Abstract
Recent data suggests that reactions of nitrite with ferric hemoglobin are potentially important in heme-protein dependent NO signaling. Our group and others are evaluating the role of reductive nitrosylation reactions in the generation of N2O3 as a signaling molecule. The latter reaction is hypothesized to involve reactions on NO, nitrite and methemoglobin to form N2O3 in a anydrase reaction. Of potential importance to these reactions is the affinity of methemoglobin for nitrite and the reactivity of nitrite bound methemoglobin with nitric oxide. In this paper we review work related to the electronic structure of nitrite bound methemoglobin and its dissociation constant. We present new data using electron paramagnetic resonance spectroscopy which confirm that methemoglobin has a much higher affinity for nitrite, under certain conditions, than reported in classical observations. Interestingly the affinity is greatest at lower pH and low nitrite:methemoglobin ratios. These data suggest additional interesting chemistry in the reaction of nitrite with ferric and ferrous heme species. Moreover, this reaction could serve as a paradigm for ferric heme reactions with nitrite.
Introduction
In 2003, Cosby and coworkers showed that infusions of slightly supraphysiological levels of nitrite led to increased nitric oxide production and increased blood flow that was further increased during exercise [1]. These data suggested that nitrite is converted to nitric oxide under deoxygenated conditions and the authors proposed that the activity is due to nitrite reductase activity of deoxygenated hemoglobin [1]. Several other mechanisms have been explored to explain nitrite reduction under physiological or therapeutic conditions including those involving myoglobin [2], xanthine oxidase [3; 4; 5; 6; 7], endothelial nitric oxide synthase [8; 9], cytochrome oxidase [10; 11; 12], and cytochrome c [13; 14]. It is likely that each of these mechanisms are important under different conditions in different tissues. However, several studies examining the interaction of hemoglobin and nitrite in aortic ring preparations [15; 16], the ability of hemoglobin-based substitutes to vasodilate when infused with nitrite [17], and human nitrite infusion studies that included inhibition of alternate enzymatic mechanisms, such as xanthine oxidase [18], all suggest a major role for deoxygenated hemoglobin in effecting nitrite mediated vasodilation.
A major challenge, however, to the notion that red cell hemoglobin reduces nitrite to NO which leads to increased blood flow is that hemoglobin is a potent scavenger of NO, thus limiting NO escape from red blood cell. The primary reaction within the red blood cell will be the reaction of NO with oxygenated hemoglobin to form methemoglobin (MetHb) and nitrate, eliminating NO bioactivity. The kinetics of this reaction are governed by a bimolecular rate constant of 6–8 × 107 M−1s−1 [19; 20; 21] so that the half-life of NO in an oxygenated red blood cell (RBC) is about 0.5 microseconds. During this time, NO could only diffuse about 0.02 μm (assuming an intraerythrocytic diffusion constant of 1000 μm2 s−1). Some NO may bind to deoxygenated hemoglobin but once it comes off, it still finds itself surrounded by hemoglobin. Thus, many have argued that NO formed in the red blood cell cannot get out without going through additional chemical reactions to form more stable intermediate species. This has also been suggested by computational analysis where only 0.1 picomolar NO was calculated to escape the red cell from 1 μM nitrite [22].
One hypothesis that has been proposed to explain nitrite mediated export of NO bioactivity from RBCs is that some intermediate species is formed during the reaction such as N2O3 that can escape the red cell or export NO activity through the intermediacy of a nitrosothiol [22; 23; 24; 25]. This proposal is consistent with the observation of S-nitrosohemoglobin formation that was observed during nitrite infusions [1]. Two ways that N2O3 could form include the following reactions:
| (1) |
| (2) |
where HbFeII-NO+ represents NO bound to MetHb (HbFeIII-NO) and the NO+ bound to ferrous heme character of this resonance structure is emphasized. The term HbFeIII-NO2− in Equation 2 is nitrite bound to MetHb. The nature of these reactions has been explored in the context of reductive nitrosylation [24; 25]. Reductive nitrosylaton begins with reduction of HbFeII-NO+ through the nucleophilic attack by H2O forming nitrite (Equation 3), with subesequent nitrosylation of the ferrous heme by excess NO (Equation 4).
