Abstract
The effects of brain-derived neurotrophic factor (BDNF) on cocaine-seeking are brain region-specific. Infusion of BDNF into subcortical structures, like the nucleus accumbens and ventral tegmental area, enhances cocaine-induced behavioral sensitization and cocaine seeking. Conversely, repeated administration of BDNF antiserum into the nucleus accumbens during chronic cocaine self-administration attenuates cocaine-induced reinstatement. In contrast, BDNF infusion into the dorsomedial prefrontal cortex immediately following a final session of cocaine self-administration attenuates relapse to cocaine seeking after abstinence, as well as cue- and cocaine prime-induced reinstatement of cocaine-seeking following extinction. BDNF-induced alterations in the ERK-MAP kinase cascade and in prefronto-accumbens glutamatergic transmission are implicated in BDNF’s ability to alter cocaine seeking. Within 22 hr after infusion into the prefrontal cortex, BDNF increases BDNF protein in prefrontal cortical targets, including nucleus accumbens, and restores cocaine-mediated decreases in phospho-ERK expression in the nucleus accumbens. Furthermore, three weeks after BDNF infusion in animals with a cocaine self-administration history, suppressed basal levels of glutamate are normalized and a cocaine-prime-induced increase in extracellular glutamate levels in the nucleus accumbens is prevented. Thus, BDNF may have local effects at the site of infusion and distal effects in target areas that are critical to mediating or preventing cocaine-induced dysfunctional neuroadaptations.
Keywords: neurotrophin, ERK MAP kinase, cocaine, self-administration, reinstatement, glutamate, dopamine, Brain-derived neurotrophic factor (BDNF), Cocaine self-administration, Glutamate, Neurotrophins, Nucleus accumbens, Prefrontal cortex
1. BDNF is a neurotrophic peptide that mediates synaptic plasticity including drug-induced neuroadaptations
BDNF, a member of the neurotrophin polypeptide family that includes nerve growth factor, neurotrophin-3, and neurotrophin 4/5, is the most widely and abundantly expressed neurotrophin in the nervous system (Thoenen, 1995). Like other neuropeptides, BDNF is synthesized as a pro-peptide (32 KDa) that is proteolytically processed into a smaller (13 KDa), mature form. This cleavage is thought by some to occur after secretion by the extracellularly-located tissue plasminogen-activated plasmin (Lu, 2003); however, others have reported that CNS neurons store and secrete mature, not pro-BDNF, in response to excitatory input (Matsumoto et al., 2008). The significance is that pro-BDNF binds and activates p75 pro-apoptotic receptors whereas mature BDNF binds and activates pro-survival tropomyosin receptor kinase B (TrkB) receptors (Bibel and Barde, 2000). The latter results in receptor dimerization, tyrosine-phosphorylation that provides docking sites for adapter molecules, internalization, and down-regulation (Sommerfield et al., 2000; Du et al., 2003; Nagappan and Lu, 2005). BDNF activation triggers changes in TrkB-mediated intracellular signaling and transcription factor activity (Lu, 2003; Patapoutian and Reichart, 2001). Through these mechanisms, BDNF expression is associated with neuronal activity and synaptic plasticity.
Many stimuli that induce neuronal activity in a calcium-dependent manner increase bdnf mRNA and BDNF protein expression via CREB activation (Morimoto et al., 1998; Shieh et al., 1998; Shieh and Ghosh, 1999; Lu, 2003). Following transcription, bdnf mRNA can be trafficked to active synapses for translation (Steward and Schuman, 2001; Tongiorgi et al., 1997). Release of BDNF via synapsin-associated mechanisms and subsequent TrkB receptor activation are associated with increased glutamatergic activity (Hartmann et al., 2001; Jovanovic et al., 2000; Balkowiec and Katz, 2002). Furthermore, BDNF promotes both early and late-phase longterm potentiation (LTP), promotes dendritic protein synthesis, and increases dendritic spine formation (Bramham et al., 1996; Kang and Schuman, 1996; Massaoudi et al., 1998). BDNF regulates spine formation by inhibiting miR-134, a micro-RNA that inhibits the translation of Lim kinase 1, a protein kinase that regulates actin filament activity (Schratt et al., 2006).
BDNF may also regulate drug-induced long-term neuroadaptations that encompass alterations in molecular components at the synapse, changes in gene expression, and modifications of behavioral output. Integration of dopaminergic and glutamatergic input to medium spiny neurons in the striatum, including the nucleus accumbens (NAc), and pyramidal cells in the prefrontal cortex (PFC) generates molecular changes in dendritic spines that activate a variety of intracellular cascades important for associative learning (Kennedy, 2000; Hyman, 2005; Girault, 2007). Repeated alterations in calcium influx, phosphorylation-dephosphorylation events, and the activation of immediate early genes and transcription factors culminate in changes in neuronal structure and synaptic composition that ultimately modify behavior. Repeated exposure to psychostimulants increases dendritic branching and dysmorphic dendritic spines in the NAc and PFC in rats during extended abstinence (Robinson et al., 2001); however, the molecular cascades underlying these responses have not been elaborated. Thus, a significant question is how the cellular substrates of plasticity undergo dynamic alterations in drug addiction and how BDNF modifies these changes.
