Abstract
Stress-like symptoms are an integral part of acute and protracted drug withdrawal, and several lines of evidence have shown that dysregulation of brain stress systems, including the extrahypothalamic corticotropin-releasing factor (CRF) system, following long-term drug use is of major importance in maintaining drug and alcohol addiction. Recently, two other neuropeptide systems have attracted interest, the nociceptin/orphanin FQ (N/OFQ) and orexin/hypocretin (Orx/Hcrt) systems. N/OFQ participates in a wide range of physiological responses, and the hypothalamic Orx/Hcrt system helps regulate several physiological processes, including feeding, energy metabolism, and arousal. Moreover, these two systems have been suggested to participate in psychiatric disorders, including anxiety and drug addiction. Dysregulation of these systems by chronic drug exposure has been hypothesized to play a role in the maintenance of addiction and dependence. Recent evidence demonstrated that interactions between CRF-N/OFQ and CRF-Orx/Hcrt systems may be functionally relevant for the control of stress-related addictive behavior. The present review discusses recent findings that support the hypotheses of the participation and dysregulation of these systems in drug addiction and evaluates the current understanding of interactions among these stress-regulatory peptides.
Keywords: corticotropin-releasing factor, orexin/hypocretin, nociceptin/orphanin FQ, addiction, reinstatement, relapse, allostasis, stress, anxiety
1. Introduction
Stress has an established role in the initiation and maintenance of drug abuse and is a major determinant of relapse in abstinent individuals (Brown et al., 1995; Marlatt, 1985; McKay et al., 1995; Sinha, 2001; Wallace, 1989). The significance of stress in drug-seeking behavior and reinforcement has been extensively studied and documented in the animal literature. Stressors can facilitate the acquisition of drug self-administration and increase drug intake in animals (e.g., Sarnyai et al., 2001). Stress also elicits the recovery of extinguished drug- and alcohol-seeking behavior (Le and Shaham, 2002; Shaham et al., 2000; Shaham et al., 2003) in reinstatement models of relapse and enhances the effects of drug cue exposure on reinstatement (Liu and Weiss, 2002). Moreover, the motivating effects of stress are persistent and can precipitate “relapse” despite many weeks of abstinence (Shalev et al., 2001).
In addition to the direct role of stress in drug seeking and consumption, dysregulation of brain stress systems following long-term drug use is important in maintaining drug and alcohol addiction. Chronic drug use produces neuroadaptive changes, both in systems mediating the acute reinforcing effects of drugs of abuse and other neural systems, notably brain systems that regulate behavioral and emotional responses to stress. These latter changes, originally referred to as “between-system” neuroadaptations (Koob and Le Moal, 1997), are thought to represent excitatory adaptive responses to chronic drug or alcohol exposure. These changes are hypothesized to oppose the acute reinforcing and hedonic effects of drug or alcohol exposure, leading to anxiety, mood disturbances, and adverse somatic symptoms that accompany acute withdrawal. Moreover, drug-induced neuroadaptations are hypothesized to be responsible for the persistence of these symptoms, albeit at reduced intensity, during protracted withdrawal (Meyer, 1996). This neuroadaptive activation of brain stress systems has been implicated as a mechanism responsible for the development of an allostatic state underlying compulsive drug taking, loss of control over drug intake, and vulnerability to relapse (e.g, Koob and Le Moal, 2001).
Research aimed at understanding the neural basis of the stress-addiction link has focused predominantly on corticotropin-releasing factor (CRF), a major molecule regulating stress and arousal in the mammalian central nervous system. In addition to the CRF system, two other neuropeptide systems with a role in stress and arousal have received growing attention with respect to their role in drug and alcohol addiction: the nociceptin/orphanin FQ (N/OFQ) opioid-like peptide system and the orexin/hypocretin (Orx/Hcrt) system (e.g., Boutrel et al., 2005; Ciccocioppo et al., 1999b; Ciccocioppo et al., 2000; Harris et al., 2005). These systems have attracted interest because of emerging evidence demonstrating interactions between CRF and N/OFQ and Orx/Hcrt systems that may be functionally relevant for the control of stress-related addictive behavior.
This paper reviews the literature on the respective roles of CRF, N/OFQ, and Orx/Hcrt in drug addiction and evaluates the current understanding of interactions among these stress-regulatory peptides relevant for drug addiction.
2. CRF: Role in Stress and Addiction
CRF is a 41-amino acid peptide that interacts with two G-protein-coupled CRF receptors, CRF1 and CRF2, that are positively coupled to adenylate cyclase via the Gs protein (Zorrilla and Koob, 2004; Zorrilla and Koob, 2005). CRF is the primary activator of the hypothalamic-pituitary-adrenal (HPA) “stress” axis via anterior pituitary CRF1 activation, leading to increased adrenocorticotropic hormone (ACTH) secretion that ultimately stimulates glucocorticoid production and release from the adrenal gland (Vale et al., 1981). Circulating glucocorticoids exert fine negative feedback control on the HPA axis at several levels, including transcriptional modulation of paraventricular nucleus (PVN) CRF-producing neurons (Kageyama and Suda, 2009). CRF not only regulates HPA axis activity, but also functions as a neurotransmitter in extrahypothalamic brain regions. CRF in the amygdala, bed nucleus of the stria terminalis (BNST), and septum has been implicated in the integration of emotional responses to stress . CRF neurotransmission has also been identified in the ventral tegmental area (VTA) where it appears to play an important role in the regulation of appetitive and motivational states (Wang et al., 2005; Wang et al., 2007; Ungless et al., 2003; Rodaros et al., 2007). Unlike their effects on the regulation of CRF in the PVN, glucocorticoids appear to upregulate the activity of CRF systems in the extended amygdala, forming a feed-forward circuit (Schulkin et al., 1998). Stress-related addictive behavior is partially related to activation of the HPA axis by CRF (for review, see Goeders, 2002; Sarnyai et al., 2001). However, extrahypothalamic, non-neuroendocrine CRF neurotransmission in brain regions that regulate behavioral and emotional responses to stress plays a significant independent role in addictive behavior (e.g., Sarnyai et al., 2001).