| (3) |
| (4) |
Fernandez and Ford discovered that reductive nitrosylation is catalyzed by nitrite and hypothesized that this catalysis involved nitrite reacting with NO bound MetHb to form N2O3 [25]. We subsequently examined the kinetics of nitrite catalysis of reductive nitrosylation and, based on that and other analysis, proposed that N2O3 could be made via the reaction of NO with nitrite bound MetHb (Equation 2) [24]. The viability of this reaction has recently been supported by studies of glass embedded MetHb [26]. Interestingly, it has recently been observed that nitrophorin 7, a salivary ferric hemoprotein from a blood-feeding insect, can catalzye the convertion of nitrite to NO, suggesting similar interesting biological signaling properties of a ferric hemoprotein and nitrite [27].
We hypothesized that the reaction of MetHb-nitrite with NO to form N2O3 could constitute a mechanism of nitrite mediated export of NO activity from the red blood cell. Thus, the nature of the MetHb bond and its reactivity have become an area of considerable interest. Performing density functional theory calculations, we found that the nitrite could bind to the ferric heme with either N-bound (FeIII-nitro) or O-bound (FeIII-O-nitrito) configuration, with the nitro form being more stable by about 7 kcal mol−1 [24]. Some configurations of the O-nitrito form were found to have some FeII-NO2• character, and we therfore hypothesized that it is this form that can react rapidly with NO. One may suggest that the fact that this species is less stable than nitro form by 7 kcal mol−1 makes it energetically unfeasible, given that a Boltzman distribution would predict that only one in one-hundred thousand MetHb-nitrite molecules would have this conformation. However, 7 kcal mol−1 is about the energy of a single strong H-bond which would not be included in the density functional theory calculations. Indeed, recent studies using x-ray crystallography have shown that MetHb-nitrite crystallizes in the O-nitrito mode, suggesting that this is a viable or perhaps favored binding mode in solution under certain conditions [28].
Another important factor that needs to be considered in evaluating the feasibility of the MetHb-nitrite + NO reaction contributing to nitrite mediated NO activity export from the RBC is the affinity of nitrite for metHb. The lower the affinity, the less MetHb-nitrite there would be available for the reaction to proceed and the less likely it would be important in NO activity export. Based on analysis of EPR data, we reported that the affinity of nitrite for MetHb is much higher under some conditions than has been reported previously [24]. The methods used in the EPR experiments and conclusions drawn have been challenged [29] and we have presented a short, formal reply arguing that our original methods and conclusions are valid [30].
In this paper, we present new data and analysis exploring the binding characteristics of MetHb-nitrite. We have performed more EPR experiments and present results supporting complex binding of nitrite to MetHb with the affinity being much higher than previously reported under different experimental conditions, suggesting that the interaction of nitirte with ferric hemoglobin is not simple.
Experimental Procedures
Sodium nitrite was purchased from Fisher Scientific and inositol hexaphosphate (IHP), sodium salt, from Sigma Chemicals (St. Louis, MO). Methemoglobin was prepared by treatment of hemoglobin with 5-fold excess concentration of potassium ferricyanide followed by passing through G25 columns (PD-10, GE Healthcare) and dialysis against PBS at the appropriate pH (pH 6.5 or pH 7.4). Nitrite bound hemoglobin was prepared by incubating measured amounts of nitrite with MetHb at 37 °C (unless otherwise noted) for five minutes before freezing for EPR analysis. When IHP was used, the pH of the stock solution was adjusted to the desired value as addition of IHP to a buffer solution often lowers the pH.
EPR spectroscopy was used to determine the dissociation constant (Kd=[MetHb][nitrite]/[MetHb-nitrite]) of nitrite-bound methemoglobin at various concentrations of nitrite. EPR measurements of low spin (nitrite-bound methemoglobin) or high spin (methemoglobin) species were carried out at either 5 K or 15 K at varying microwave powers ranging from 1 mW to 0.1 mW (indicated in the text), at microwave frequency 9.37 GHz, modulation amplitude 10 G, sweep time of 168 s and time constant of 81.92 ms. The signals obtained from EPR were imported into the simulation program SimFonia (Bruker Inc. Version 1.29, 1976,) in which g-values were varied to give the best fit to experimental spectra. The double integrals of the species used to fit the experimental spectra were used to calculate concentrations and Kd values for nitrite-bound methemoglobin.