2. Addictive drugs alter endogenous BDNF mRNA/protein expression in the mesocorticolimbic system
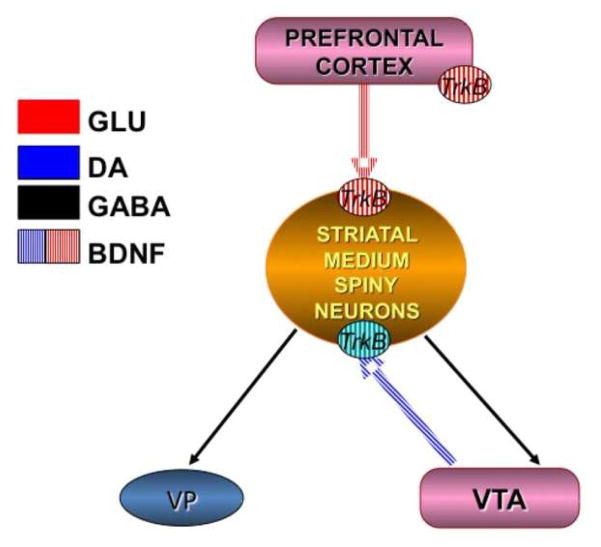
Key brain areas that are altered by drugs of abuse, particularly psychostimulants, are implicated in the reinstatement of drug-seeking. These structures include the PFC and its downstream target, the NAc. Both of these structures receive dopamine (DA) innervation from the ventral tegmental area (VTA) via the mesocorticolimbic DA (“reward”) pathway. Many cocaine-induced neuroadaptations that are thought to drive reinstatement of cocaine-seeking are manifested by alterations in the plasticity of the PFC-NAc pathway. BDNF is expressed in this pathway; in fact, cortical pyramidal neurons arising from the PFC are the predominant source of BDNF within the striatum, including the NAc (Altar et al., 1997). Figure 1 illustrates the expression and transport of BDNF within the mesocorticolimbic pathway where it signals through TrkB receptors expressed on medium spiny neurons in the striatum and on cortical pyramidal cells and interneurons.
Figure 1.
BDNF expression within the meso-corticolimbic system. BDNF is expressed in glutamatergic pyramidal neurons arising from the PFC as well as dopaminergic neurons arising from the VTA, both of which synapse on GABAaergic medium spiny neurons within the NAc. BDNF itself is transported anterogradely to the striatum where it binds to TrkB receptors on medium spiny neurons. Labeling studies have demonstrated that a majority of BDNF expression within the NAc arises from the PFC, not the VTA. Glu=glutamate, DA=dopamine, GABA=gamma-aminobutyric acid.
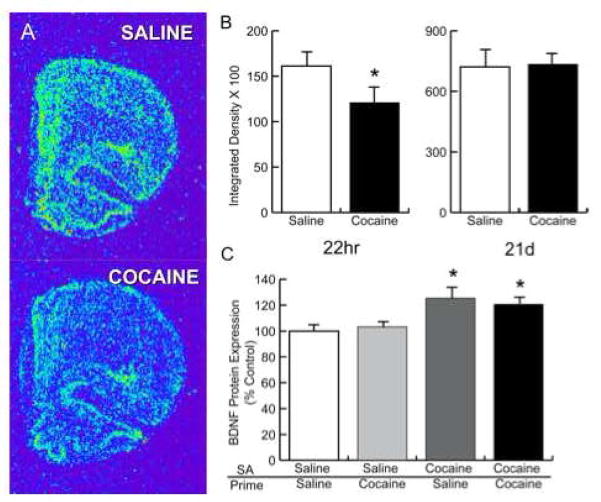
Under several conditions, endogenous bdnf mRNA and protein are differentially regulated in mesolimbic and cortical neurons in response either to acute administration of addictive drugs or during extended periods of abstinence from prolonged drug administration. For instance, acute administration of cocaine, amphetamine, or alcohol transiently induces bdnf mRNA expression in the medial PFC, cingulate cortex, and/or striatum 45 min-4 h after drug-administration (McGough et al., 2004; Kerns et al., 2005; Le Foll et al., 2005; Fumagalli et al., 2007, 2009; Saylor and McGinty, 2008). This acute induction of bdnf mRNA may represent the initial stages of cortical plasticity that lay the foundation for subsequent psychostimulant-induced alterations in intracellular signaling and altered neurotransmission. However, repeated, random stress prevents this cocainae-induced increase (Fumagalli et al., 2009). After repeated administration of cocaine, amphetamine, or alcohol, the expression of bdnf mRNA and BDNF protein are also transiently increased in forebrain structures (Meredith et al., 2002; McGough et al., 2004; Fumagalli et al., 2007). In contrast however, 22 hrs, but not 21 days, after the end of cocaine self-administration, bdnf mRNA levels in the dorsomedial PFC are decreased (Fig 2A,B). In addition, a persistent BDNF protein response develops in mesolimbic and cortical structures and lasts for extended durations during abstinence from cocaine. Following self-administration of cocaine, BDNF protein is induced in mesolimbic structures including the VTA, NAc and the amygdala at extended time points of abstinence (Grimm et al., 2003). In addition, BDNF protein is induced in the dorsomedial PFC 21 d after cocaine self-administration whether or not a cocaine priming injection was administered 30 min prior to sacrifice (Fig 2C). Given that BDNF is transported in the cortical glutamatergic projections from the PFC to the NAc (Altar et al., 1997) and that this pathway regulates relapse to drug-seeking (McFarland et al., 2003; Fuchs et al., 2004), variable expression of BDNF in this network of reciprocally interconnected structures suggests that BDNF may constitute a critical component of cocaine-induced plasticity in mesolimbic and cortical neurons.
Figure 2.