Generally confirming a role for CRF in addictive behavior, manipulations that interfere with CRF transmission reduce the effects of stress on drug seeking and intake. For example, CRF1 antagonist administration blunts the acquisition of cocaine-induced conditioned place preference (Lu et al., 2003), reduces conditioned reinstatement of cocaine seeking (Gurkovskaya and Goeders, 2001), attenuates footshock-induced reinstatement of cocaine seeking , and reverses stress-induced renewal of cocaine-induced conditioned place preference (for review, see Koob, 2008; Boutrel, 2008; Cleck and Blendy, 2008). Conversely, intracranial administration of CRF reinstates cocaine-seeking behavior (Brown et al., 2009; Erb et al., 2006). Moreover, blockade of central CRF transmission by CRF receptor antagonists or CRF antisera blocks cocaine-induced anxiety-like behavior, locomotor activity, and stress-induced cross-sensitization with psychostimulants (Lu et al., 2003; Przegalinski et al., 2005; Sarnyai et al., 1992; Sarnyai et al., 1995).
2.1. Drug-induced neuroadaptive dysregulation of the HPA axis
Stress-related addictive behavior is partially related to changes in HPA axis activity (e.g., Goeders, 2002; Sarnyai et al., 2001). For example, plasma corticosterone levels are increased in animals self-administering cocaine (Mantsch et al., 2000). More importantly, plasma corticosterone levels before acquisition of low-dose cocaine self-administration correlate with subsequent drug-reinforced responding (Mantsch et al., 2001). Thus, basal HPA axis activity may predict individual vulnerability to initial drug use, and individual differences in HPA axis responses to drug intake may represent a moderating vulnerability factor , when high doses of the drug are self-administered (Koob and Kreek, 2007). Furthermore, in rats with extended access to cocaine self-administration, both downregulation of HPA axis activity and impaired glucocorticoid receptor function have been observed (Mantsch et al., 2007). Specifically, dysregulation of glucocorticoid receptor-mediated negative feedback on HPA axis activity has been reported, which may contribute to drug-induced stress system alterations (Mantsch et al., 2007). Similar observations have been reported following chronic alcohol exposure , after which decreased activity of hypothalamic CRF neurons (Lee et al., 2000) and reduced anterior pituitary responsiveness to CRF (Dave et al., 1986) are evident. These preclinical findings are consistent with the clinical literature demonstrating that alcoholics and drug addicts show reduced HPA axis activity or a blunted HPA axis response to acute stress (Wand and Dobs, 1991).
2.2. Drug-induced neuroadaptive dysregulation of extrahypothalamic CRF
Repeated or long-term exposure to drugs of abuse leads to adaptations in the extrahypothalamic CRF circuitry with functional and behavioral consequences relevant for the development of drug addiction. This has been shown at several levels of analysis for psychostimulants, opiates, and ethanol. The focus here will be on the effects of chronic cocaine and ethanol. Following chronic cocaine treatment, neuroplasticity in the central nucleus of the amygdala (CeA) occurs via a CRF-dependent mechanism (Fu et al., 2007; Pollandt et al., 2006). Moreover, repeated cocaine administration cross-sensitizes locomotor activity and c-fos responses in the CeA to intracerebroventricular CRF administration (Erb et al., 2003; Erb et al., 2005; Erb and Brown, 2006). Behavioral evidence of CRF system neuroadaptation has been obtained in rats voluntarily self-administering cocaine, specifically in rats with a history of escalated cocaine intake associated with daily extended access (6 h/day) to intravenous cocaine (Mantsch et al., 2008). Rats showing escalated cocaine intake exhibit behavioral signs consistent with cocaine dependence. These signs include (i) increased motivation to obtain cocaine, measured by progressive-ratio responding, (ii) vertical shifts in the cocaine dose-response function, suggesting an increased hedonic set point (Ahmed and Koob, 1998; Ahmed and Koob, 1999; Wee et al., 2007), (iii) anhedonia-like symptoms upon cocaine withdrawal, measured by elevations in brain stimulation reward thresholds (Ahmed et al., 2002), iv) resistance to extinction, and (v) facilitated reinstatement of cocaine seeking (Ahmed and Cador, 2006). In these animals, stressors and intracerebroventricular CRF administration more effectively reinstate cocaine-seeking behavior than in rats without a history of escalation (Mantsch et al., 2008). Additionally, CRF1 antagonists reduce cocaine intake in rats with a history of extended daily cocaine access compared with non-escalated rats, suggesting increased activity of the CRF receptor system (Specio et al., 2008).
A second line of evidence illustrating dysregulation of extrahypothalamic CRF function by chronic drug use derives from intracranial microdialysis studies in which withdrawal from chronic ethanol, cocaine, cannabinoid, and morphine treatments was shown to result in substantial elevations in extracellular CRF levels in the amygdala (Merlo-Pich et al., 1995; Richter and Weiss, 1999; Rodriguez de Fonseca et al., 1997; Weiss et al., 2001) and BNST (Olive et al., 2002). This hypersecretion of CRF in the amygdala is an effect common to withdrawal from all major drugs of abuse studied to date (Weiss et al., 2001) and is associated with aversive and anxiety-like behavioral manifestations that are reversible by functional antagonism of CRF transmission (Basso et al., 1999; Sarnyai, 1998).
The alcohol literature also indicates the involvement of CRF neurotransmission in the medial raphe nucleus (MRN) in addictive behavior, particularly stress-induced ethanol seeking and relapse. CRF microinjection into the MRN elicits marked reinstatement of extinguished alcohol seeking behavior (Le et al., 2002). Conversely, intra-MRN administration of the nonselective CRF receptor antagonist D-Phe CRF12–41 prevents footshock stress-induced reinstatement of ethanol seeking and reduces stress-induced c-fos expression in the CeA (Funk et al., 2003), confirming a significant role for MRN CRF systems in alcohol seeking induced by stress.
More recently, CRF neurotransmission in the VTA has been identified as an important participant in the regulation of appetitive and motivational states. Specifically, footshock stress increases extracellular CRF levels in the VTA, and, similar to stress, intra-VTA infusion of CRF elicits reinstatement of cocaine-seeking behavior (Wang et al., 2005). The behavioral actions of CRF were associated with increased glutamate and dopamine release in the VTA, but only in cocaine-experienced rats, suggesting that cocaine self-administration leads to neuroadaptive changes in CRF neurotransmission within the VTA (Wang et al., 2005). Consistent with this hypothesis are findings showing that application of CRF to VTA slice preparations facilitates excitatory glutamatergic responses of dopamine neurons (Ungless et al., 2003) and that the magnitude and duration of the CRF-induced potentiation of N-methyl-D-aspartate (NMDA) receptor-mediated neurotransmission is significantly enhanced by repeated cocaine exposure (Hahn et al., 2009). Interestingly, in the VTA, in contrast to other brain regions where CRF1 receptors play a primary role, the effects of CRF are blocked by selective CRF2, but not CRF1, receptor antagonists (Ungless et al., 2003; Wang et al., 2007). The actions of CRF on dopamine transmission in the VTA may also involve the CRF binding protein (a protein that binds free CRF or urocortin-1 with high affinity; Behan et al., 1995). CRF6–33, a fragment without known receptor agonist activity that competitively displaces CRF from the CRF binding protein, prevented CRF from stimulating VTA dopamine transmission and eliciting cocaine seeking in rats (Ungless et al., 2003; Wang et al., 2007). These findings suggest a role not only for CRF2 receptors in the VTA, but also the CRF binding protein in the regulation of addictive behavior.
