Abstract
It is unknown whether facilitation and inhibition of stimulus processing represent one or two mechanisms in auditory attention. We performed electrophysiological experiments in humans to address these two competing hypothesis. Participants performed an attention task under low or high memory load. Facilitation and inhibition were measured by recording electrophysiological responses to attended and ignored sounds and comparing them to responses to these same sounds when attention was considered to be equally distributed towards all sounds. We observed two late frontally distributed components: a negative one in response to attended sounds, and a positive one to ignored sounds. These two frontally distributed responses had distinct timing and scalp topographies and were differentially affected by memory load. Taken together these results provide evidence that attention-mediated top-down control reflects the activity of distinct facilitation and inhibition mechanisms.
Introduction
It is generally accepted that top-down signals are important for cognitive control enabling selective attention to environmental inputs. These top-down signals are thought to be at the origin of the task-dependent modulation of neural activity in sensory cortices leading to enhancement of processing of task-relevant information. Consistent with this hypothesis, auditory selective attention has been shown to modulate the processing of both relevant (Hillyard et al., 1973; Woldorff and Hillyard, 1991) and irrelevant stimuli (Bidet-Caulet et al., 2007; Donald, 1987; Michie et al., 1993; Schroger and Eimer, 1997). These effects have been observed at early sensory processing stages at multiple levels of the auditory system including primary (Bidet-Caulet et al., 2007) and secondary (e.g. Jancke et al., 1999; Pugh et al., 1996) auditory cortical areas, the brain stem (Lukas, 1980, 1981) and as peripherally as the cochlea (Giard et al., 1994 but see Michie et al., 1996); but also at later processing stages (e.g. representation in memory) (Giard et al., 2000; Näätänen, 1982, 1992). Despite this extensive body of research on auditory attention (reviewed in Giard et al., 2000), it is unknown whether enhanced vs. reduced sound processing by attention result from a unitary gain control mechanism which regulates activity either up or down along one continuum; or from the net activity of distinct top-down facilitation and inhibition mechanisms.
In the visual modality, working memory impairment in normal aging has been associated with a specific deficit in suppression of irrelevant stimulus processing -whereas enhancement of task-relevant activity was preserved- with both fMRI (Gazzaley et al., 2005) and electroencephalography, EEG (Gazzaley et al., 2008). These results suggest that reduction and enhancement in sensory processing of irrelevant and relevant visual stimuli, respectively, rely on distinct mechanisms. Moreover, an fMRI study in young adults observed that the extent to which distractors are inhibited can be determined by the availability of cognitive resources (de Fockert et al., 2001). Cognitive resources were manipulated in a dual task protocol where subjects performed, at the same time, an attention and a memory tasks. This visual study showed that the more difficult the memory task, the less cognitive resources are available to perform the attention task, and the less the distractors are inhibited. However, this work did not assess to what extent the availability of cognitive resources affects the processing of relevant information (de Fockert et al., 2001). These results suggest that, in the visual modality, attention-mediated facilitation and inhibition rely on distinct mechanisms that would be differentially affected by the amount of available cognitive resources, and thus the difficulty of a memory task in dual task protocol. More precisely, facilitation seems not to be affected by the memory task difficulty, whereas inhibition is most likely to decrease with increasing memory task difficulty.
In the current study, we tested whether attention-mediated facilitation and inhibition can operate independently at the late selection stages, by modulating the amount of available cognitive resources in an auditory selective attention task. We measured facilitation and inhibition by comparing the electrophysiological responses to the same sounds when they were attended or ignored, respectively, to the responses to these sounds in a control condition in which attention was considered to be equally distributed towards all sounds (Bidet-Caulet et al., 2007; Luck et al., 1994; Quinlan and Bailey, 1995; Schroger and Eimer, 1997). The availability of cognitive resources was modulated by varying the difficulty (or load) of a concurrent sound memorization task. The hypothesis was that if independent facilitation and inhibition mechanisms support auditory selective attention, these mechanisms should have different electrophysiological signatures, should not be correlated, and should be differentially affected by the memory task difficulty.
Materials and Methods
Subjects
Sixteen paid subjects (5 female, 1 left-handed, aged 18-30 years) participated in this experiment. All subjects were free from neurological or psychiatric disorder, and had normal hearing. All subjects gave written informed consent in accordance to our study protocol approved by the University of California, Berkeley Committees on Human Research.
Stimuli and Tasks
Subjects had to perform an attention and a memory tasks at the same time (dual task protocol).