Results
Saturation of MetHb-nitrite EPR
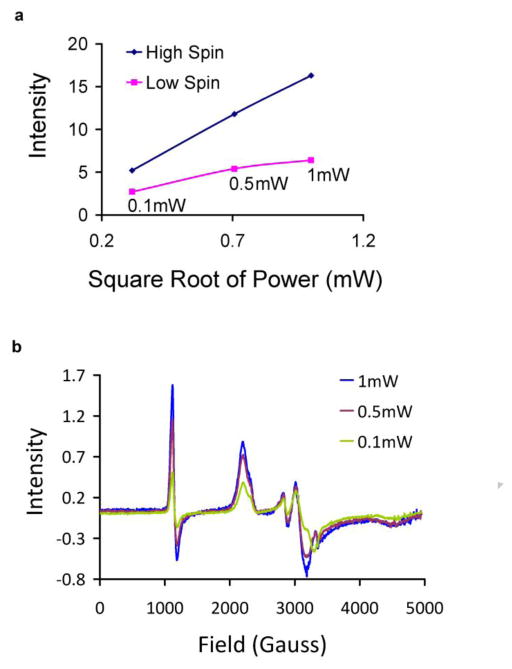
One expects that when a ligand binds to MetHb, it might form a low spin species, detectable by EPR. In our earlier studies, we did not detect a low spin MetHb-NO2− species by EPR and considered that this could occur secondary to EPR silence, like that reported for some other ferric heme-nitrite species [31; 32]. However, in subsequent studies we have found that the apparent EPR-silence that we observed was largely or entirely due to saturation of the low spin species. At the high microwave powers used (10 mW), this saturation effect would greatly increase the high spin species around 1100 gauss relative to the low spin signals at higher field values, making them appear to be silent. Figure 1 demonstrates that there is substantial saturation of low spin signal, even at much lower microwave powers when working at 5K. The high spin signal measured around g=6, corresponding to unbound MetHb, shows the expected microwave power dependence when there is no saturation with the signal increasing linearly as the square root of the microwave power. On the other hand, the low spin signal does not obey this dependence and saturation is clear even at 1 mW microwave power (Figure 1a). The saturation is also clear upon visual inspection of the spectra (Figure 1b). The relative heights of the low spin peak at 2214 gauss increases relative to the high spin peak at 1124 gauss as the microwave power is lowered.
Figure 1. Evidence of power saturation at 5K for low spin MetHb-nitrite.
(a) EPR peak intensities of high spin MetHb and low spin MetHb-nitrite measured at 5K using different microwave powers. MetHb-nitrite was prepared in 0.01 M PBS buffer at pH 6.5 by adding 10 mM nitrite to 200 μM MetHb. (b) Wide field EPR scans of MetHb-nitrite measured at 5K under varying microwave powers. Same samples as described for panel a. The high spin MetHb peak recorded for Figure 3a is at ~ 1124 gauss and the lowest field peak for the low spin MetHb-nitrite peak is at ~2214 gauss.