2.3. Drug-induced neuroadaptation and genetic factors in the dysregulation of extrahypothalamic CRF: implications for addiction
Perhaps the most extensive and compelling evidence of a link between extrahypothalamic CRF dysregulation, heightened stress sensitivity, and exacerbated drug seeking and intake can be found in the ethanol literature.
Similar to the effects of other drugs of abuse, a factor in the dysregulation of extrahypothalamic CRF dysfunction is chronic ethanol use itself. Chronic ethanol exposure produces functional adaptation of the CRF system in the CeA (Weiss et al., 2001) and BNST (Olive et al., 2002). Withdrawal from chronic ethanol exposure profoundly elevates extracellular CRF levels (Merlo-Pich et al., 1995; Richter and Weiss, 1999; Rodriguez de Fonseca et al., 1997; Weiss et al., 2001). This hypersecretion of CRF results in aversive and anxiety-like behavioral effects. Microinjection of CRF receptor antagonists into the CeA reverses increased anxiety-like reactions and ethanol self-administration observed in ethanol-dependent rats during acute withdrawal but does not alter these behaviors in ethanol nondependent rats (Funk et al., 2006; Rassnick et al., 1993; Valdez et al., 2002). Moreover, stress-induced reinstatement of ethanol seeking is exacerbated in a CRF antagonist-reversible manner (D-Phe CRF12–41) in rats with a history of ethanol dependence (Liu and Weiss, 2002). These findings indicate that adaptations occur not only in the regulation of CRF release, but also CRF receptors. Systemic administration of selective CRF1 antagonists or CRF1 deletion effectively reduces anxiety-like behavior during acute ethanol withdrawal, revealing a role for the CRF1 receptor in these adaptations (Breese et al., 2004; Knapp et al., 2004; Overstreet et al., 2004; Timpl et al., 1998). Similarly, nonpeptide CRF1 antagonists reduce increased ethanol self-administration typically associated with acute withdrawal from repeated, intermittent passive ethanol exposure in rats at doses that do not modify ethanol intake in nondependent rats (Funk et al., 2007; Sabino et al., 2006).
A second line of evidence supporting the link between dysregulation of extrahypothalamic CRF function and high ethanol intake is innate dysfunction of the CRF system identified in rats genetically selected for high ethanol preference. In early studies, ethanol-naive Sardinian alcohol-preferring (sP) rats exhibited an innate upregulation of CRF function in the CeA, reflected by elevated basal CRF output. Additionally, these animals showed increased behavioral sensitivity to stress and anxiogenic stimuli, measured on the elevated plus maze, compared with their alcohol-nonpreferring (sNP) couterparts (Richter et al., 2000). Thus, these alcohol-preferring rats show abnormalities in stress sensitivity and CRF function in the CeA that resemble those in chronic ethanol-exposed genetically heterogeneous rats (i.e., Wistar rats not selected for ethanol preference). Notably, however, despite these abnormalities in stress responsiveness in sP rats, the CRF1 antagonist LWH-63 was shown to suppress only withdrawal-induced but not spontaneous ethanol intake in this line of rats (Sabino et al., 2006). Nonetheless, LWH-63 and other CRF1-selective antagonists produced clear anti-stress-like actions in nondependent sP rats, such as attenuation of stress-induced suppression of ethanol intake (Sabino et al., 2006). Therefore, innate upregulation of the CRF system in sP rats may convey vulnerability to high alcohol consumption, but further dysregulation of the CRF system by chronic alcohol exposure may exacerbate CRF1 receptor-dependent high alcohol drinking in these animals. Similarly, increased alcohol intake may be related to additional CRF1 gene single nucleotide polymorphisms (SNPs), such as those found in the sP-derived Marchigian Sardinian alcohol-preferring (msP) rat line (Fonareva et al., 2009; Hansson et al., 2006).
Perhaps the most direct evidence for similarities in addiction-relevant consequences of both genetically determined and chronic ethanol-induced abnormalities in extrahypothalamic CRF function (and other brain stress-systems; see below) comes from studies with msP alcohol-preferring rats in which high alcohol preference co-segregates with increased sensitivity to stress, creating a phenocopy of the postdependent phenotype in nonselected Wistar rats (Ciccocioppo et al., 1999a; Hansson et al., 2006). A screen for differential gene expression identified an innate upregulation of the Crhr1 transcript encoding the CRF1 receptor in several limbic brain areas of msP rats, including the CeA, medial nucleus of the amygdala (MeA), basolateral nucleus of the amygdala (BLA), and hippocampal regions, was associated with a genetic polymorphism of the Crhr1 promoter and accompanied by increased CRF1 receptor density. Interestingly, in this line of rats, voluntary ethanol consumption led to a reduction of anxiety-like and depression-like behaviors (Ciccocioppo et al., 1999a; Ciccocioppo et al., 2006) associated with significant downregulation of the Crhr1 transcript in the CeA, MeA, and nucleus accumbens (Hansson et al., 2007). These findings suggest that msP rats may consume large amounts of ethanol to self-medicate a “negative affective state” resulting from excess extrahypothalamic CRF1 receptor activity. Consistent with this hypothesis, the selective CRF1 antagonist antalarmin was devoid of effects on ethanol self-administration in nonselected Wistar rats but significantly suppressed this behavior in msP rats. Similarly, antalarmin did not significantly attenuate footshock stress-induced reinstatement of ethanol seeking in Wistar rats but fully blocked the effects of footshock on reinstatement in msP rats (Hansson et al., 2006). Overall, these findings suggest a causal role of CRF1 receptor upregulation in the behavioral phenotype of msP rats.
The findings in msP rats described above demonstrate that the Crhr1 genotype and Crhr1 expression regulate alcohol-taking behavior and interact with environmental stress to reinstate alcohol seeking. Considering the similarities between genetically determined and chronic ethanol-induced abnormalities in the function of extrahypothalamic CRF stress systems discussed earlier, chronic ethanol intoxication may upregulate the Crh1 transcript, CRF1 receptors, or CRF peptide levels in genetically nonselected Wistar rats, resulting in an “ethanol-dependent” behavioral phenotype similar to msP rats, characterized by high alcohol intake and stress sensitivity.