In the attention task, subjects were randomly presented with successive monaural standard (50-ms duration) and duration deviant (100-ms duration) band-pass noises (5-semitone wide, 5 ms rise/fall times) in each ear, and binaural pure tone in both ears (carrier frequency 988 Hz, 50-ms duration). In one ear, the standard and deviant sounds were low-pitch noises (554-740 Hz). In the other ear, the standard and deviant sounds were high-pitch noises (1319-1760 Hz). The loudness of these noises was matched by previous subjective matching in 11 subjects. The sound pitches presented in each ear were balanced across blocks. In each block (about 25 s), 49 sounds were played: 20 standards and 3 deviants in each ear (41% and 6% probability in each ear, respectively), and 3 pure tones (6% probability). The inter-stimulus-interval (ISI) between 2 successive sound onsets varied between 300 and 500 ms. Subjects had to perform 3 different detection tasks. They either had to pay attention to the left (right) ear and press the right button of a joystick when they heard a duration deviant in the left (right) ear; or they had to press the right button when they heard a binaural sound (control condition). Thus, in the two first conditions, half of the standards were considered as attended (in the attended ear) and half were considered as ignored (in the unattended ear). In the control condition, all standards (in both right and left ears) were considered as “control” standards.
The memory task consisted in the memorization of a sequence of four 5-harmonic sounds (100-ms duration, 5 ms rise/fall times). Subjects were presented with this sequence, then performed the attention task, and finally were presented with a second sequence they had to compare to the first one. They had to keep the short sequence in memory while performing the attention task. To construct the sequences, 4 different sounds were used with the following fundamental frequencies: 1724, 4023, 5747, or 8046 Hz. When the memory task was easy the first sequence was the 4-time repetition of one of these sounds, and the second was either the same (left button press) or a sequence of the 4 different sounds (right button press). When the memory task was difficult the first sequence was a sequence of the 4 different sounds, and the second was either the same (left button press) or a sequence of the 4 different sounds in a different order (right button press). Three memory conditions were considered: no, easy or difficult memory task.
Procedure
Participants were seated in a sound-attenuated EEG recording room. Sounds were delivered using Presentation software (Neurobehavioral Systems, Albany, NY, USA), through earphones at an intensity level judged comfortable by the subjects (about 60 dB SPL). The experiment started with a familiarization with the sounds and tasks and participants were trained on the attention and memory tasks separately. EEG was then recorded while subjects performed 12 blocks of the attention task (4 in each attention condition) for each memory condition, resulting in a total of 160 attended standards, 160 ignored standards and 160 standards in the control condition. The blocks were run by memory condition, the order of memory conditions was balanced across subjects. The order of the 12 attention blocks was the same for each memory condition, and was balanced across participants using a Latin-square design. During all the experiment, subjects were instructed to perform as well and as fast as possible, and to favor accuracy in the memory task if it was difficult to perform both tasks correctly. They were also asked to keep their eyes fixated on a centrally presented cross and to minimize any eye movements and blinks while performing the tasks.
Statistical Analysis of Behavioral data
In the attention task, a button press within the interval of 200-1000 ms after target onset was considered a correct response, and a press at any other time was counted as a false alarm. Reaction times, percentage of correct responses and number of false alarms were averaged across attention conditions for each memory condition, separately. The effect of the memory task difficulty on these measures was assessed using a repeated-measure one-way analysis of variance (ANOVA) with memory difficulty (3 levels: no, easy, difficult) as within-subject factor. When necessary, ANOVA results were corrected with the Greenhouse-Geisser procedure (epsilon and corrected P are reported). Significant effects were explored using 2-tailed paired t-tests, we used the Bonferroni correction to correct the P-value for multiple comparison.
EEG recording
EEG data were recorded from 64 electrodes using the ActiveTwo system (BioSemi, the Netherlands). Vertical and horizontal eye movements were recorded from electrodes placed at both external canthi and below the left eye. Data were amplified (-3dB at ∼819 Hz low-pass, DC coupled), digitized (1024 Hz), and stored for offline analysis. Data were referenced offline to the average potential of two earlobe electrodes.
EEG data analysis
Trials contaminated with eye movements, eye blinks or excessive muscular activity were excluded from further analysis. Trials corresponding to standards after a target, before or after a button press were also excluded. In seven subjects, the flat or excessively noisy signals at one or two electrodes were replaced by their values interpolated from the remaining adjacent electrodes. Averaging, locked to standard onset, was done separately for each attention condition (attended, ignored and control) in each memory condition (no, easy, difficult memory task). At least 108 trials were averaged for each participant, for each condition. With this procedure, the average acoustic content of the sounds was the same for all obtained event-related potentials (ERPs), only the attention orientation and the memory task difficulty varied. ERPs were corrected with a -100 to 0 ms baseline before standard onset, and were digitally filtered (low-pass 35 Hz). Since the shortest ISI was 300 ms, only the -100 to 300 ms time-window was retained for further analysis. ERP scalp topographies were computed using spherical spline interpolation (Perrin et al., 1989; Perrin et al., 1987).
Statistical Analysis of ERPs to standards
To compare ERPs to attended and ignored standards, we conducted a permutation test on ERP mean amplitude in successive 10-ms time-windows at each electrode between all attended and ignored standards (collapsing memory conditions) with correction for multiple tests (see Supplementary Methods and Supplementary Fig. 1).