In situ hybridization histochemistry performed on frontal sections after the end of cocaine self-administration demonstrates that Bdnf mRNA in the dorsomedial PFC was downregulated after 22 hr (A,B-left), but not after 21 days (B-right), of cocaine abstinence. C. BDNF protein expression was increased in the PFC 21 days after the end of cocaine self-administration in abstinent rats. SA=self-administration condition in which rats received cocaine in response to active lever presses or yoked-saline. Prime=i.p. injection of saline or cocaine given 30 min. before euthanasia. *p<0.05.
3. BDNF modifies drug–induced behaviors
Ventral midbrain or NAc BDNF infusions enhance motor activity, cocaine reinstatement after extended abstinence, as well as sensitization to cocaine and cocaine-conditioned cues (Altar et al., 1992; Guillin et al., 2001; Martin-Iversen, et al., 1994; 1996; Horger, et al., 1999; Lu et al., 2004; Bahi et al., 2008). These reports suggest that elevated BDNF is associated with enhancement of dopamine neurotransmission, motor activation, and goal-directed behavior. Consistent with this idea, microinfusion of antisera against BDNF or TrkB receptors into NAc attenuated acute METH-induced increases in dopamine extracellular levels and psychostimulant-induced motor activity (Narita et al., 2003). Further, alcohol-induced increases in bdnf mRNA expression negatively regulate alcohol-associated behaviors and alcohol consumption (Janak et al., 2006). Consistent with these data, bdnf antisense oligonucleotide infusions into the central or medial, but not the basolateral, amygdala, increased alcohol intake, which was prevented by co-infusion of BDNF (Pandey et al., 2006). Conversely, bdnf heterozygous mice display increased conditioned place preference and increased locomotor sensitization to alcohol and show a prolonged elevation in alcohol consumption following a period of abstinence (McGough et al., 2004). Similarly, acute amphetamine stimulates more locomotor behavior that lasts longer in bdnf +/− mice than in WT mice (Dluzen et al., 2001; Saylor and McGinty, 2008). Further, bdnf +/− mice show similar depletions in tyrosine hydroxyalse immunoreactvity and DA tissue concentrations in response to a neurotoxic regimen of METH (Boger et al., in press; Dluzen et al., 2001). However, in one report (Joyce et al., 2004), DAT binding and TH-ir were less affected in bdnf +/− mice than in WT mice after a high dose METH binge (mortality, thermal response, age, and sex of the mice were not reported).
4. The effects of exogenous BDNF on drug-seeking are brain region-specific
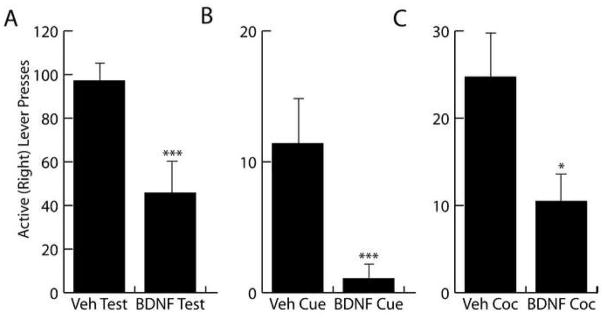
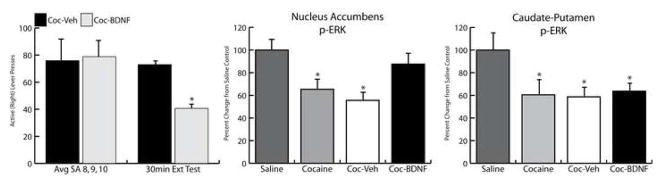
Infusion of BDNF into subcortical structures, like the NAc and VTA, enhances cocaine and opioid seeking (Lu et al., 2004; Graham et al., 2007; Vargas-Perez et al., 2009). Conversely, repeated administration of BDNF antiserum into the NAc during chronic cocaine self-administration attenuates cocaine-induced reinstatement (Graham et al., 2007). Collectively, these studies implicate BDNF activity in the VTA and NAc in long-term modulation of cocaine-induced behavior, possibly by interacting with enhanced dopamine neurotransmission or VTA GABAergic signaling (Vargas-Perez et al., 2009). These findings and the drug-induced alterations in BDNF expression observed during drug abstinence (described above) have led some investigators to speculate that BDNF activity promotes vulnerability to drug addiction and that therapeutic approaches that inhibit BDNF signaling may decrease individuals’ motivation to seek cocaine (Graham et al., 2007; Tsai, 2007). However, this conclusion is based only on altered BDNF expression and effects in subcortical brain regions and does not take into consideration that the effects of BDNF on drug seeking are site-specific. For example, in models of depression, BDNF has an anti-depressant profile when infused into the hippocampus and a pro-depressant profile when infused into the VTA (Shiriyama et al., 2002; Eisch et al., 2003). Similarly, in contrast to the pro-drug seeking effects of subcortical BDNF infusion described above, our data indicate that BDNF infusion into the dorsomedial PFC suppresses cocaine seeking. Specifically, intra-PFC infusion immediately following a final session of cocaine self-administration attenuates relapse to cocaine seeking after abstinence, as well as cue- and cocaine prime-induced reinstatement of cocaine-seeking following extinction (Fig 3-Berglind, et al., 2007). In this paradigm, rats self-administered 0.6 mg/kg, i.v. cocaine 2h/day for 10 days. Immediately after the final cocaine self-administration session, rats received a single, bilateral infusion of BDNF or vehicle within the prelimbic cortex (0.75 μg/side). The rats that received an intra-PFC BDNF infusion demonstrated significant attenuation in extinction responding, cue and cocaine-induced reinstatement of drug seeking. Furthermore, intra-PFC BDNF infusions decreased extinction responding and prevented a cocaine-induced decrease in phospho-ERK activity in the NAc, but not in the caudate-putamen (CPu), detected after one day of abstinence, revealing a trans-synaptic regulation of NAc neurons by BDNF activity in the PFC (Fig 4). Taken in isolation, this evidence suggests that BDNF, rather than a BDNF antagonist, would have possible therapeutic value in cocaine addiction. However, consideration of our findings in combination with Graham and colleagues (2007) and Lu and colleagues (2004) powerfully argue for a diverse and complex role of BDNF in relapse to cocaine seeking. Our findings should not be viewed as contradictory to those of Graham or Lu and colleagues; rather they illustrate the site-specific effects of exogenous BDNF. Furthermore, they underscore the complexity of endogenous BDNF activity and serve to caution the promotion of theories about BDNF’s possible therapeutic value in the treatment of cocaine addiction.