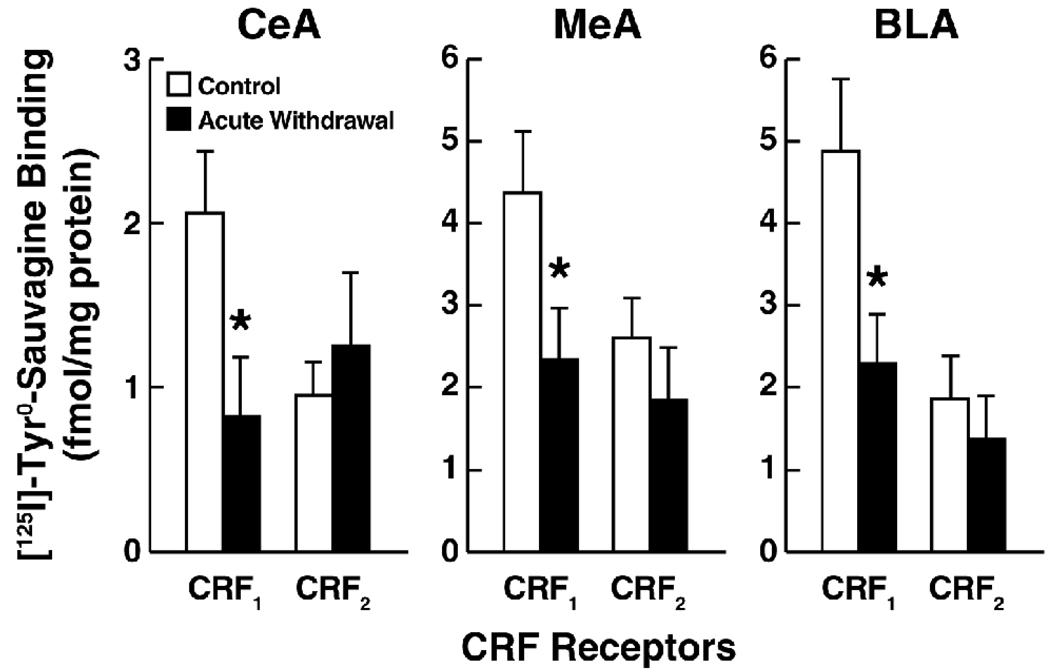
Confirming this hypothesis, alcohol intake and stress sensitivity increased in Wistar rats with a history of ethanol dependence. These behavioral changes were accompanied by upregulation of Crhr1 transcript expression in the BLA and MeA, upregulation of CRF messenger RNA (mRNA) in the CeA, and downregulation of Crhr2 expression in the BLA (Sommer et al., 2008). These findings suggest that neuroadaptations encompassing CRF function in the amygdala contribute to the high ethanol drinking and high stress responsivity of postdependent Wistar rats. Somewhat consistent with these findings, postdependent Wistar rats, tested 8 h following withdrawal from a 4 week ethanol vapor dependence induction procedure, exhibited significantly decreased CRF1 binding in the CeA, MeA, and BLA (Fig. 1), an effect that remained unaltered for at least 3 weeks (data not shown). Thus, increased Crhr1 gene expression may represent a compensatory response to the decrease in membrane CRF1 binding elicited by chronic ethanol exposure. Given the well-documented increase of CRF peptide availability in the amygdala postdependence (Zorrilla et al., 2001), the decrease in membrane CRF1 binding may be attributable to agonist-induced receptor activation and internalization, which is profound and occurs rapidly (within 5 min of activation; Reyes et al., 2006, 2008; Perry et al., 2005). Considering these findings, the decrease in CRF1 receptor membrane levels in the presence of increased CRF peptide tissue content and CRF1 synthesis may reflect the consequences of sustained CRF1 receptor stimulation driven by high CRF peptide availability. The consistent observation of higher efficacy of CRF1 receptor antagonists in postdependent rats is accounted for by the prevention of sustained CRF1 stimulation in these animals. A second, independent explanation for the increased behavioral efficacy of CRF1 antagonists in postdependent animals, tentatively supported by electrophysiological data , is that CRF1 signaling efficiency may be enhanced, possibly because of adaptation of signal transduction mechanisms. A final alternative explanation is that the increased CRF antagonist efficacy in ethanol-dependent and postdependent animals results from a more favorable receptor occupancy profile achieved by CRF1 receptor antagonists in the presence of fewer membrane receptors. The latter interpretation is perhaps less likely because it predicts that CRF1 antagonists reduce ethanol self-administration in nondependent animals given a sufficiently high dose (and degree of receptor occupancy). However, as discussed above, “anti-drinking” effects have typically not been observed even at the highest CRF antagonist doses in nondependent animals. Indeed, even doses of the CRF1 antagonist R121919 that almost fully (~85%) occupy brain CRF1 receptors in ethanol-naive rats (Gutman et al., 2003) did not alter ethanol self-administration in nondependent animals (Sabino et al., 2006; Funk et al., 2007.)
Figure 1.
Reduced amygdala CRF1, but not CRF2, receptor binding in rats during acute ethanol withdrawal following chronic, intermittent ethanol vapor exposure. Panels show mean (± SEM) specific CRF1 and CRF2 binding in the central nucleus of the amygdala (CeA), medial nucleus of the amygdala (MeA), and basolateral amygdala (BLA) determined by quantitative autoradiography. Total specific binding of the CRF1/CRF2 receptor radioligand [125I]-Tyr0-sauvagine was defined using a saturating concentration of the subtype-nonselective peptide antagonist D-Phe CRF12–41 (10 mM). CRF1 vs. CRF2 receptor binding was defined using the highly selective nonpeptide CRF1 antagonist R121919, in which residual specific binding of [125I]-Tyr0-sauvagine that was not displaced by R121919 (1 mM) was defined as CRF2 binding, and R121919-sensitive binding was defined as CRF1 binding. Autoradiography was performed on coronal brain sections (20 µm) obtained from male Wistar rats during acute withdrawal (8 h) from a 28 day intermittent ethanol vapor inhalation procedure (n = 5; 14 h on; 10 h off; target blood alcohol levels, 200–225 mg%) and from air-exposed control rats (n = 5). Optical densitometry was performed using ImageJ (NIH), and values were normalized to prefabricated autoradiography microscales (Amersham).
Thus, substantial evidence implicates increased sensitivity to stress associated with dysregulation of extrahypothalamic CRF function as a major determinant of vulnerability to and maintenance of high alcohol intake. Moreover, dysregulation of extrahypothalamic CRF function leading to enhanced stress sensitivity can preexist as a genetically determined condition or develop as a result of chronic high-dose alcohol exposure.