Second, we performed a two-way repeated-measure ANOVA with memory difficulty (3 levels: no, easy, difficult) and attention condition (3 levels: attended, ignored, control) as within-subjects factors, on 1 fronto-central group of electrodes (Fz, F1, F2, FCz, FC1 and FC2), in 3 successive 50-ms time-windows (150-200, 200-250 and 250-300 ms). The selection of electrodes and time-windows of interest was based on results in previous EEG studies on auditory selective attention and on the permutation test results in the present study. Significant effects were explored using post-hoc permutation tests.
We assessed topography differences on the difference between ERPs to attended and control standards, and the difference between ERPs to control and ignored standards (collapsing memory conditions). To avoid any bias from amplitude effect, these difference values were first normalized for each subject using a division by the norm of the vector in electrode space (McCarthy and Wood, 1985). We then used two different methods to assess topographical differences. First, we performed a two-way repeated-measure ANOVA with attention effect (2 levels: “attended – control” and “control – ignored”) and electrode group (2 levels: anterior frontal and posterior frontal) as within-subjects factors, on the 250-300 ms time-window. The anterior frontal group included Fz, F1 and F2 electrodes, and the posterior frontal, FCz, FC1 and FC2 electrodes. The second method consisted in computing the center of mass of components. In physics, the center of mass of a system of particles is the point at which the system's whole mass can be considered to be concentrated, and is a function only of the positions and masses of the particles that compose the system. Applied to ERPs, ERP amplitudes at each electrode are considered as the masses, and the electrode coordinates as the positions of the particles (Manjarrez et al., 2007). We computed the center of mass for the “attended – control” and “control – ignored” effects, from the mean ERP value in the 250-300 ms time-window from 21 frontal electrodes (Fpz, AFz, Fz, FCz, Cz, F1, FC1, C1, Fp1, AF3, F3, FC3, C3, F2, FC2, C2, Fp2, AF4, F4, FC4, C4) in each subject. We used a repeated-measure ANOVA with attention (2 levels: “attended – control” and “control – ignored”) and coordinates (3 levels: x, y and z) as within-subjects factors, to compare the coordinates of the centers of mass. Significant effects were explored using post-hoc permutation tests.
All data analyses were performed with ELAN-Pack software developed at INSERM U821 (Lyon, France).
Results
We used a dual task protocol to orthogonally manipulate attention and cognitive resources. For the attention task, we adapted a classic auditory attention protocol by adding a third condition (control condition) in which attention was considered as equally distributed to all sounds. We measured with electroencephalography (EEG) the effects of three distinct levels of attention by comparing the event-related potentials (ERPs) to the same sounds when they were attended (in the attended ear), ignored (in the opposite, non-attended ear) or during a control condition. The availability of cognitive resources was modulated by varying the difficulty of a concurrent sound memorization task (3 difficulty levels: no, easy or difficult memory task). Our hypothesis was that if attention-mediated facilitation and inhibition are distinct mechanisms, they would be differentially affected by the difficulty of the memory task.
Behavioral data
Participants performed better in the easy (99.0% of correct responses) than in the difficult (82.1%) memory task (t16 = 4.65, P = 0.0003). These results indicate that manipulation of the memory load was effective.
We observed a significant effect of memory task difficulty on performance in the attention task, both in terms of percentage of correct responses (F2,30 = 6.0, ε = 0.781, P = 0.012) and reaction times (F2,30 = 4.5, ε = 0.932, P = 0.023), but not in the number of false alarms (F2,30 = 2.7, ε = 0.771, P = 0.098) in the attention task (Table 1). Post-hoc t-tests showed that the percentage of correct responses was lower during the difficult than during the easy memory task (t16 = 3.00, P = 0.027) or when there was no memory task (t16 = 2.88, P = 0.033). Subjects were also faster to detect the targets when there was no memory task than when the memory task was difficult (t16 = 3.06, P = 0.024). These results indicate that the higher the memory load, the worse the attention performance.
Table 1. Effect of the memory task difficulty on the attention task performances.
Mean percentage of correct responses, mean number of false alarms and mean reaction time (and their standard error to mean, s.e.m.) in the attention task are indicated as a function of the memory task difficulty.
| Memory task | ||||
|---|---|---|---|---|
| No | Easy | Difficult | ||
| Attention task | Correct response % | 93.40 ±1.35 | 91.60 ±1.71 | 88.50 ±2.3 |
| Number of false alarms | 0.50 ±0.13 | 0.62 ±0.18 | 1.19 ±0.31 | |
| Reaction time (ms) | 422 ±17 | 444 ±22 | 448 ±21 | |
Scalp EEG - Standards: Main attention effect (Attended vs. Ignored)
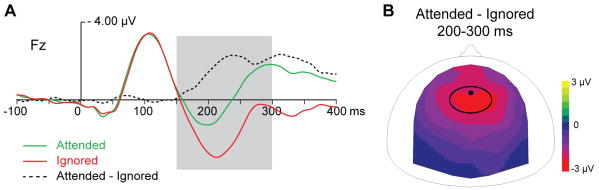
Previous studies investigating auditory selective attention compared ERPs to attended and ignored (unattended) standard sounds and found a negative frontally distributed activity (called Nd) starting around 100-150 ms (reviewed in Giard et al., 2000). We confirmed these results by performing an analysis of our data using permutation tests over all 64 electrodes and 10-ms time-windows between 0 and 300 ms (with correction for multiple comparisons), comparing ERPs to attended and ignored standards, independently of the memory conditions. We found that ERPs to attended and ignored standards begin to differ around 150 ms (Fig. 1a and Supplementary Fig. 1 online) and that this difference is reflected in a negative frontally distributed component maximal over fronto-central electrodes (Fig. 1b).
