tissue tumors that also differed from white non-Hispanic patients (p < 0.0001). White Hispanic patients were more likely to have tumors > 5 cm in maximal diameter compared to white non-Hispanic patients (p < 0.042). A greater proportion of white Hispanic cases were diagnosed in more recent years compared to the other groups (data not shown; p < 0.0001), possibly due to changes in the SEER program to include more regions with higher Hispanic populations.
Differences in Overall Survival by Race and Ethnicity
There was a statistically significant difference in OS by race and ethnicity (p-value = 0.031, Figure 1). This difference appeared to be driven mainly by inferior OS for black patients (5-year OS 40.7% with 95% CI 25.6 – 55.3%) compared to white non-Hispanic patients (5-year OS 52.2% with 95% CI 49.1 – 55.1%; p = 0.015). OS for white Hispanic patients (51.8% with 95% CI 44.0 – 59.0%) and Asian patients (47.8% with 95% CI 35.5 – 59.1%) did not differ from OS for white non-Hispanic patients (p = 0.29 and 0.09, respectively).
Figure 1.
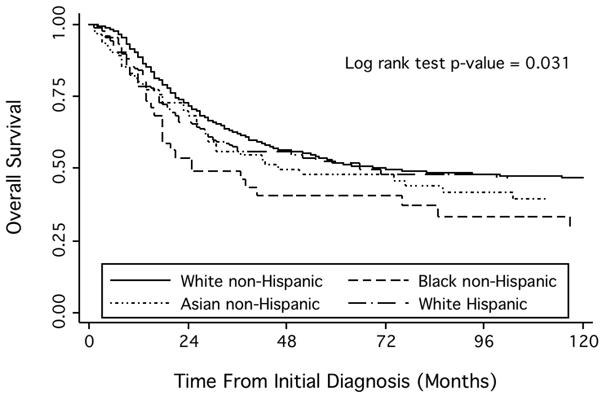
Kaplan-Meier estimates of overall survival by race and ethnicity among patients with Ewing sarcoma.
Using a Cox proportional hazards model stratified by metastatic status and controlling for age, year of diagnosis, and pelvic site, black, Asian, and white Hispanic patients each demonstrated an inferior OS compared to white non-Hispanic patients. The hazard ratios (HR) of death for black, Asian and white Hispanic patients compared to white non-Hispanic patients were 1.84 (p = 0.003), 1.48 (p= 0.018) and 1.41 (p= 0.005), respectively (Table 2). A second model was constructed that also controlled for tumor size, but this model included only approximately 42% of the patients due to missing data. In this sensitivity model, black and white Hispanic patients continued to have significantly higher hazard ratios for death compared to white non-Hispanic patients (HR = 2.34, p = 0.001; HR = 1.46, p = 0.023; respectively). The hazard ratio for death for Asian patients remained 1.48 (p= 0.08), but this result was no longer statistically significant.
Table 2.
Racial and ethnic differences in overall survival in patients with Ewing sarcoma from Cox proportional hazards model controlling for age, pelvic tumor site, metastatic disease, and year of diagnosis. Values in parenthesis reflect a sensitivity model that also controlled for tumor size.
| Race/Ethnicity | Hazard Ratio | 95% Confidence Interval | P - value |
|---|---|---|---|
| White Non-Hispanic | Reference | Reference | |
| White Hispanic | 1.41 (1.46) | 1.11 – 1.79 (1.05 –2.03) | 0.005 (0.023) |
| Asian Non-Hispanic | 1.48 (1.48) | 1.07 – 2.05 (0.95 – 2.29) | 0.018 (0.08) |
| Black Non-Hispanic | 1.84 (2.34) | 1.23 – 2.76 (1.41 – 3.89) | 0.003 (0.001) |
Cox proportional hazard models were also constructed to analyze race and ethnicity separately. The results from these models are in concordance with our findings from the final combined race/ethnicity model, demonstrating higher HR of death for black and Asian patients compared to white patients and for Hispanic compared to non-Hispanic patients.
Discussion
This is the most comprehensive analysis of racial and ethnic differences in patient characteristics and outcome for patients with ES. In univariate analysis, OS was significantly worse for black patients than white non-Hispanic patients. In addition, after controlling for recognized prognostic factors, OS was significantly worse for black, Asian, and white Hispanic patients compared to white non-Hispanic patients. These differences in outcome may broadly reflect socio-economic differences, biologic differences, or a combination of these factors. Socio-economic differences could encompass treatment disparities, delays in diagnosis, differences in protocol adherence, and differences in local control strategies. Biologic differences may include variations in tumor aggressiveness as well as host factors, such as pharmacogenomic differences in drug metabolism.16 The contribution of each of these factors to the observed differences cannot be ascertained using the SEER database and will require further study.