Simulation of MetHb-nitrite EPR
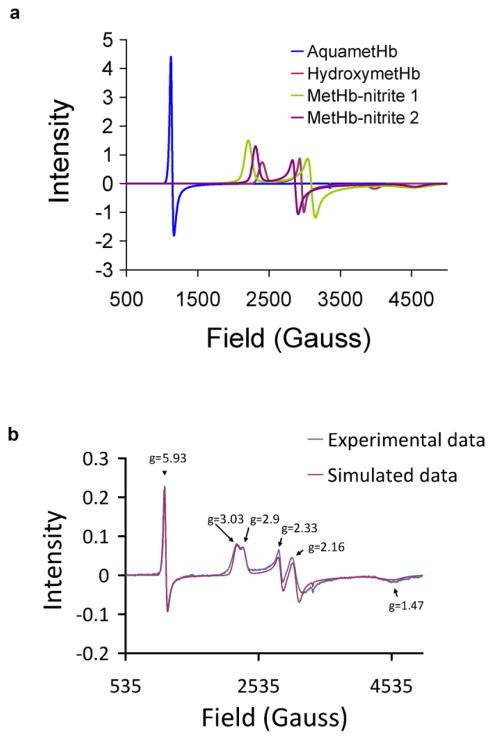
In previous work, we calculated the dissociation constant of MetHb-nitrite by simply examining the high spin MetHb signal, assuming that any disappearance of this signal was due to formation of MetHb-nitrite [24]. In subsequent work, Schwab et al calculated the dissociation constant by examination of simulations of the low spin MetHb-nitrite signal [29]. Schwab et al also showed that all of the high spin MetHb signal that disappears due to nitrite binding to MetHb can be accounted for by formation of MetHb-nitrite, affirming our assumption [29]. We therefore concluded that both methods are equivalent and that the complex behavior of nitrite binding to MetHb results in low dissociation constants (high affinity) under some conditions [30]. Here, we employ a method similar to Schwab et al to reconfirm these facets of nitrite binding to MetHb. Figure 2 shows simulation of the MetHb-nitrite spectrum using similar g-values as those determined by Schwab et al [29]. Figure 2a shows the simulated basis spectra used which generally include two forms of MetHb (a high spin aquaMetHb form and a low spin hydroxyMetHb form) as well as two low spin MetHb-nitrite forms. Figure 2b shows an example of simulated data.
Figure 2. Simulation of MetHb-nitrite EPR.
(a) The four basis spectra for simulations are shown (b)Experimental EPR spectrum (blue) was taken at 15K and 0.8mW microwave power. MetHb-nitrite was prepared in 0.01M PBS buffer at pH 7.4 by adding 10mM nitrite to 200uM MetHb. Three separate species were simulated with SimFonia and added together to produce the simulated spectrum. These species include a high spin MetHb species with principal g values of 5.93, 5.93, 1.99 and two low spin MetHb-Nitrite species with principal g values of 3.03, 2.33, 1.47 and 2.90, 2.16, and 1.47. (g values are close to that determined by Schwab et. al. [29]). The double integrals of the three simulated spectra were used to calculate the concentration of the corresponding species which were subsequently used to calculate the Kd values for MetHb-nitrite under the conditions used.
Dissociation constants for MetHb-nitrite
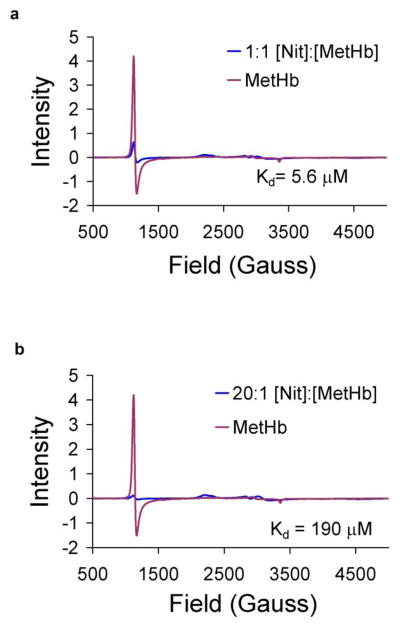
As described earlier, the dissociation constant of MetHb-nitrite decreases as the ratio of nitrite to MetHb decreases [24]. Figure 3 shows spectra before and after adding nitrite to 300 μM MetHb in a 1:1 (Figure 3a) and 20:1 (Figure 3b) nitrite:MetHb ratio at pH 6.5 (incubated, here, at 0 °C). The dissociation constants for these MetHb-nitrite samples were determined to be 5.6 μM and 190 μM, with the lower value corresponding to the lower nitrite:MetHb ratio. This value is similar to that we reported earlier (7 μM when 100 μM nitrite was added to 85 μM MetHb at pH 6.5) [24]. It is worth noting that if the dissociation constant of 1800 M that has been determined by others [29] were to apply to the conditions of Figure 3a, one would expect the MetHb signal to decrease by only 13%, and this is clearly not the case.