Figure 1. Main attention effect on ERPs to standards.
(A) Mean ERPs at Fz electrode. ERPs to attended and ignored standards are depicted in green and red, respectively. The difference between ERPs to attended and ignored standards is represented by a dashed black line; the shaded area corresponds to the 150-300 ms period, used for further analysis, when this difference is significant (see Supplementary Fig. 1). (B) Scalp topography (top view) of the mean difference between ERPs to attended and ignored standards (200-300 ms). The black dot indicates the position of the Fz electrode and the black oval surrounds the fronto-central group of electrodes used for further analysis of ERPs to standards.
Following these and previous authors, we focused our analysis of ERPs to standard stimuli on a fronto-central group of electrodes (Fz, F1, F2, FCz, FC1 and FC2) and on 3 successive 50-ms time-windows between 150 and 300 ms.
Scalp EEG - Standards: influence of the memory task difficulty on attention effects
We examined the ERPs to attended, control and ignored standards across three conditions of no, easy or difficult memory task (Supplementary Fig. 2 online) and performed a two-way ANOVA on ERP mean amplitude, with memory difficulty (no, low and high) and attention condition (attended, control, ignored) as factors. On the three 50-ms time-windows, we found a significant main effect of attention, but not of the memory task difficulty (see Table 2). We also found a significant interaction between attention condition and the memory task difficulty between 200 and 250 ms, but not for the other time-windows. To assess whether these results are independent of the control condition, we performed the same statistical analysis excluding the control condition: a two-way ANOVA on ERP mean amplitude, with memory difficulty (no, low and high) and attention condition (attended and ignored) as factors. We obtained similar results with and without factoring in the control condition (see Table 2).
Table 2. Effect of the memory task difficulty and attention conditions on the ERP amplitude.
Results of the two-way ANOVA on ERP mean amplitude, with memory difficulty (no, low and high) and attention condition as factors, for the three tested timewindows. Statistical values (F, ε and P) of attention and memory difficulty main effects and of attention by memory interaction effect are indicated with the control condition included (attention condition factor with 3 levels: attended, control and ignored) and with the control condition excluded (attention condition factor with 2 levels: attended and ignored). Significant effects are highlighted in grey.
| 150-200ms | 200-250ms | 250-300ms | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | ε | P | F | ε | P | F | ε | P | ||
| Control condition included | attention | 13.2 | 0.996 | <0.001 | 38.6 | 0.893 | <0.001 | 40.2 | 0.934 | <0.001 |
| memory difficulty | 0.1 | 0.987 | 0.865 | 0.9 | 0.978 | 0.387 | 1.9 | 1.000 | 0.165 | |
| attention x memory | 1.7 | 0.984 | 0.171 | 2.7 | 0.855 | 0.048 | 1.4 | 0.933 | 0.246 | |
| Control condition excluded | attention | 22.6 | 1.000 | <0.001 | 49.1 | 1.000 | <0.001 | 66.8 | 1.000 | <0.001 |
| memory difficulty | 0.5 | 0.963 | 0.609 | 1.7 | 0.984 | 0.203 | 1.5 | 0.953 | 0.247 | |
| attention x memory | 2.9 | 0.963 | 0.072 | 3.2 | 0.984 | 0.050 | 2.2 | 0.953 | 0.128 | |
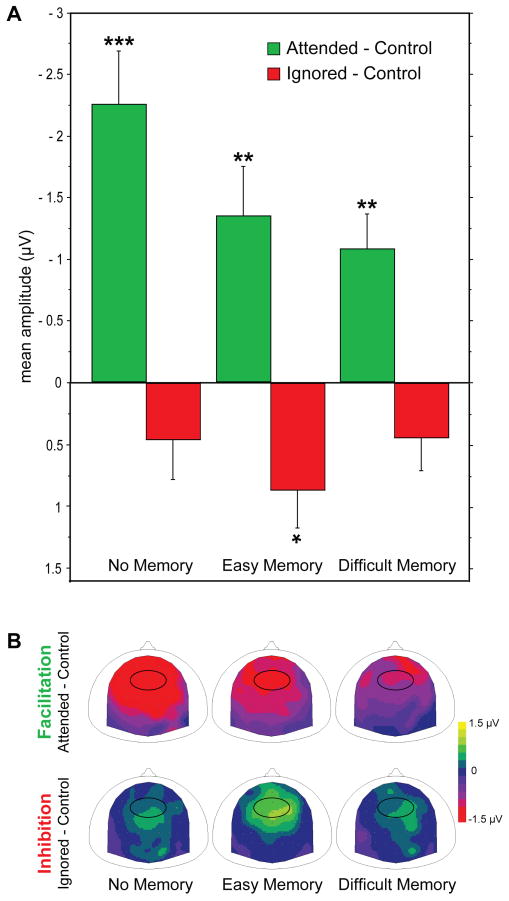
To further investigate the effect of the memory task on attention modulations between 200 and 250 ms, we assessed, for each memory difficulty, the amplitude of the facilitatory (ERP difference between attended and control standards) and inhibitory (ERP difference between ignored and control standards) attention effects (Fig. 2). We found that amplitudes of ERPs to attended and control standards were significantly different in all memory conditions (P < 0.004), whereas the amplitudes of ERPs to ignored and control standards significantly differed in the easy memory task only (P = 0.018). These results indicate that the memory task difficulty differentially affects facilitation and inhibition mechanisms.