The influence of race and ethnicity on survival of children with cancer has not been fully analyzed.13 Some studies have reported less of an impact of race and ethnicity on outcomes,12, 17 while others have reported clear differences in survival by race and ethnicity.18, 19 For example, poorer outcome for specific types of acute lymphoblastic leukemia have been reported in black and Hispanic children compared to white children.18, 19 As in the current study, the etiology for these differences is not clear and may include a combination of factors such as biological differences and differential access to healthcare. For example, black children were significantly more likely than white children to have higher-risk prognostic features in childhood ALL including certain immunophenotypes and the t(1;19) chromosomal translocation.17 In addition, higher incidence of leukemia8 and fewer cases of the favorable TEL-AML1 translocation were noted among Hispanic children.20 These differences in outcomes reinforce the need to understand these differences and to try to adjust treatment to reduce outcome disparities among racial and ethnic groups.13 This point is particularly important in Ewing sarcoma in which the standard treatment approach has been defined from clinical trials enrolling mainly white non-Hispanic patients. These approaches may not be optimal for patients from other racial or ethnic groups.
While the SEER database allowed for evaluation of a relatively large number of patients with a rare tumor, the use of this database also resulted in a number of major limitations. First, race, ethnicity, and tumor histology could not be confirmed. Second, in order to develop a large enough cohort of patients with different race and ethnicity groups for analysis, we included patients treated in the 1970's. Given that the treatment of ES has evolved since the 1970's, some of our findings may not apply in the setting of current practice. Third, a substantial amount of data is missing from the SEER database. We had intended to evaluate for differences in treatment by race and ethnicity. Unfortunately, data on local control were of limited quality, with approximately half of patients without any apparent local control. Data on the use of chemotherapy and on the use of high-dose chemotherapy with autologous stem cell rescue were also not available.
Tumor size is a recognized prognostic factor in Ewing sarcoma.21–23 Tumor size was not available for approximately half of the analyzed population. Due to these large numbers of missing values, our final model did not control for tumor size. Approximately 90% of patients had complete data for other confounding variables and were included in these final models. In a sensitivity model that also controlled for tumor size, white Hispanic and black patients continued to demonstrate a significantly inferior outcome compared to white non-Hispanic patients. In this sensitivity model, Asian patients did not demonstrate a statistical difference from the reference group. This lack of difference may reflect small patient numbers, particularly since the point estimate of the hazard ratio did not change but the confidence interval widened.
A higher proportion of white Hispanic patients with ES were registered in SEER over the last decade compared to white non-Hispanic patients with ES. This effect might be explained in part by the growing US Hispanic population. Furthermore, the SEER program has been specifically adding more population-based cancer registries with a higher census of Hispanic residents. Since outcomes for patients with ES have improved over time,24 we controlled for the changing demographic of the SEER database by controlling for year of diagnosis.
This study demonstrates differences in OS according to race and ethnicity even after accounting for known prognostic factors in Ewing sarcoma. Although the reasons for these differences remain unknown, several factors suggest that biologic differences may at least contribute to these differences. First, the striking difference in incidence by race suggests a genetic component to this disease. Second, patients other than white non-Hispanic patients were more likely to present with soft tissue rather than bone tumors. Finally, a previous group demonstrated that Japanese patients had a higher frequency of somatic loss of chromosome 19 compared to white patients.25 Further investigations into the biologic and genetic differences in ES tumors according to race and ethnicity are required. Similarly, further studies into possible environmental differences and health care disparities are required to evaluate the role of these factors in explaining the observed outcome differences.
Condensed abstract.
Patient and tumor characteristics in Ewing sarcoma differ by race and ethnicity. Overall survival also differs by race and ethnicity even after controlling for known prognostic factors.
Acknowledgments
Support: Supported by the Campini Foundation, Hope Street Kids, and NIH/NCRR/OD UCSF-CTSI Grant Number KL2 RR024130. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures: None
Previous presentation: Presented in part at the 2009 American Society of Pediatric Hematology/Oncology (ASPHO) Annual Meeting; San Diego, CA.