Figure 3.
An intra-PFC infusion of BDNF attenuated extinction responding, cue- and cocaine-induced reinstatement of cocaine-seeking. (A) After 6 days of abstinence, the BDNF-treated rats exhibited significantly fewer active lever presses during the post-abstinence test than the vehicle-treated rats (***P=0.001). (B) The BDNF-treated rats exhibited significantly fewer active (***P<0.001) lever presses during the cue-induced reinstatement test than vehicle-treated rats. (C) The BDNF-treated rats exhibited significantly fewer active lever presses during the cocaine-induced reinstatement test than the vehicle-treated rats (*P<0.05). Modified from Berglind et al., 2007.
Figure 4.
Intra-PFC BDNF infusion suppressed cocaine-seeking behavior (A) and normalized phospho-(p)ERK expression levels in the NAc (B), but not the caudate-putamen (C) of rats after a 30 min extinction test 22 hrs after the final cocaine SA session. *p<0.05. Avg SA 8, 9, 10=average number of active lever presses on the last 3 days of cocaine self-administration; 30 min Ext Test=30 min extinction test 22 hr after the 10th self-administration session; Coc-Veh=cocaine self administration and intra-PFC vehicle infusion; Coc-BDNF= cocaine self administration and intra-PFC BDNF infusion. Modified from Berglind et al., 2007.
5. BDNF regulates glutamatergic neurotransmission in the prefronto-accumbens pathway
The susceptibility to drug relapse and other addictive behaviors is thought to depend on long-term neuroadaptations in mRNA, proteins, and phospho-proteins in meso-cortico-basal ganglia circuitry (Nestler, 2005; Kalivas & Volkow, 2005). These persistent neuroadaptive changes encompass alterations in molecular components at the synapse, changes in gene expression, and altered behavioral output. The PFC, a critical region for goal-directed behaviors and impulse control, is one of the brain regions in which these changes take place. Imaging studies of addicts have revealed decreased basal activity during drug withdrawal (Goldstein and Volkow, 2002) and large increases in metabolic activity in the PFC following exposure to drug cues (Grant et al., 1996; Maas et al., 1998; Childress et al., 1999).
Reinstatement of drug seeking after extinction in rodent studies appears to be driven primarily by disturbances in glutamatergic transmission in the prefronto-accumbens pathway specifically a suppression of basal levels that is associated with an excessive cocaine prime-induced overshoot of glutamate release in the NAc (McFarland et al., 2003; Kalivas, 2004). BDNF augments glutamatergic transmission in the cerebral cortex and hippocampus where its expression is closely linked to activity-dependent synaptic plasticity (Lessman, 1998; Lu, 2003; Matsumoto et al., 2001). Although dopaminergic afferents to the striatum, including NAc, also contain BDNF, the primary source of BDNF protein levels in the striatum is the cortico-accumbens pathway. BDNF potentiates excitatory neurotransmission (Lu and Chow, 1999) by increasing synaptic vesicle docking at excitatory synapses (Tyler and Pozzo-Miller, 2001), enhancing glutamate release (Lessman et al., 1998), and postsynaptic NMDA receptor activity (Levine et al., 1995; 1998). BDNF regulation of glutamatergic signaling contributes to the neuronal plasticity underlying learning and memory (Tyler et al., 2002) by inducing early- and late-phase LTP (Kang and Schuman, 1995; Massaoudi et al., 1998) and dendritic spine growth (Horch et al., 1999).
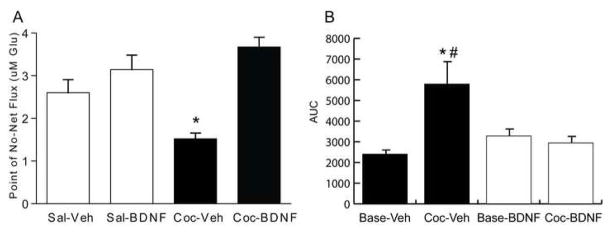
The glutamatergic pathway arising in the dorsomedial PFC and projecting to the NAc core is a critical component of the reward circuitry that underlies reinstatement to cocaine-seeking. In addition to attenuating reinstatement to cocaine-seeking, BDNF infusion into the dorsomedial PFC elevates BDNF and phospho-ERK expression in the NAc (Berglind et al., 2007). Moreover, intra-PFC BDNF prevents the cocaine self administration-induced reduction in basal extracelluar glutamate, as well as the cocaine prime-induced increase in extracellular glutamate levels in the NAc up to three weeks after BDNF infusion (Fig 5-Berglind et al., 2009). These data suggest that intra-PFC BDNF attenuates reinstatement to cocaine-seeking by normalizing cocaine-induced neuroadaptations that alter glutamate neurotransmission within the NAc.