3. Nociceptin/Orphanin FQ
N/OFQ is a 17-amino acid peptide that shows structural homology with opioid peptides, particularly dynorphin A (Meunier et al., 1995; Reinscheid et al., 1995). N/OFQ binds with high affinity at opioid receptor-like 1 (ORL-1), now included in the opioid receptor family and renamed the NOP receptor (nociceptin/orphanin peptide receptor), but does not activate μ,κ, or δ opioid receptors. N/OFQ and its receptor are widely distributed in the central nervous system, with the highest density in the CeA, BNST, medial prefrontal cortex (mPFC), VTA, lateral hypothalamus (LH), nucleus accumbens, and brain stem areas, including the locus coeruleus (LC) and dorsal raphe (DR; Darland et al., 1998; Neal et al., 1999). Interestingly, N/OFQ has been found to act in the brain as a functional antiopioid peptide by blocking opioid-induced supraspinal analgesia (Mogil et al., 1996a; Mogil et al., 1996b; Morgan et al., 1997), morphine-induced conditioned place preference (Ciccocioppo et al., 2000; Murphy et al., 1999), and morphine-induced increases in extracellular dopamine levels in the nucleus accumbens (Di Giannuario and Pieretti, 2000). Moreover, N/OFQ inhibits stress-induced ethanol seeking (Martin-Fardon et al., 2000) and exerts general anti-stress-like effects by acting as a functional antagonist of extrahypothalamic CRF transmission (Ciccocioppo et al., 2003a; Ciccocioppo et al., 2003b).
3.1. Role in drug and alcohol addiction
Manipulation of the N/OFQ system by pharmacological agents or gene deletion modifies the reinforcing effects of psychostimulants (Kotlinska et al., 2002; Kotlinska et al., 2003; Sakoori and Murphy, 2004; Sakoori and Murphy, 2008; Zhao et al., 2003), morphine (Ciccocioppo et al., 2000; Murphy et al., 1999; Sakoori and Murphy, 2004), ethanol (Ciccocioppo et al., 1999b; Kuzmin et al., 2003; Sakoori and Murphy, 2008), and nicotine (Sakoori and Murphy, 2009) measured by the acquisition of conditioned place preference and consumption (bottle choice drinking paradigm) tests. A role of N/OFQ in the regulation of psychostimulant-induced behavioral sensitization has also been described. Specifically, intracerebroventricular or intra-VTA administration of N/OFQ before repeated cocaine or amphetamine treatments prevents the development of locomotor sensitization, suggesting that the VTA may be a substrate of N/OFQ actions (Kotlinska et al., 2003; Lutfy et al., 2002). Moreover, intra-VTA administration of N/OFQ reduces dopamine levels in the nucleus accumbens (Murphy and Maidment, 1999). Altogether these findings suggest that N/OFQ participate in regulating mesolimbic dopamine neurotransmission and possibly the rewarding effects of several drugs of abuse.
The role of N/OFQ in drug reward has been studied most extensively with ethanol. Activation of the N/OFQ system (i) blunts the reinforcing and motivating effects of ethanol across a range of behavioral measures, including ethanol self-administration (Ciccocioppo et al., 1999b), conditioned place preference (Kuzmin et al., 2003), and conditioned reinstatement (Ciccocioppo et al., 2004), (ii) reverses increased anxiety-like behavior associated with neuroadaptive changes in N/OFQ function associated with a history of ethanol dependence (Aujla et al., 2007), (iii) attenuates alcohol withdrawal symptoms (Stopponi et al., 2009), and (iv) produces substantial anti-anxiety-like and anti-stress-like effects in animal models of anxiety (Rodi et al., 2008), CRF-induced anorexia (Ciccocioppo et al., 2001; Ciccocioppo et al., 2003b), and stress-induced reinstatement of ethanol seeking (Martin-Fardon et al., 2000). Importantly, activation of the N/OFQ system failed to block stress-induced reinstatement of cocaine seeking (Martin-Fardon et al., 2000). The reasons for the lack of effect of N/OFQ on stress-induced reinstatement of cocaine seeking are presently not clear. However, emerging evidence suggests that the N/ OFQ system may be differentially affected by alcohol and psychostimulants. For example, acute ethanol administration increases NOP receptor density in cortical areas, whereas acute administration of cocaine does not (Rosin et al., 2003). Furthermore, under chronic treatment conditions, alcohol administration decreases N/OFQ brain tissue levels (Lindholm et al., 2002), whereas repeated cocaine administration increases N/OFQ immunoreactivity in the brain (Lutfy et al., 2008).
3.2. Genetic predisposition to alcohol abuse
Data from both animals and humans implicate innate dysregulation of the N/OFQ system as a possible factor in alcoholism. msP rats exhibit traits frequently associated with alcoholism, including high sensitivity to stress, a high anxiety phenotype, and depression-like symptoms. These behavioral characteristics of msP rats are ameliorated by alcohol consumption (Ciccocioppo et al., 1999a). Ethanol self-administration in msP rats is highly sensitive to suppression by N/OFQ and N/OFQ analogs (Ciccocioppo et al., 1999b; Ciccocioppo et al., 2000; Ciccocioppo et al., 2004; Economidou et al., 2006), suggesting that hypofunction of the N/OFQ system may be a factor in high alcohol intake by msP rats. Apparently contradicting this hypothesis, msP rats exhibit elevated expression of N/OFQ and NOP receptor mRNA, with particularly high levels in the BNST and CeA (Economidou et al., 2008), and significantly increased NOP receptor binding in the CeA, BNST, VTA, and several cortical structures, indicating an innate overall upregulation of the N/OFQ-NOP system (Economidou et al., 2008). However, msP rats show a distinct pattern of N/OFQ functional abnormalities in the CeA where N/OFQ-stimulated [35S]GTPγS binding was significantly lower in msP than Wistar rats, despite elevated NOP receptor expression and binding in the msP line (Economidou et al., 2008). Thus, “uncoupling” of the NOP receptor from G-protein-mediated signal transduction in the CeA may lead to regionally selective hypofunction of the N/OFQ system which facilitates alcohol drinking. This hypothesis is supported by data showing that stimulation of NOP receptors in the CeA by site-specific microinjection of N/OFQ reduces alcohol self-administration in msP rats (Economidou et al., 2008).