Figure 3. EPR of MetHb and MetHb-nitrite taken at 15K and 0.8mW microwave power.
(a) 300uM MetHb was prepared in 0.01M PBS buffer at pH 6.5 at 0 °C. 300uM nitrite was added for a [Nit]:[Met] ratio of 1:1. (b) 6mM nitrite was added for a [Nit]:[Met] ratio of 20:1. Kd values were calculated by fitting simulations of the high spin MetHb and two low spin MetHb-nitrite species to the experimental data and calculating concentrations of each species from the simulations. The Kd value increases as the [Nit]:[Met] ratio increases.
Figure 4 shows a plot of the dissociation constant of MetHb-nitrite vs ratio of nitrite to MetHb at pH 6.5 and 7.4 at 37 degrees celsius. As the ratio of nitrite to MetHb decreases the dissociation constant decreases, similar to what we reported previously for experiments at room temperature [24]. The dissociation constants at pH 6.5 are dramatically lower than those at pH 7.4, reaching a minimum of 51 μM when the ratio of nitrite:MetHb is 1:1, a bit higher than that reported previously for a similar ratio measured at room temperature (7 M μ[24]), but still substantially lower than previously published values.
Figure 4. Dissociation constants for four different ratios of [Nitrite]:[Met] at pH 6.5 and pH 7.4 at 37 °C.
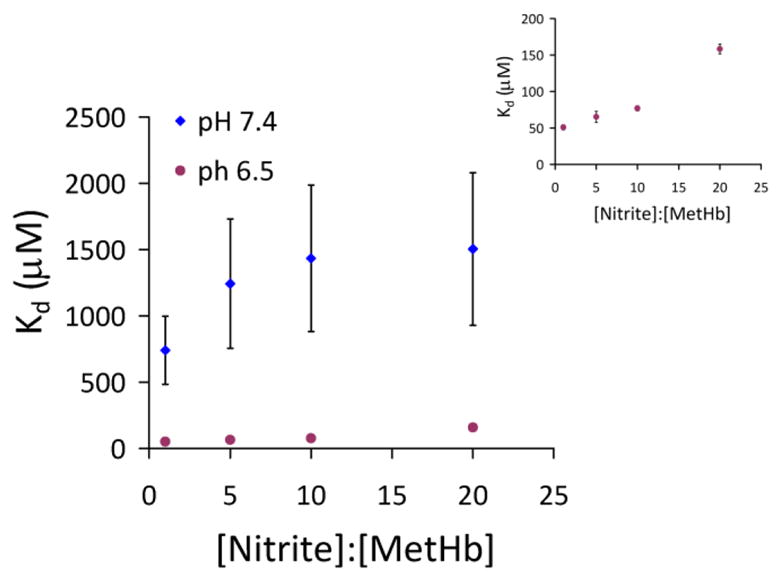
MetHb-nitrite was prepared in 0.01M PBS buffer by adding 300 μM, 1.5 mM, 3mM, or 6mM nitrite to 300 μM methemoglobin. Dissociation constants (Kd) were calculated by fitting simulations of the high spin MetHb, the low spin MetHb, and the two low spin MetHb-nitrite species to the experimental data and calculating concentrations of each species from the simulations. Inset shows Kd values for samples prepared at pH 6.5 on a smaller scale.
In addition to pH, other effectors influence nitrite binding to MetHb. Figure 5 shows the effect of allosteric effectors inositol hexaphosphate (IHP) and N-ethylmalemide (NEM). The shape of the low spin MetHb EPR signal is different when NEM or IHP are added (Figure 5a). The difference in the shape of the spectrum can be accounted for by a difference in the relative amounts of the two low spin MetHb-nitrite species with the ratio of MetHb-nitrite species 1 to MetHb species 2 (see Figure 5a) being 2.6 when IHP is added and 3.4 when NEM is added. NEM and IHP also have dramatically different affects on the MetHb-nitrite dissociation constants with IHP greatly increasing the affinity of MetHb for nitrite compared to NEM (Figure 5b). The data shown were taken on samples prepared at pH 7.4. Similar trends were noted when experiments were repeated at pH 6.5, but the effects were smaller (perhaps due the effect of low pH already making the samples T-state).