Figure 5.
A. BDNF normalized basal extracellular glutamate levels in the NAc of rats with a cocaine self-administration history. Intra-PFC BDNF treatment significantly increased the point of no net flux, indicating an increase in the basal extracellular glutamate concentrations in the rats compared with those that received intra-PFC vehicle infusions *p<0.05. B. Average area under the curve (AUC) values before and after a cocaine challenge in rats with a cocaine history. Veh-Base: average baseline AUC value for rats that received an intra-PFC vehicle infusion; Coc- Veh: average AUC value after the cocaine challenge for rats that received a vehicle infusion BDNF-Base: average baseline AUC value for rats that received an intra-dmPFC BDNF infusion; Coc- BDNF: average AUC value after the cocaine challenge for rats that received an intra-PFC BDNF infusion. * p<0.05 vs. Coc- BDNF; # p<0.05 vs. Veh-Base. Modified from Berglind et al., 2009.
6. Prefrontal cortical Trk receptor binding is necessary for the expression of BDNF’s suppressive effect on cocaine-seeking
Despite the finding that intra-PFC BDNF infusion suppresses cocaine relapse, the specific site(s) of action and molecular-cellular substrates of BDNF’s behavioral effects are not fully understood. It is possible that BDNF has local effects at the site of infusion (PFC) as well as distal effects in PFC target areas like the NAc. Infusion of exogenous BDNF into the PFC can have implications for local postsynaptic neuroplasticity in PFC neurons. Interactions between extracellular BDNF and TrkB can promote postsynaptic survival and growth signaling (Goggi et al., 2003), prolonged neuroplasticity in the form of LTP (Bramham & Messaoudi, 2005), and synaptic scaling (Rutherford et al., 1998; Leslie et al., 2001). However, there is evidence that BDNF may also have more widespread effects via its neurotransmitter-like activities (Blum and Konnerth, 2005). It has been demonstrated that exogenous BDNF can be internalized and become available for activity-dependent secretion (Santi et al., 2006; Dean et al., 2009), and that intracellular BDNF can undergo axonal transport (Altar and DiStefano, 1998) and calcium-dependent release (Sadakata et al., 2004) from presynaptic terminals. Taken together, this evidence suggests that BDNF has the capability to regulate neuroplasticity both locally in the PFC as well as distally in target regions of PFC projections.
Information obtained to date indicates that exogenous BDNF infused into the PFC 1) increases BDNF protein expression in the NAc and other PFC targets such as the amygdala (Fig 6), (2) restores cocaine-mediated decreases in p-ERK expression in the NAc, and (3) prevents cocaine-mediated aberrations in extracellular glutamate levels in the NAc (Berglind et. al., 2007; 2009). These data support the notion that intra-PFC BDNF’s suppressive effect on cocaine seeking depends on normalization of cocaine-mediated plasticity in the PFC-NAc pathway. However, this does not rule out the possibility that local BDNF-TrkB interactions in the PFC precede subsequent distal effects in target structures such as the NAc. Therefore, we investigated the site and mechanism of action of intra-PFC BDNF’s suppressive effects on cocaine-seeking behavior.
Figure 6.
BDNF protein expression levels, detected by ELISA, are significantly elevated in the NAc, basolateral amygdala (BLA), and central nucleus of the amygdala (CeA) of naïve rats 22 hrs after infusion of BDNF in to the dorsomedial PFC. N=6 per group.mPFC=medial prefrontal cortex; NAc=nucleus accumbens; lCPu=lateral caudate-putamen; mCPu=medial caudate-putamen. *p<0.05 vs. Vehicle-infused.
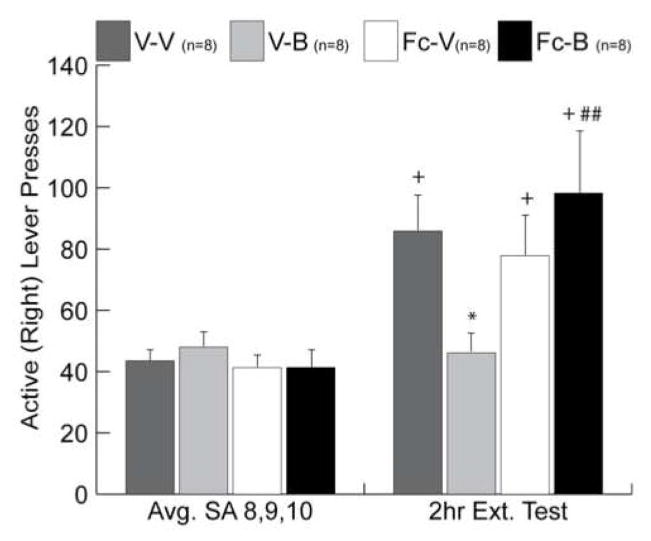
Given that TrkB receptor binding and activation have been shown to be necessary for the expression of BDNF’s behavioral effects in vivo (Ou and Gean, 2006), we determined the extent to which the attenuation of cocaine-seeking following intra-PFC BDNF depends on local TrkB signaling. Infusion of the extracellular BDNF scavenger TrkB-Fc 20 minutes prior to BDNF in the dorsomedial PFC completely reversed BDNF’s suppressive effect on cocaine-seeking 22 hrs post-infusion (Fig 7). However, lever pressing of rats that received an intra-PFC infusion of TrkB-Fc followed by vehicle was not significantly different than vehicle-vehicle-treated rats, indicating that scavenging endogenous BDNF in the PFC did not affect cocaine-seeking. This lack of effect of TrkB-Fc alone is consistent with the finding that bdnf mRNA is decreased in the PFC 22 hr after the end of cocaine self administration (see Fig 2).