Figure 2. Effect of the memory task difficulty on attention effects.
(A) Mean ERP amplitudes (fronto-central group, 200-250 ms) of attention-mediated facilitation (green) and inhibition (red) effects as a function of the memory task difficulty (no, easy, difficult). Facilitation and inhibition effects are represented as the mean difference between ERPs to attended and control, and to ignored and control standards, respectively. Error bars represent 1 s.e.m. Stars indicate significant differences assessed by permutation post-hoc tests of the interaction (attention by memory) effect (*: P < 0.05; **: P < 0.01; ***: P < 0.001). (B) Scalp topographies (top view) of the attention effects (200-250 ms): facilitation (mean difference between ERPs to attended and control standards) and inhibition (mean difference between ERPs to ignored and control standards). The black oval surrounds the fronto-central electrode group used for computation of mean amplitudes and statistical analysis represented in A.
Scalp EEG - Standards: timing of attention facilitation and inhibition
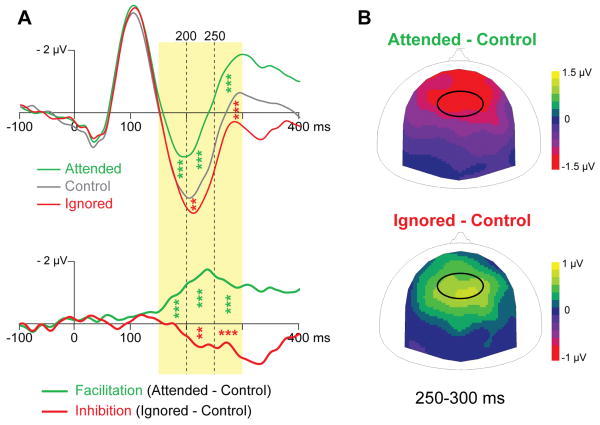
We assessed the timing and amplitude of the facilitatory and inhibitory attention effects, independent of memory load. ERP amplitudes to attended and control standards were different between 150 and 200 ms, (P = 0.001), between 200 and 250 ms (P = 0.0001), and between 250 and 300 ms (P = 0.0001). Amplitudes of ERPs to ignored and control standards were not different between 150 and 200 ms (P = 0.85), but were significantly different between 200 and 250 ms (P = 0.002) and between 250 and 300 ms (P = 0.0003). These results, in combination with the ones observed in the attention by memory interaction, suggest that facilitation and inhibition mechanisms have different timing: facilitation starts as early as 150 ms after stimulus onset in all memory conditions, whereas inhibition begins around 200 ms in the easy memory condition and not before 250 ms in the other memory conditions (Fig. 3a).
Figure 3. Timing and topography of attention-mediated facilitation and inhibition.
(A) Mean ERPs at the fronto-central electrode group to attended, control and ignored standards are depicted in green, grey and red, respectively. The differences between ERPs to attended and control (facilitation), and to ignored and control (inhibition) standards are represented below by green and red lines respectively. Shaded areas correspond to the 50-ms windows, in the 150-300 ms period, used for statistical analysis. Stars indicate significant differences assessed by permutation post-hoc tests of the main attention effect (**: P < 0.01; ***: P < 0.001). (B) Scalp topographies (top view) of facilitation (mean difference between ERPs to attended and control standards) and inhibition (mean difference between ERPs to ignored and control standards) between 250 and 300 ms. The black oval surrounds the fronto-central electrode group used for ERP computation and statistical analysis represented in A.
Scalp EEG - Standards: topographies of attention facilitation and inhibition
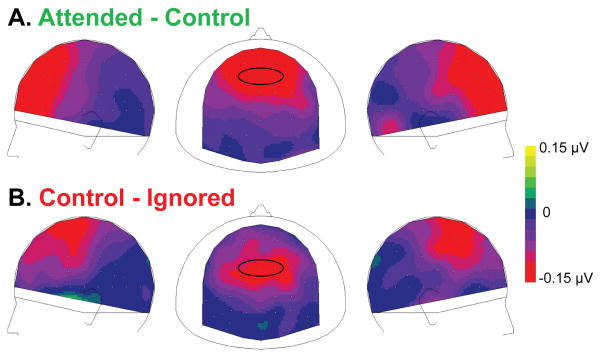
We also observed that the inhibitory component had a more posterior scalp distribution than the facilitatory component (Fig. 2 and 3b). Since facilitatory and inhibitory components were both found to be active between 250 and 300 ms, we used this time-window to test if the topographies of these two components were different.