Figure 5. Nitrite binding to MetHb in the presence of allosteric affectors.
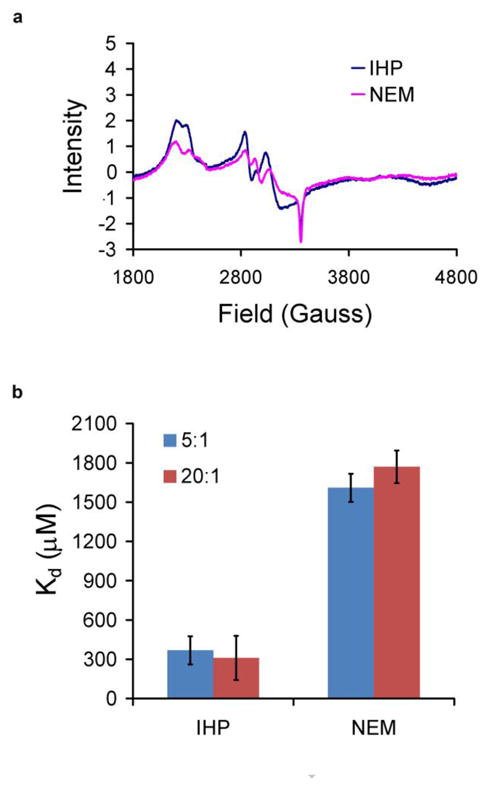
(a) Nitrite (1.5 mM) was added to MetHb (300 μM) that had been incubated with 3 mM NEM or IHP at pH 7.4. The high field EPR spectra are shown demonstrating significant differences in the shape of the spectra. (b) The dissociation constants were obtained from EPR spectra on samples as described for panel “a” where the ratio of nitrite to MetHb is 5:1 and also for when 6 mM nitrite was added (nitrite to MetHb ratio of 20:1).
Discussion
We have confirmed that nitrite binding to MetHb is complex, being affected by allosteric effectors and relative concentrations of reactants. In 1976, Rodkey published a dissociation constant of 1.1 mM at pH 7.1 using 800 fold excess nitrite to heme [33]. Schwab et al obtained a similar dissociation constant (1.8 mM) testing a range of nitrite:MetHb ratios in Hepes buffer at pH 7.4 [29]. These values are much higher than those we have reported under some conditions [24; 30] and those obtained here. We hold that rather than involving simple reaction, with a single association and dissociation rate, nitrite binding to MetHb is complex and may play important roles in nitrogen oxide biology.
By performing EPR measurements at lower microwave power and operating at 15 K rather than 5 K as we did previously [24], the low spin MetHb-nitrite signals was observable. As reported by Schwab et al [29], there are two distinct low spin MetHb-nitrite signals observable by EPR and these can be simulated using anisotropic g-values for each one. It is interesting to speculate on the nature of the two MetHb-nitrite species. One possibility is that the two species correspond to nitrite bound to hemes in alpha or beta subunits of MetHb. Using x-ray crystallography, Richter-Addo and coworkers found that the binding in each subunit is different [28]. Thus, one low spin species may be for nitrite bound in the alpha subunits and the other may be for that in the beta subunits. A potentially more interesting possibility would be if one species corresponded to the N-bound (FeIII-nitro) and the other was the O-bound (FeIII-O-nitrito) form. Although the uncommon O-nitrito form was seen in x-ray crystallography [28], density functional theory found that the N-nitro form is more stable [24; 34]. Although the apparent discrepancy may be explained by hydrogen bonding favoring the O-nitrito form, it is also possible that some Hb conformations (other than that which forms crystals that give the O-nitrito form) can have substantial N-nitro binding under some conditons.