Figure 7.
Pre-infusion with a BDNF scavenger, TrkB/Fc, prevented the suppressive effect of intra-PFC BDNF on cocaine-seeking during day 1 of extinction responding. V-V=vehicle-vehicle; V-B=vehicle-BDNF; Fc-V=TrkB/Fc-vehicle; Fc-B=TrkB/Fc-BDNF. Avg SA 8,9,10=average number of active lever presses on the last 3 days of cocaine self-administration; 2hr Ext Test= 2hr extinction test 22 hr after the 10th self-administration session. *p<0.05 vs. V-V; ##=p<0.005 vs. V-B; +=p<0.05 vs. Avg SA 8,9,10 within each group.
In further experiments, we assessed whether BDNF’s suppressive effect on cocaine relapse is mediated by Trk receptor binding by infusing the Trk inhibitor, K252a, into the dorsomedial PFC 20 min before BDNF. K252a blocked BDNF’s suppressive effect on cocaine-seeking following 6 days of abstinence from cocaine self- administration and in tests of cue and cocaine prime-induced reinstatement (Whitfield et al., 2008). These data suggest that exogenous BDNF infused into the PFC binds surface-expressed Trk receptors, and that this BDNF-Trk interaction is necessary for BDNF’s suppressive effect on cocaine-seeking. Collectively, the reversal of the suppressive behavioral effects of intra-PFC BDNF with both the TrkB-Fc and K252a compounds demonstrates that BDNF’s effects are, in part, local to the PFC and depend on TrkB receptor activation.
7. BDNF suppresses cocaine-seeking by altering TrkB-mediated intracellular signaling in the prefrontal cortex
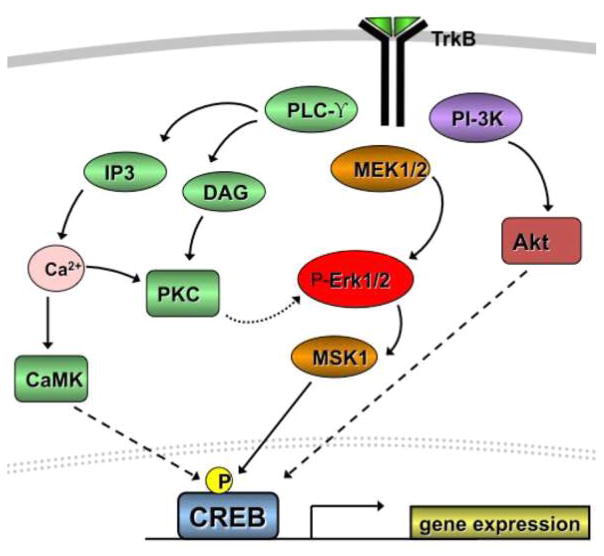
A critical point at which BDNF likely intersects with the effects of psychostimulants is at the level of intracellular signaling. Alterations in TrkB-mediated activation of several distinct intracellular signaling cascades, including the mitogen-activated protein kinase (MAPK), the phospholipase C-γ (PLC-γ), and phosphoinositol-3 kinase (PI3K) cascades, provide a means by which BDNF mediates persistent neuroplasticity (Patapoutian and Reichardt, 2001). These cascades converge on nuclear transcription factors such as CREB and ELK to regulate gene expression and induce neuroadaptations. Figure 8 illustrates the downstream signaling cascades of TrkB activation in neurons.
Figure 8.
Schematic of BDNF/TrkB signal transduction. Activation of the TrkB receptor initiates downstream changes in CREB-dependent gene transcription via PI-3K, ERK/MAPK, and PLC-γ signaling.
Most notably, activation of each of these cascades has been independently implicated in the CNS response to acute and chronic administration of psychostimulants, and in many cases, governs the expression of psychostimulant-related behaviors. For example, MAPK signaling in the ventral striatum is a necessary contributor to the expression of cocaine-mediated induction of immediate early genes, behavioral sensitization and reward (Girault et al., 2007; Valjent et al. 2000; 2004; 2005), as well as to the consolidation and reactivation of cocaine conditioned place preference (Miller & Marshall, 2005; Valjent et al., 2006). Moreover, VTA MAPK signaling is necessary for a BDNF-mediated inhibition of morphine or cocaine-induced tyrosine hydroxylase expression (Berhow et al., 1995; 1996) and enhancement of cocaine-seeking during abstinence (Lu et al., 2004). Additionally, it has been shown that acute administration of cocaine induces PLC-γ in the NAc and that anti-BDNF infusion blocks this effect (Graham et al., 2007). PFC and NAc PI3K signaling may also be involved, given that experimenter-administered cocaine induced PI3K in the NAc (Zhang et. al, 2006), and antagonism of PI3K with LY294002 intracerebroventricularly decreased cocaine-mediated behavioral sensitization (Izzo et al., 2002).