We first performed a two-way ANOVA on normalized ERP mean amplitude (averaging across memory conditions), with electrode group (anterior or posterior frontal) and attention effect (“attended – control” and “control – ignored”) as factors (Fig. 4). We found a significant interaction between electrode groups and attention effects (F1,15 = 15.3, ε = 1.000, P = 0.001), suggesting that facilitation and inhibition mechanisms have distinct topographies.
Figure 4. Comparison of facilitation and inhibition topographies.
Scalp topographies (left, top and right views) of the normalized mean attention effects (250-300 ms). A. Mean difference between normalized ERPs to attended and control standards. B. Mean difference between normalized ERPs to control and ignored standards. The black ovals surround the anterior and posterior frontal groups of electrodes used for statistical analysis of the topography differences.
Second, we computed the center of mass for “attended – control” and “control – ignored” effects, from the mean ERP value in the 250-300 ms time-window from 21 frontal electrodes. We obtained the mean coordinates X=0.55, Y=10.16, Z=11.00 for the facilitatory component, and X=0.24, Y=2.70, Z=3.51 for the inhibitory component. Using a two-way ANOVA with attention (2 levels: “attended – control” and “control – ignored”) and coordinates (3 levels: X, Y and Z) as factors, we found a significant effect of attention (P = 0.050), of coordinates (P = 0.008), and attention by coordinates interaction (P = 0.044). The centers of mass of the facilitatory and inhibitory components were found to be different in their Y coordinates (P < 0.05), but not in their X or Z coordinates (P > 0.26), suggesting that the topography of the facilitatory component is more anterior than the topography of the inhibitory one.
It is noteworthy that these results are independent of the control condition since this condition is subtracted to extract both the facilitatory (attended - control) and the inhibitory (control - ignored) components, and the remaining difference can only be attributed to a difference between the ERPs to attended and ignored sounds. In this analysis, the control condition is only used to eliminate the overlapping P2 response.
Discussion
We found two frontally distributed components; a negative one in response to attended standard sounds (facilitatory component), and a positive one to ignored standard sounds (inhibitory component). These frontal electrophysiological responses have distinct timing and topographies, and are differentially modulated by the difficulty of the memory task. These results provide evidence that auditory attention is enabled by distinct facilitation and inhibition mechanisms.
We first observed a negative frontally distributed ERP component onsetting at about 150 ms that differentiated attended and ignored standard sounds. This response probably corresponds to components of the Nd or PN (“Processing Negativity”) described in several previous studies (reviewed in Giard et al., 2000). This component is felt to index late selective attention mechanisms, involved in controlling and maintaining the representation of stimuli according to their behavioral relevance (Giard et al., 2000; Näätänen, 1982, 1992) and can be elicited without being preceded by N1 enhancement (Näätänen et al., 1978), as was observed in the present experiment. Indeed, we observed no difference in ERPs to attended and ignored sounds during the first 150 ms, probably until sufficient information is processed in order to decide whether the sound belongs to the task-relevant or to the task-irrelevant ear, in agreement with the findings and theory of Näätänen and colleagues (Näätänen, 1982, 1992).
To dissociate facilitatory and inhibitory components, we used a control condition in which the participants had to detect binaural pure tones. We acknowledge that the perfect control condition is elusive but we considered the current choice better than a passive task (what is the subject actually doing?) or a visual task (inter-modal attention is then involved). Furthermore, this control task has already been shown to be valuable in understanding the mechanisms of auditory selective attention in intracranial recordings (Bidet-Caulet et al., 2007). We assume that, in this control condition, participants' auditory attention was equally distributed towards all monaural standard sounds. Indeed, to detect these binaural pure tones, they had to pay attention to the auditory modality, but they did not need to actively ignore the other sounds since the pure tones were quite salient and the task was easy. The control condition did not require selective attention, but necessitated broad auditory attention towards all sounds to be correctly performed. One can argue that the control task we used actually required the inhibition of the standard monaural noises. In this case, we might be underestimating the inhibitory component. More importantly, to further address the issue of the control condition, we reanalyzed the data independently of the control condition. This analysis did not affect the effect of the memory difficulty manipulation: processing of attended and ignored sounds are differentially affected by the memory difficulty. Moreover, the topographical differences are independent of the control condition since the control condition is subtracted to extract both the facilitatory and inhibitory components.