At the clinical level, recent association studies suggest a possible link between alcoholism and polymorphisms in the gene encoding the NOP receptor. One study revealed a link between alcoholism and two SNPs in the N/OFQ gene (Xuei et al., 2008). This observation was corroborated by findings showing that the gene encoding the NOP receptor (SNP rs6010718) is significantly associated with both Type I and Type II alcoholism in a Scandinavian sample of alcoholics (Huang et al., 2008). Thus, genetic variants of the N/OFQ and NOP genes may be linked to vulnerability for alcohol dependence.
3.3. Dysregulation of the N/OFQ system following ethanol dependence
The hypothesis that N/OFQ system dysregulation is a factor in high alcohol intake is consistent with recent findings showing that Wistar rats with a history of ethanol dependence exhibit neuroadaptive changes in the N/OFQ system that are associated with increased stress sensitivity and alcohol intake. Wistar rats were trained to operantly self-administer 10% ethanol and then subjected to induction of ethanol dependence using a 3 week ethanol vapor inhalation procedure. In subsequent tests of ethanol self-administration conducted 1 week following withdrawal, the ethanol intake-reducing effects of N/OFQ emerged in postdependent rats, whereas nondependent controls rats remained less sensitive to the effects of N/OFQ (Fig. 2).
Figure 2.
Effects of N/OFQ on ethanol-reinforced responses in ethanol nondependent (n = 7) and postdependent (right panel: Dependent) Wistar rats (n = 9). Rats self-administered ethanol on a fixed-ratio schedule in 30 min sessions in which each reinforced response resulted in the delivery of 0.1 ml of 10% (w/v) ethanol. A mixed-factorial ANOVA did not reveal significant main effects of ethanol dependence history (F1,14 = 0.6, not significant) or an ethanol dependence history × dose interaction (F3,42 = 0.2, not significant). However, a main effect of dose was detected (F3,42 = 4.0, p < 0.01). Separate one-way ANOVAs revealed a significant effect of N/OFQ in postdependent (F3,24 = 4.2, p < 0.01) but not nondependent rats (F3,18 = 0.9, p = 0.4). For postdependent rats, Newman-Keuls post hoc tests revealed significant differences between vehicle- (0 µg) and N/OFQ-treated rats at all doses tested (*p < 0.05).
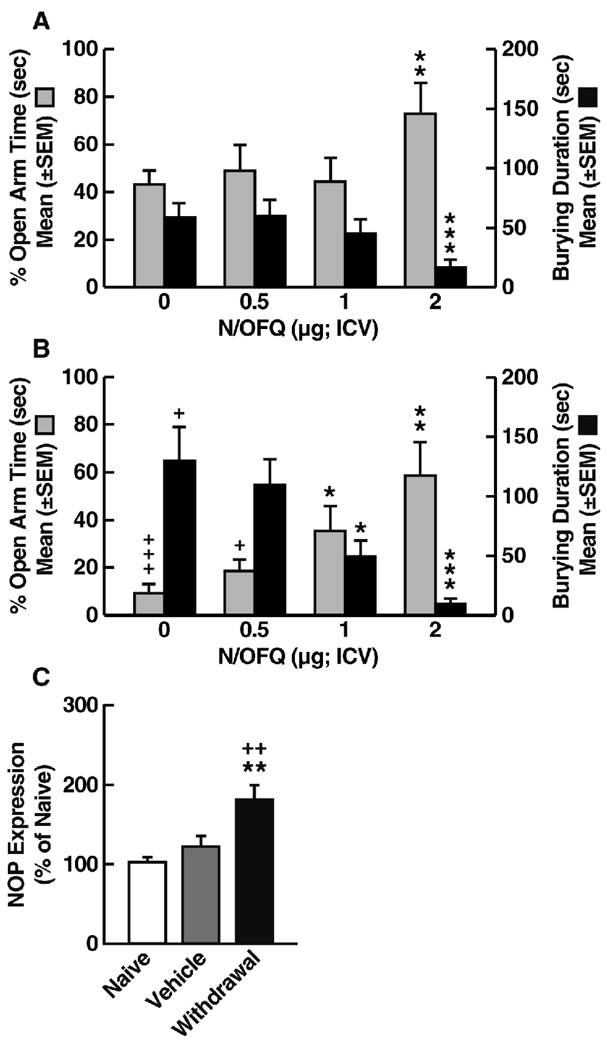
Further evidence of N/OFQ system dysregulation following chronic ethanol exposure has been obtained in tests of the effects of N/OFQ on anxiety-like behavior measured by the shock-probe defensive burying and elevated plus maze tests. In rats made dependent by repeated intragastric ethanol intubation and tested 1 week following ethanol withdrawal (Braconi et al., in press), N/OFQ dose-dependently modified the behavior of Wistar rats in a manner consistent with anxiolytic actions (Fig. 3A and B). These effects, however, emerged at lower doses in postdependent compared with nondependent animals (Fig. 3A and B), consistent with increased sensitivity to the anxiolytic action of N/OFQ in postdependent Wistar rats (Aujla et al., 2007). Notably, the anxiolytic effects of N/OFQ in these tests were more prominent in postdependent rats, whereas N/OFQ produced only small reductions in anxiety-like behavior in nondependent animals (see Fig. 3A and B for comparison). This scenario is similar to the anxiolytic effects of CRF antagonists (Valdez et al., 2002) and suggests that ethanol-induced neuroadaptations convey increased sensitivity to the actions of these neuropeptides. Indeed, in these studies, neuroadaptive changes in the N/OFQ system were evident in rats with a history of ethanol dependence, demonstrated by enhanced NOP gene expression in the BNST (Fig. 3C). A second line of evidence for ethanol-induced neuroadaptive changes in N/OFQ function comes from electrophysiological findings in CeA slice preparations in which N/OFQ decreased the facilitation of GABAA neurotransmission by ethanol, and this effect was significantly increased in the CeA of ethanol-dependent rats (Roberto and Siggins, 2006). The upregulation of N/OFQ function in major stress-regulatory sites revealed by these studies is likely to be relevant for the increased sensitivity to the effects of N/OFQ on ethanol intake and anxiety-like behavior in postdependent rats, as well as for the reported reversal of ethanol withdrawal symptoms by N/OFQ (Stopponi et al., 2009), although this hypothesis remains to be confirmed.
Figure 3.