The dissociation constants determined at 37 °C show the same dependence on nitrite to MetHb ratio as reported earlier, decreasing as nitrite/MetHb decreases [24]. They also show the same pH dependence with a higher affinity at pH 6.5 compared to 7.4 (Figure 4). The dissociation constants at pH 6.5 are quite small reaching a minimum of 51 μM when the ratio of nitrite:MetHb is 1:1. The values obtained here at 37 °C and pH 7.4 are higher than those obtained at room temperature. When experiments were repeated at room temperature for this study, more similar values as those obtained previously were obtained (185 μM obtained here and 75 μM obtained previously [24] for a 1:1 MetHb:nitrite ratio).
The pH dependence shows that protons appear to increase the binding affinity of MetHb for nitrite and so does IHP. This suggests that T-state MetHb has a higher nitrite affinity than R-state MetHb. It is interesting to speculate that these allosteric effects are responsible, at least partially, for the observed dependence of Kd on [nitrite], where higher concentrations of nitrite push the MetHb towards R-state. This could also explain the lack of [nitrite] dependence when NEM or IHP are used (Figure 5b) as these allosteric effectors would lock the protein in one state or the other. In any case, our results demonstrate the complex behavior of nitrite binding to MetHb and show that, under some conditions, the binding affinity is high.
Acknowledgments
This work was supported by NIH grants HL058091, HL078786, HL62198, HL090503, and funding from the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu XL, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Medicine. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 2.Shiva S, Huang Z, Grubina R, Sun JH, Ringwood LA, MacArthur PH, Xu XL, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation Research. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 3.Li HT, Samouilov A, Liu XP, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction - Evaluation of its role in nitric oxide generation in anoxic tissues. Journal of Biological Chemistry. 2001;276:24482–24489. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric Oxide Production from Nitrite Occurs Primarily in Tissues Not in the Blood: CRITICAL ROLE OF XANTHINE OXIDASE AND ALDEHYDE OXIDASE. Journal of Biological Chemistry. 2008;283:17855–17863. doi: 10.1074/jbc.M801785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb AJ, Milsom AB, Rathod KS, Chu WL, Qureshi S, Lovell MJ, Lecomte FMJ, Perrett D, Raimondo C, Khoshbin E, Ahmed Z, Uppal R, Benjamin N, Hobbs AJ, Ahluwalia A. Mechanisms Underlying Erythrocyte and Endothelial Nitrite Reduction to Nitric Oxide in Hypoxia Role for Xanthine Oxidoreductase and Endothelial Nitric Oxide Synthase. Circulation Research. 2008;103:957–U114. doi: 10.1161/CIRCRESAHA.108.175810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zweier JL, Wang PH, Samouilov A, Kuppusamy P. Enzyme-Independent Formation of Nitric-Oxide in Biological Tissues. Nature Medicine. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 7.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. Febs Letters. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 8.Gautier C, van Faassen E, Mikula I, Martasek P, Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to NO under anoxia. Biochemical and Biophysical Research Communications. 2006;341:816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cellular and Molecular Life Sciences. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. Febs Letters. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- 11.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metabolism. 2006;3:277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Castello PR, Woo DK, Ball K, Wojcik J, Liu L, Poyton RO. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proceedings of the National Academy of Sciences. 2008;105:8203–8208. doi: 10.1073/pnas.0709461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu S, Azarova NA, Font MD, King SB, Hogg N, Gladwin MT, Shiva S, Kim-Shapiro DB. Nitrite Reductase Activity of Cytochrome c. Journal of Biological Chemistry. 2008;283:32590–32597. doi: 10.1074/jbc.M806934200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YR, Chen LC, Liu X, Li HT, Zweier JL, Mason MP. Involvement of protein radical, protein aggregation, and effects on NO metabolism in the Hypochlorite-mediated oxidation of mitochondrial cytochrome c. Free Radical Biol Med. 2004;37:1591–1603. doi: 10.1016/j.freeradbiomed.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. American Journal of Physiology-Heart and Circulatory Physiology. 2007;293:H2565–H2572. doi: 10.1152/ajpheart.00759.2007. [DOI] [PubMed] [Google Scholar]