Although these and other studies have characterized the involvement of MAPK, PI3K, and PLC-γ signaling at the mesolimbic level, there is little information about the role of prefrontal cortical intracellular signaling in the expression of psychostimulant-mediated behaviors during self-administration and relapse. Therefore, we investigated the contribution of PFC MAPK and PI3K signaling to the expression of BDNF’s suppressive effect on cocaine-seeking behavior. Infusion of the MAPK/ERK kinase (MEK1/2) inhibitor, U0126, 20 minutes prior to intra-PFC BDNF blocked BDNF’s suppressive effect on cocaine-seeking following 6 days of abstinence and in tests of cue- and cocaine prime-induced reinstatement (Whitfield et al., in press). In contrast, infusion of the PI3K inhibitor, wortmannin, had no effect on BDNF’s suppression of cocaine-seeking during these tests. Interestingly, neither intra-PFC infusion of U0126 nor infusion of wortmannin followed by vehicle infusion uniquely altered cocaine-seeking behavior. These data indicate that the intracellular mechanisms by which intra-PFC BDNF suppresses cocaine-seeking depend on MAPK, but not PI3K, signaling in PFC neurons. In summary, these studies elucidate a mechanism whereby intra-PFC BDNF binds and activates TrkB receptors, which, in turn, predominantly activates MAPK, not PI3K, signaling in PFC neurons in order to normalize cocaine-mediated plasticity and suppress addictive cocaine-seeking behavior.
8. Summary
In summary, BDNF expression is associated with neuronal activity and synaptic plasticity. BDNF enhances synaptic transmission and promotes LTP by increasing dendritic protein synthesis and dendritic spine formation. BDNF also regulates drug-induced long-term neuroadaptations that encompass alterations in molecular components at the synapse, changes in gene expression, and modifications of behavioral output. The effects of BDNF on cocaine-seeking are brain region-specific. BDNF may have local effects at the site of infusion and distal effects in target areas that are critical to mediating or preventing cocaine-induced dysfunctional neuroadaptations.
Acknowledgments
Funded by P50 DA015369, RO1 DA03982, T32 DA07288, F31 DA023743, and F31 DA018500
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- CREB
cAmp response element binding protein
- DA
Dopamine
- ERK
extracellular-regulated kinase
- MAPK
mitogen activated protein kinase
- MEK
mitogen activated extracellular-regulated protein kinase
- GDNF
Glial cell line-derived neurotrophic factor
- NAc
Nucleus accumbens
- PFC
Prefrontal cortex
- PLCγ
Phospholipase C gamma
- PI3K
phosphoinositide 3-kinase
- Trk
tropomyosin receptor kinase
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci USA. 1992;8:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsay RM, Wiegand SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–437. doi: 10.1016/s0166-2236(98)01273-9. [DOI] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacol. 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, Lalumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine- induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DS, Lindsay RM, Nestler EJ. In uence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–979. doi: 10.1016/0306-4522(95)00207-y. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (Extracellular Signal Regulated Kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel M, Barde Y. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system. Physiol. 2005;20:10–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- Boger HA, Samuvel DJ, Saylor AJ, Bender TS, McGinty JF, Zaman V, Huang P, Middaugh LD, Jayanthi LD, Rohrer B, Granholm AC, Ramamoorthy S. Effects of partial genetic deletion of brain-derived neurotrophic factor on dopaminergic function and motor behavior in mice during aging. Genes, Brain & Behavior. doi: 10.1111/j.1601-183X.2010.00654.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. NeuroMolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Southard T, Sarvey JM, Herkenham M, Brady LS. Unilateral LTP triggers bilateral increases in hippocampal neurotrophin and trk receptor mRNA expression in behaving rats: evidence for interhemispheric communication. J Comp Neurol. 1996;368:371–382. doi: 10.1002/(SICI)1096-9861(19960506)368:3<371::AID-CNE4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley D, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Liu H, Dunning FM, Chang PY, Jackson MB, Chapman ER. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nature Neuroscience. 2009;12:767–776. doi: 10.1038/nn.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Gao X, Story GM, Anderson LI, Kucera J, Walro JM. Evaluation of nigrostriatal dopaminergic function in adult +/+ and +/− BDNF mutant mice. Exp Neurol. 2001;170:121–128. doi: 10.1006/exnr.2001.7698. [DOI] [PubMed] [Google Scholar]
- Du J, Feng L, Zaitsev E, Je HS, Liu XW, Lu B. Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ in ux. J Cell Biol. 2003;163:385–395. doi: 10.1083/jcb.200305134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. BDNF in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:94–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacol. 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Caffino L, Racagni G, Riva MA. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur Neuropsychopharmacol. 2009;19:402–408. doi: 10.1016/j.euroneuro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Goggi J, Pullar IA, Carney SL, Bradford HF. Signalling pathways involved in the short-term potentiation of dopamine release by BDNF. Brain Res. 2003;968:156–61. doi: 10.1016/s0006-8993(03)02234-0. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;9:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–97. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horch HW, Kruttgen A, Portbury SD, Katz LC. Destabilization of cortical BDNF dendrites and spines by BDNF. Neuron. 1999;23:353–364. doi: 10.1016/s0896-6273(00)80785-0. [DOI] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Izzo E, Martin-Fardon R, Koob GF, Weiss F, Sanna PP. Neural plasticity and addiction: PI3-kinase and cocaine behavioral sensitization. Nat Neurosci. 2002;5:1263–1264. doi: 10.1038/nn977. [DOI] [PubMed] [Google Scholar]