Effects of N/OFQ on anxiety-like behavior in nondependent (A) and postdependent (B) Wistar rats measured in the elevated plus maze (% Open Arm Time, left y-axis) and defensive burying test (Burying Duration, right y-axis). Postdependent animals exhibited increased anxiety-like behavior measured by both the elevated plus maze and defensive burying test (N/OFQ, 0 µg). N/OFQ dose-dependently decreased anxiety-like behavior in both models of anxiety, with anxiolytic-like effects appearing at a lower dose (1 µg) in postdependent compared with nondependent rats. This observation is consistent with increased sensitivity to the anxiolytic action of N/OFQ in postdependent Wistar rats. For both behavioral tests, significant differences were found between the two groups (two-way ANOVA; elevated plus maze, F1,68 = 9.9, p < 0.01; defensive burying, F1,68 = 7.4, p < 0.05), with significant effects of N/OFQ dose (elevated plus maze, F3,68 = 6.3, p < 0.001; defensive burying, F3,68 = 12.08, p < 0.001). A group (dependent, postdependent) × dose interaction was also confirmed in the defensive burying test (F3,68 = 3.1, p < 0.05). *p < 0.05, **p < 0.01, compared with N/OFQ (0 µg); +p < 0.05, +++p < 0.001, compared with nondependent (n = 9–10 animals/group; Newman-Keuls post hoc test). (C) NOP receptor mRNA levels in the BNST of postdependent (Withdrawal) and nondependent (Vehicle) Wistar rats. Data were obtained using real-time reverse transcription polymerase chain reaction and compared with mRNA expression in experimentally naive age-matched controls (100%). **p < 0.01, compared with Vehicle; ++p < 0.01, compared with naive (n = 6 animals/group).
4. Orexin/Hypocretin
Orexin A (hypocretin-1) and orexin B (hypocretin-2) are recently discovered hypothalamic neuropeptides that regulate a range of physiological processes, including feeding, energy metabolism (Willie et al., 2001), and arousal (Sutcliffe and de Lecea, 2002; Taheri et al., 2002). Two Orx/Hcrt receptors have been identified: Hcrt-r1 and Hcrt-r2. Major Orx/Hcrt neuron populations are found in the LH, a brain region long associated with reward and motivation (for review, see DiLeone et al., 2003), perifornical hypothalamus (PFA), and dorsomedial hypothalamus (DMH). Orx/Hcrt neurons in the DMH and PFA project to brain stem nuclei and have a major role in the regulation of arousal and modulation of stress responses (Baldo et al., 2003; Winsky-Sommerer et al., 2004).
Rapidly growing evidence indicates a possible central role of Orx/Hcrt neurons in the LH in drug addiction. These neurons project to the paraventricular thalamus (PVT), nucleus accumbens shell, ventral pallidum (VP), VTA, CeA, and BNST (Baldo et al., 2003; Peyron et al., 1998) and were originally implicated in the regulation of feeding behavior (Edwards et al., 1999; Haynes et al., 2000; Haynes et al., 2002; Sakurai et al., 1998). Recent evidence, however, shows that Orx/Hcrt neurons in the LH play a significant role in the modulation of reward function and, particularly, drug-directed behavior (Harris et al., 2005).
4.1. Recruitment of the Orx/Hcrt system by drugs of abuse
Orx/Hcrt neurons in the LH become activated by stimuli associated not only with food but also morphine and cocaine reward (Harris et al., 2005). Similarly, the expression of conditioned place preference induced not only by food, but also morphine and cocaine, is associated with activation of Orx/Hcrt neurons in the LH (Harris et al., 2005). Consistent with these observations, intra-VTA microinjection of orexin-A produces renewal of morphine-induced conditioned place preference, whereas administration of the Hcrt-r1 antagonist SB334867 decreases the expression of morphine-induced conditioned place preference (Harris et al., 2005). SB334867 also blocks the acquisition of cocaine-induced behavioral sensitization and blocks the potentiation of excitatory currents by cocaine in VTA dopamine neurons (Borgland et al., 2006). Blockade of Hcrt-r1 decreases ethanol (Lawrence et al., 2006) and nicotine self-administration (Hollander et al., 2008), inhibits cue-induced reinstatement of ethanol (Lawrence et al., 2006) and cocaine seeking (Smith et al., 2009), and attenuates stress-induced reinstatement of cocaine (Boutrel et al., 2005) and ethanol seeking (Richards et al., 2008). Thus, behavioral and functional evidence indicates a role for Orx/Hcrt signaling in the neurobehavioral and motivational effects of cocaine and other drugs of abuse (Borgland et al., 2006; for review, see Bonci and Borgland, 2009).
4.2. Differential recruitment of the Orx/Hcrt system by drugs and natural rewards
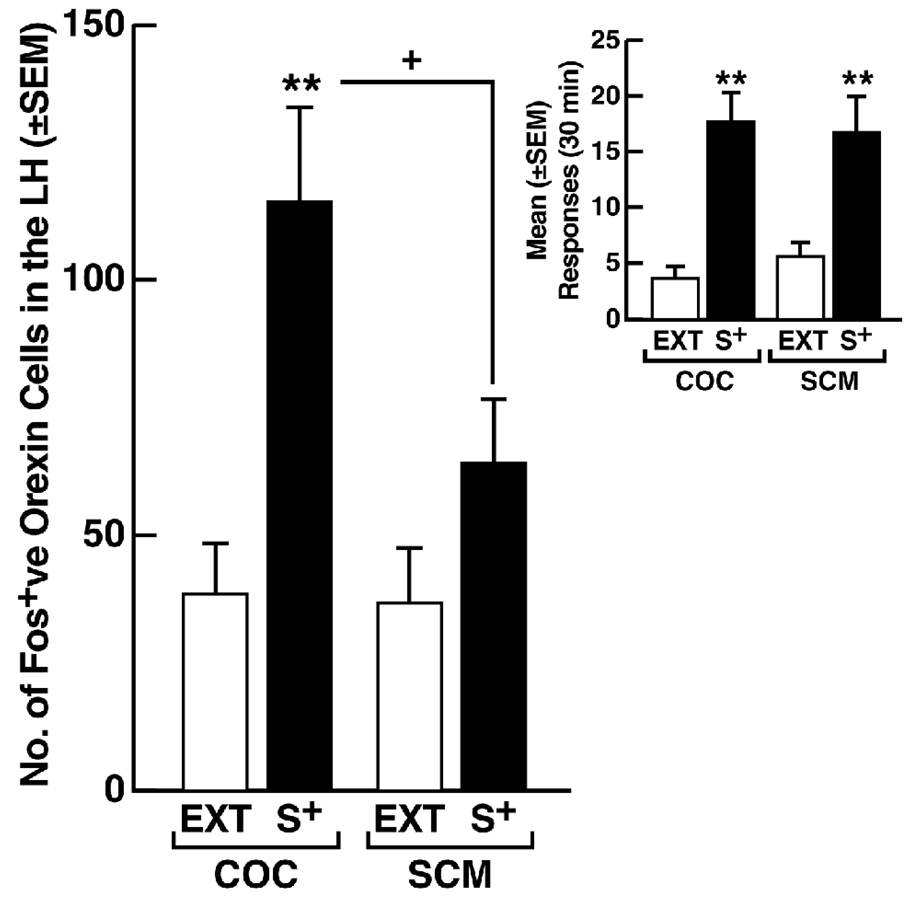
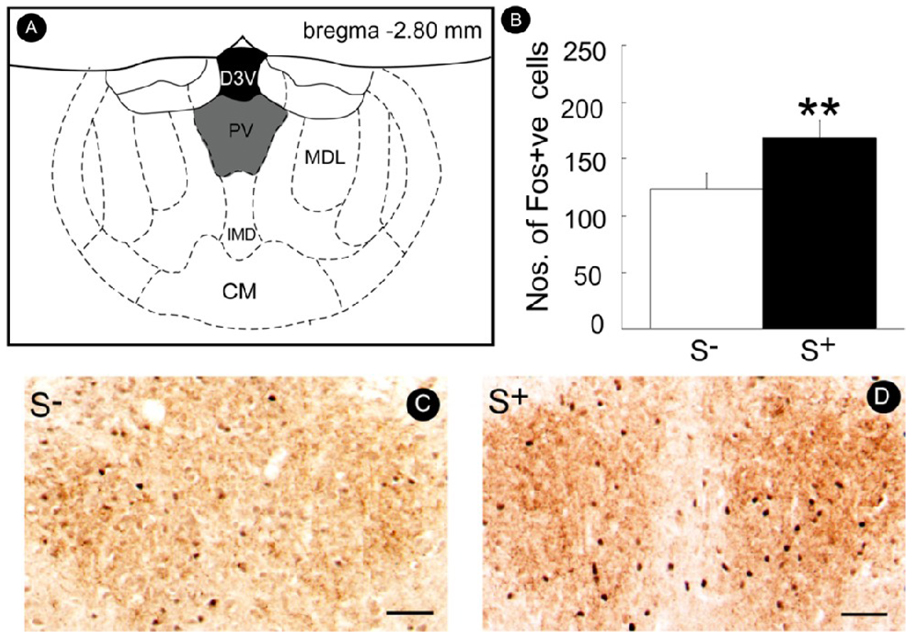