- 17.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, III, Schechter AN, Gladwin MT. Nitrite Infusion in Humans and Nonhuman Primates. Endocrine Effects, Pharmacokinetics, and Tolerance Formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 19.Doyle MP, Hoekstra JW. Oxidation of Nitrogen-Oxides by Bound Dioxygen in Hemoproteins. Journal of Inorganic Biochemistry. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 20.Eich RF, Li TS, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 21.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 22.Jeffers A, Xu XL, Huang KT, Cho M, Hogg N, Patel RP, Kim-Shapiro DB. Hemoglobin mediated nitrite activation of soluble guanylyl cyclase. Comparative Biochemistry and Physiology a-Molecular & Integrative Physiology. 2005;142:130–135. doi: 10.1016/j.cbpb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 23.Robinson JM, Lancaster JR. Hemoglobin-mediated, hypoxia-induced vasodilation via nitric oxide - Mechanism(s) and physiologic versus pathophysiologic relevance. American Journal of Respiratory Cell and Molecular Biology. 2005;32:257–261. doi: 10.1165/rcmb.F292. [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by a concerted nitrite reductase and anhydrase activity of hemoglobin. Nature Chemical Biology. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez BO, Ford PC. Nitrite catalyzes ferriheme protein reductive nitrosylation. Journal of the American Chemical Society. 2003;125:10510–10511. doi: 10.1021/ja036693b. [DOI] [PubMed] [Google Scholar]
- 26.Navati MS, Friedman JM. Reactivity of Glass-Embedded Met Hemoglobin Derivatives toward External NO: Implications for Nitrite-Mediated Production of Bioactive NO. Journal of the American Chemical Society. 2009;131:12273–12279. doi: 10.1021/ja903364h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He CM, Knipp M. Formation of Nitric Oxide from Nitrite by the Ferriheme b Protein Nitrophorin 7. Journal of the American Chemical Society. 2009;131:12042–12403. doi: 10.1021/ja9040362. [DOI] [PubMed] [Google Scholar]
- 28.Yi J, Safo MK, Richter-Addo GB. The nitrite anion binds to human hemoglobin via the uncommon O-nitrito mode. Biochemistry. 2008;47:8247–8249. doi: 10.1021/bi801015c. [DOI] [PubMed] [Google Scholar]
- 29.Schwab DE, Stamler JS, Singel DJ. Nitrite-methemoglobin inadequate for hypoxic vasodilation. Nature Chemical Biology. 2009;5:366–366. doi: 10.1038/nchembio0609-366. [DOI] [PubMed] [Google Scholar]
- 30.Goetz BI, Wang P, Shields HW, Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Ghosh A, Hogg N, Gladwin MT, Kim-Shapiro DB. Nitrite-methemoglobin inadequate for hypoxic vasodilation Reply. Nature Chemical Biology. 2009;5:367–367. doi: 10.1038/nchembio0609-366. [DOI] [PubMed] [Google Scholar]
- 31.Day EP, Peterson J, Bonvoisin JJ, Young LJ, Wilkerson JO, Siegel LM. Magnetization of the Sulfite and Nitrite Complexes of Oxidized Sulfite and Nitrite Reductases - Electron-Paramagnetic-Res Silent Spin S=1/2 States. Biochemistry. 1988;27:2126–2132. doi: 10.1021/bi00406a046. [DOI] [PubMed] [Google Scholar]
- 32.Young LJ, Siegel LM. On the Reaction of Ferric Heme-Proteins with Nitrite and Sulfite. Biochemistry. 1988;27:2790–2800. doi: 10.1021/bi00408a020. [DOI] [PubMed] [Google Scholar]
- 33.Rodkey FL. Mechanism for Conversion of Oxyhemoglobin to Methemoglobin by Nitrite. Clinical Chemistry. 1976;22:1986–1990. [PubMed] [Google Scholar]
- 34.Novozhilova IV, Coppens P, Lee J, Richter-Addo GB, Bagley KA. Experimental and density functional theoretical investigations of linkage isomerism in six-coordinate {FeNO}(6) iron porphyrins with axial nitrosyl and nitro ligands. Journal of the American Chemical Society. 2006;128:2093–2104. doi: 10.1021/ja0567891. [DOI] [PubMed] [Google Scholar]