Using this control condition, we found that the Nd response can be dissociated into two distinct components: (i) a negative ERP component in response to attended standards, with onset at about 150 ms, with an anterior frontal scalp distribution; and (ii) a positive ERP component in response to ignored standards, with onset between 200 and 250 ms, with a fronto-central scalp distribution. These findings are consistent with results from several previous scalp EEG studies dissociating the Nd component into two facilitatory and inhibitory sub-components, using control conditions in the auditory modality (Donald, 1987; Melara et al., 2002; Schroger and Eimer, 1997) or in the visual modality (Alho et al., 1987; Alho et al., 1994; Berman et al., 1989; Degerman et al., 2008; Michie et al., 1990; Michie et al., 1993). These researches found a positive response or “rejection positivity” to unattended sounds compared to the control condition, starting later in latency than the negative response to attended sounds. It has been suggested in some of this previous work that the topographies of facilitatory and inhibitory components are different (Degerman et al., 2008; Donald, 1987; Melara et al., 2002). In the current paper, these two components are directly compared and dissociated. The distinct scalp topographies provide support that different brain sources support the facilitatory and inhibititory components. However, we cannot precisely infer the brain origin of these components from the present data. These components most likely reflect neural activity from the auditory cortices in the superior temporal lobes and/or from frontal areas, as it has been suggested for the Nd components (Alcaini et al., 1995; Degerman et al., 2008; Giard et al., 1988; Woldorff et al., 1993).
To test if these facilitatory and inhibitory components correspond to two functionally distinct mechanisms or are generated by a single control mechanism, we manipulated the availability of cognitive resources. The hypothesis was that the control of facilitation and inhibition mechanisms requires cognitive resources, and that if these two mechanisms are independently controlled they should not covary according to the amount of available cognitive resources. It has been shown, previously, that increasing the load on executive functions, such as increasing memory, decreases the availability of cognitive resources to perform other cognitive task, such as an attention task (Lavie, 2005). We manipulated the availability of cognitive resources by varying the difficulty (or load) of a concurrent sound memorization task. We found that facilitation and inhibition mechanisms in auditory selective attention are differentially modulated by the memory difficulty, providing evidence for distinct functional roles, as reported in the visual modality (Gazzaley et al., 2008; Gazzaley et al., 2005). More precisely, we found that the availability of cognitive resources differentially influenced the timing of attention-mediated facilitation and inhibition mechanisms: facilitation starts at the same latency (150 ms) in all memory loads, whereas inhibition is activated at 200 ms for low memory load (easy memory task), and after 250 ms for no and high (difficult memory task) memory loads.
In a previous visual study employing fMRI, brain activation by distracting sounds was found to be larger under high rather than low memory load, suggesting a reduction of inhibition mechanisms under high memory load (de Fockert et al., 2001). Accordingly, our findings show that the inhibition mechanism is delayed from low to high memory load conditions. Thus, the less cognitive resources are available, the later the inhibition mechanisms are activated and the more distractors are processed. We did not observe inhibition before 250 ms in the no memory condition likely because of the ease of the attention task. These results extend the cognitive load theory (Lavie, 2005) to the auditory modality, but importantly, we have also shown using the time resolution of electrophysiology, that the availability of cognitive resources influences late selection processes (after the first steps of the sensory analysis) which control access to memory and response. When cognitive resources are available, distractor inhibition can be activated early (as early as 200 ms). Late attention-mediated inhibition mechanisms also seem to be influenced by the task difficulty: they are delayed when the task is easy even if the cognitive resources are available.
The present study provides new insights on the brain mechanisms of selective attention: late selection of the relevant stream of stimuli relies on the engagement of distinct attention-mediated facilitation and inhibition mechanisms. Sustained facilitatory and inhibitory frontally distributed components represent distinct cognitive processing of the attended and ignored streams of sounds, enhancing the rapid and accurate detection of targets without interference by distracting stimuli. These findings provide evidence that, at a late selection stage, attention operates by employing distinct facilitation and inhibition mechanisms.