References
- 1.Gurney JGS, AR, Bulterys M. Malignant bone tumors. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975–1995. 1999:99–110. [Google Scholar]
- 2.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008:2575–96. doi: 10.1002/cncr.23866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson SE, Lorentzon R. The geographic variation of the incidence of malignant primary bone tumors in Sweden. J Bone Joint Surg Am. 1974;56(3):592–600. [PubMed] [Google Scholar]
- 4.Fraumeni JF, Jr., Glass AG. Rarity of Ewing's sarcoma among U.S. Negro children. Lancet. 1970;1(7642):366–7. doi: 10.1016/s0140-6736(70)90754-3. [DOI] [PubMed] [Google Scholar]
- 5.Li FP, Tu JT, Liu FS, Shiang EL. Rarity of Ewing's sarcoma in China. Lancet. 1980;1(8180):1255. doi: 10.1016/s0140-6736(80)91719-5. [DOI] [PubMed] [Google Scholar]
- 6.Dorfman HD, Czerniak B. Bone cancers. Cancer. 1995;75(1 Suppl):203–10. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 7.Guo W, Xu W, Huvos AG, Healey JH, Feng C. Comparative frequency of bone sarcomas among different racial groups. Chin Med J (Engl) 1999;112(12):1101–4. [PubMed] [Google Scholar]
- 8.Howe HL, Wu X, Ries LA, Cokkinides V, Ahmed F, Jemal A, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006;107(8):1711–42. doi: 10.1002/cncr.22193. [DOI] [PubMed] [Google Scholar]
- 9.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Haynes MA, Smedley BD. The unequal burden of cancer: An Assessment of NIH Research and Programs for Ethnic Minorities and the Medically Underserved. National Academy Press; Washington, D.C.: 1999. [PubMed] [Google Scholar]
- 11.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–57. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 12.Castellino SM, Casillas J, Hudson MM, Mertens AC, Whitton J, Brooks SL, et al. Minority adult survivors of childhood cancer: a comparison of long-term outcomes, health care utilization, and health-related behaviors from the childhood cancer survivor study. J Clin Oncol. 2005;23(27):6499–507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia S. Influence of race and socioeconomic status on outcome of children treated for childhood acute lymphoblastic leukemia. Curr Opin Pediatr. 2004;16(1):9–14. doi: 10.1097/00008480-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–21. [PubMed] [Google Scholar]
- 15.SEER- Surveillance, Epidemiology, and End Results Program . NIH Publication No. 05-4772. 2005. [Google Scholar]
- 16.Suarez-Kurtz G. Pharmacogenomics in admixed populations. Trends Pharmacol Sci. 2005;26(4):196–201. doi: 10.1016/j.tips.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Pui CH, Sandlund JT, Pei D, Rivera GK, Howard SC, Ribeiro RC, et al. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA. 2003;290(15):2001–7. doi: 10.1001/jama.290.15.2001. [DOI] [PubMed] [Google Scholar]
- 18.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100(6):1957–64. doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 19.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA. 2003;290(15):2008–14. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 20.Aldrich MC, Zhang L, Wiemels JL, Ma X, Loh ML, Metayer C, et al. Cytogenetics of Hispanic and White children with acute lymphoblastic leukemia in California. Cancer Epidemiol Biomarkers Prev. 2006;15(3):578–81. doi: 10.1158/1055-9965.EPI-05-0833. [DOI] [PubMed] [Google Scholar]
- 21.Paulussen M, Ahrens S, Dunst J, Winkelmann W, Exner GU, Kotz R, et al. Localized Ewing tumor of bone: final results of the cooperative Ewing's Sarcoma Study CESS 86. J Clin Oncol. 2001;19(6):1818–29. doi: 10.1200/JCO.2001.19.6.1818. [DOI] [PubMed] [Google Scholar]
- 22.Oberlin O, Deley MC, Bui BN, Gentet JC, Philip T, Terrier P, et al. Prognostic factors in localized Ewing's tumours and peripheral neuroectodermal tumours: the third study of the French Society of Paediatric Oncology (EW88 study) Br J Cancer. 2001;85(11):1646–54. doi: 10.1054/bjoc.2001.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauer R, Jurgens H, Burgers JM, Dunst J, Hawlicek R, Michaelis J. Prognostic factors in the treatment of Ewing's sarcoma. The Ewing's Sarcoma Study Group of the German Society of Paediatric Oncology CESS 81. Radiother Oncol. 1987;10(2):101–10. doi: 10.1016/s0167-8140(87)80052-x. [DOI] [PubMed] [Google Scholar]
- 24.Cotterill SJ, Ahrens S, Paulussen M, Jurgens HF, Voute PA, Gadner H, et al. Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol. 2000;18(17):3108–14. doi: 10.1200/JCO.2000.18.17.3108. [DOI] [PubMed] [Google Scholar]
- 25.Ozaki T, Schaefer KL, Wai D, Yokoyama R, Ahrens S, Diallo R, et al. Population-based genetic alterations in Ewing's tumors from Japanese and European Caucasian patients. Ann Oncol. 2002;13(10):1656–64. doi: 10.1093/annonc/mdf218. [DOI] [PubMed] [Google Scholar]