- Janak PH, Wolf FW, Heiberlein U, Pandey SC, Logrip ML, Ron D. BIG news in alcohol addiction: new findings on growth factor pathways BDNF, insulin, and GDNF. Alcoholism: Clinical Experimental Res. 2006;30:214–221. doi: 10.1111/j.1530-0277.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–329. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Renish L, Osredkar T, Walro JM, Kucera J, Dluzen DE. Methamphetamine-induced loss of striatal dopamine innervation in BDNF heterozygote mice does not further reduce D3 receptor concentrations. Synapse. 2004;52:11–19. doi: 10.1002/syn.10309. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, Williams RW, Miles MF. Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. J Neurosci. 2005;25:2255–2266. doi: 10.1523/JNEUROSCI.4372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Leslie KR, Nelson SB, Turrigiano GG. Postsynaptic depolarization scales quantal amplitude in cortical pyramidal neurons. J Neurosci. 2001;21:RC170–175. doi: 10.1523/JNEUROSCI.21-19-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–74. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ES, Dreyfus CF, Black IB, Plummer MR. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Chow A. Neurotrophins and hippocampal synaptic transmission and plasticity. J Neurosci Res. 1999;58:76–87. [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufmann MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-elicited cocaine craving. Am J Psychiat. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Altar CA. Spontaneous behaviours of rats are differentially affected by substantia nigra infusions of brain-derived neurotrophic factor and neurotrophin-3. Eur J Neurosci. 1996;8:1696–1706. doi: 10.1111/j.1460-9568.1996.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Todd KG, Altar CA. Brain-derived neurotrophic factor and neurotrophin-3 activate striatal dopamine and serotonin metabolism and related behaviors: interactions with amphetamine. J Neurosci. 1994;14:1262–1270. doi: 10.1523/JNEUROSCI.14-03-01262.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaoudi E, Bardsen K, Srebo B, Branham CR. Acute intra-hippocampal infusion of brain-derived neurotrophic factor induces lasting potentiation of synaptic transmission in the dentate gyrus. J Neurophysiol. 1998;79:469–499. doi: 10.1152/jn.1998.79.1.496. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Numakawa T, Adachi N, Yokomaku D, Yamagishi S, Takei N, Hatanaka H. Brain-derived neurotrophic factor enhances depolarization-evoked glutamate release in cultured cortical neurons. J Neurochem. 2001;79:22–530. doi: 10.1046/j.1471-4159.2001.00591.x. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough NNH, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D. RACK1 and brain-derived neurotrophic factor : a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–84. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Sato K, Sato S, Yamada N, Hayabara T. Time-dependent changes in neurotrophic factor mRNA expression after kindling and long-term potentiation in rats. Brain Res Bull. 1998;45:599–605. doi: 10.1016/s0361-9230(97)00459-0. [DOI] [PubMed] [Google Scholar]
- Naggapan G, Lu B. Activity-dependent modulation of the BDNF receptor TrkB: mechanisms and implications. Trends Neurosci. 2005;28:464–471. doi: 10.1016/j.tins.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine- related behaviors induced by methamphetamine. Neurosci. 2003;119:767–75. doi: 10.1016/s0306-4522(03)00099-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1845–1849. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacol. 2006;31:287–96. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–80. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Pu L, Liu QS, Poo MM. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat Neurosci. 2006;9:605–607. doi: 10.1038/nn1687. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–30. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Sadakata T, Mizoguchi A, Sato Y, Katoh-Semba R, Fukuda M, Mikoshiba K, Furuichi T. The secretory granule-associated protein CAPS2 regulates neurotrophin release and cell survival. J Neurosci. 2004;24:43–52. doi: 10.1523/JNEUROSCI.2528-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi S, Cappello S, Riccio M, Bergami M, Aicardi G, Schenk U, Matteoli M, Canossa M. Hippocampal neurons recycle BDNF for activity-dependent secretion and LTP maintenance. EMBO J. 2006;25:4372–80. doi: 10.1038/sj.emboj.7601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor AJ, McGinty JF. Amphetamine-induced locomotion and gene expression are altered in BDNF heterozygous mice. Genes, Brain and Behavior. 2008;7:906–914. doi: 10.1111/j.1601-183X.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–89. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Ghosh A. Molecular mechanisms underlying activity-dependent regulation of BDNF expression. J Neurobiol. 1999;41:127–134. [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerfield MT, Schweitgreiter R, Barde YA, Hoppe E. Down- regulation of the neurotrophin receptor TrkB following ligand binding. J Biol Chem. 2000;275:8982–8990. doi: 10.1074/jbc.275.12.8982. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Ann Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Tongiorgi E, Righi M, Cattaneo A. Activity dependent dendritic targeting of BDNF and TrkB mRNAs in hippocampal neurons. J Neurosci. 1997;17:9492–9505. doi: 10.1523/JNEUROSCI.17-24-09492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ. Increased central brain-derived neurotrophic factor activity could be a risk factor for substance abuse: Implications for treatment. Med Hypoth. 2007;68:410–414. doi: 10.1016/j.mehy.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–4258. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Poszzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–9. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–36. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–6. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting-A-Kee R, Walton CH, Hansen DM, Razava R, Clarke L, Bufalino MR, Allison DW, Steffensen SC, van der Kooy D. Ventral tegmental area BDNF induces an opiate-dependent like reward state in naïve rats. Science. 2009;324:1732–4. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Berglind WJ, Carnell A, See RRE, McGinty JF. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. Intra-dPFC infusion of K252a blocks the suppressive effect of BDNF on cocaine-seeking behavior. Program No. 561.13. 2008 Online. [Google Scholar]
- Whitfield TW, Gomez A, McGinty JF. 2009 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2009. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine relapse is ERK MAPK-dependent. In press. [Google Scholar]
- Zhang X, Mi J, Wetsel WC, Davidson C, Xiong X, Chen Q, Ellinwood EH, Lee TH. PI3 kinase is involved in cocaine behavioral sensitization and its reversal with brain area specificity. Biochem Biophys Res Commun. 2006;340:1144–50. doi: 10.1016/j.bbrc.2005.12.114. [DOI] [PubMed] [Google Scholar]