Supplementary Material
Acknowledgments
We thank O. Bertrand, A. Caclin, J. Besle and D. Morlet for helpful advice on the experimental design and statistical analysis, and D. Scabini for comments on an earlier version of the manuscript. This work was supported by the Fondation pour la Recherche Medicale (ABC), the Otto-Von-Guericke-Universität Magdeburg (CM) and NINDS Grants NS21135 and PO40813.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcaini M, Giard MH, Echallier JF, Pernier J. Selective auditory attention effects in tonotopically organized cortical areas: a topographic ERP study. Human Brain Mapping. 1995;2:159–169. [Google Scholar]
- Alho K, Tottola K, Reinikainen K, Sams M, Näätänen R. Brain mechanism of selective listening reflected by event-related potentials. Electroencephalography and Clinical Neurophysiology. 1987;68:458–470. doi: 10.1016/0168-5597(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Alho K, Woods DL, Algazi A. Processing of auditory stimuli during auditory and visual attention as revealed by event-related potentials. Psychophysiology. 1994;31:469–479. doi: 10.1111/j.1469-8986.1994.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Berman SM, Heilweil R, Ritter W, Rosen J. Channel probability and Nd: an event-related potential sign of attention strategies. Biol Psychol. 1989;29:107–124. doi: 10.1016/0301-0511(89)90033-1. [DOI] [PubMed] [Google Scholar]
- Bidet-Caulet A, Fischer C, Besle J, Aguera PE, Giard MH, Bertrand O. Effects of selective attention on the electrophysiological representation of concurrent sounds in the human auditory cortex. J Neurosci. 2007;27:9252–9261. doi: 10.1523/JNEUROSCI.1402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Degerman A, Rinne T, Sarkka AK, Salmi J, Alho K. Selective attention to sound location or pitch studied with event-related brain potentials and magnetic fields. Eur J Neurosci. 2008;27:3329–3341. doi: 10.1111/j.1460-9568.2008.06286.x. [DOI] [PubMed] [Google Scholar]
- Donald MW. The timing and polarity of different attention-related ERP changes inside and outside of the attentional focus. Electroencephalogr Clin Neurophysiol Suppl. 1987;40:81–86. [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105:13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Giard MH, Collet L, Bouchet P, Pernier J. Auditory selective attention in the human cochlea. Brain Res. 1994;633:353–356. doi: 10.1016/0006-8993(94)91561-x. [DOI] [PubMed] [Google Scholar]
- Giard MH, Fort A, Mouchetant-Rostaing Y, Pernier J. Neurophysiological mechanisms of auditory selective attention in humans. Front Biosci. 2000;5:D84–94. doi: 10.2741/giard. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Peronnet F. Several attention-related wave forms in auditory areas: a topographic study. Electroencephalography and Clinical Neurophysiology. 1988;69:371–384. doi: 10.1016/0013-4694(88)90008-9. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Jancke L, Mirzazade S, Shah NJ. Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci Lett. 1999;266:125–128. doi: 10.1016/s0304-3940(99)00288-8. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Lukas JH. Human auditory attention: the olivocochlear bundle may function as a peripheral filter. Psychophysiology. 1980;17:444–452. doi: 10.1111/j.1469-8986.1980.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Lukas JH. The role of efferent inhibition in human auditory attention: an examination of the auditory brainstem potentials. Int J Neurosci. 1981;12:137–145. doi: 10.3109/00207458108985796. [DOI] [PubMed] [Google Scholar]
- Manjarrez E, Vazquez M, Flores A. Computing the center of mass for traveling alpha waves in the human brain. Brain Res. 2007;1145:239–247. doi: 10.1016/j.brainres.2007.01.114. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Melara RD, Rao A, Tong Y. The duality of selection: excitatory and inhibitory processes in auditory selective attention. J Exp Psychol Hum Percept Perform. 2002;28:279–306. [PubMed] [Google Scholar]
- Michie PT, Bearpark HM, Crawford JM, Glue LC. The nature of selective attention effects on auditory event-related potentials. Biol Psychol. 1990;30:219–250. doi: 10.1016/0301-0511(90)90141-i. [DOI] [PubMed] [Google Scholar]
- Michie PT, LePage EL, Solowij N, Haller M, Terry L. Evoked otoacoustic emissions and auditory selective attention. Hear Res. 1996;98:54–67. doi: 10.1016/0378-5955(96)00059-7. [DOI] [PubMed] [Google Scholar]
- Michie PT, Solowij N, Crawford JM, Glue LC. The effects of between-source discriminability on attended and unattended auditory ERPs. Psychophysiology. 1993;30:205–220. doi: 10.1111/j.1469-8986.1993.tb01733.x. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Processing negativity: an evoked-potential reflection of selective attention. Psychol Bull. 1982;92:605–640. doi: 10.1037/0033-2909.92.3.605. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Attention and Brain Function. Lawrence Erlbaum Associates; Hillsdale, NJ: 1992. [Google Scholar]
- Näätänen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol (Amst) 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Giard MH, Echallier JF. Mapping of scalp potentials by surface spline interpolation. Electroencephalography and Clinical Neurophysiology. 1987;66:75–81. doi: 10.1016/0013-4694(87)90141-6. [DOI] [PubMed] [Google Scholar]
- Pugh KR, offywitz BA, Shaywitz SE, Fulbright RK, Byrd D, Skudlarski P, Shankweiler DP, Katz L, Constable RT, Fletcher J, Lacadie C, Marchione K, Gore JC. Auditory selective attention: an fMRI investigation. Neuroimage. 1996;4:159–173. doi: 10.1006/nimg.1996.0067. [DOI] [PubMed] [Google Scholar]
- Quinlan PT, Bailey PJ. An examination of attentional control in the auditory modality: further evidence for auditory orienting. Percept Psychophys. 1995;57:614–628. doi: 10.3758/bf03213267. [DOI] [PubMed] [Google Scholar]
- Schroger E, Eimer M. Endogenous Covert Spatial Orienting in Audition: “Cost-Benefit” Analyses of Reaction Times and Event-Related Potentials. The Quaterly Journal of Experimental Psychology. 1997;50A:457–474. [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, Bloom FE. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc Natl Acad Sci U S A. 1993;90:8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG, Hillyard SA. Modulation of early auditory processing during selective listening to rapidly presented tones. Electroencephalogr Clin Neurophysiol. 1991;79:170–191. doi: 10.1016/0013-4694(91)90136-r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.