Pharmacological manipulation of the Orx/Hcrt system is particularly effective in modifying the conditioned effects of drug cues in studies of conditioned place preference and reinstatement (Harris et al., 2005; Lawrence et al., 2006; Smith et al., 2009). Understanding whether Orx/Hcrt has a selective role in drug-seeking behavior or whether this role extends to motivated behavior in general is interesting. In a series of studies addressing this question, blockade of Hcrt-r1 receptors by SB334867 selectively reversed conditioned reinstatement induced by a stimulus conditioned to cocaine, without interfering with the effects of the same stimulus when conditioned to a highly palatable conventional reinforcer (Fig. 4). Moreover, although the stimuli conditioned to cocaine and the conventional reinforcer were equally effective in eliciting reinstatement (Fig. 5, inset), only the cue conditioned to cocaine recruited a larger number of Fos-positive Orx/Hcrt cells in the LH (Fig. 5). Thus, Orx/Hcrt neurons in the LH appear to preferentially regulate conditioned drug seeking compared with behavior motivated by a natural reward.
Figure 4.
Effects of the specific Hrct-r1 antagonist SB334867 (0–3 mg/kg, IP, 30 min before testing) on conditioned reinstatement induced by a cocaine (COC)-related stimulus (S+, left) or a stimulus conditioned to sweetened condensed milk (SCM; S+, right). These data provide preliminary evidence that blockade of Hrct-r1 reverses the motivational effects of cocaine-related environmental stimuli but not stimuli conditioned to a highly palatable conventional reinforcer. ++p < 0.01, compared with non-reward-related stimulus (S−); **p < 0.01, compared with 0 mg/kg (n = 6 animals/group).
Figure 5.
Recruitment of Orx/Hcrt neurons by a stimulus (S+) conditioned to cocaine (COC) but not sweetened condensed milk (SCM). Male Wistar rats were subjected to reinforcement contingencies in which responses at the active lever were differentially reinforced in the presence of a distinct discriminative stimuli SD associated with reinforcers (cocaine or SCM) availability vs. non-availability. A constant 70 dB white noise served as a discriminative stimulus (S+) for availability of the reinforcer (cocaine or SCM), while illumination of a 2.8 W house light located at the top of the chamber’s front panel served as a discriminative stimulus (S−) signaling non-availability of the reinforcer (i.e., saline solution instead of cocaine or no consequence instead of SCM). Sessions were initiated by extension of the levers into the chambers and concurrent onset of the respective SD which remained present until termination of the session by retraction of the levers. In the presence of the S+, responses at the right, active lever were reinforced by cocaine or SCM on a fixed-ratio 1 schedule and, similar to training, were followed by a 20 s timeout period signaled by illumination of a cue light above the lever. In the presence of the S−, depression of the right active lever was followed by an intermittent tone during which the lever remained inactive for 20 s. Three daily sessions (each lasting 1 h for the cocaine group and 20 min for the SCM group) separated by 30 min intervals were conducted, with two “reward” sessions and one “non-reward” session sequenced in random order. After 8 training days (i.e., a total of 16 “reward” and 8 “non-reward” sessions), both the cocaine and SCM groups were placed on extinction conditions in daily 30 min sessions during which the reinforcers and SD were withheld until a criterion of ≤ 4 responses/session for 3 consecutive days was reached. Fos-positive (Fos+ve) Orx/Hcrt neurons in the lateral hypothalamus (LH) were then counted following COC or SCM S+ presentation (reinstatement tests in which both the COC and SCM S+ elicited robust recovery of responding; see inset) and compared with Fos-positive counts obtained following the final extinction (EXT) session. Fos-positive orexin neurons: **p < 0.05, compared with EXT; +p < 0.05, COC S+ vs. SCM S+. Inset: Mean (± SEM) number of responses during reinstatement tests in the presence of the COC S+ or SCM S+. Both stimuli were equally effective in eliciting responding. **p < 0.01, compared with EXT (n = 8 animals/group).
The pharmacological and neural mapping data above are somewhat difficult to reconcile with the role of the Orx/Hcrt system in behavior motivated by food (i.e., a natural reward) in addition to its more recently discovered role in drug reward. One hypothesis concerning the control of drug-seeking behavior is that neural circuits mediating the effects of drug cues are not specific to addiction-related events, but rather are “normal” circuits activated to a greater degree, thereby creating new motivational states or tilt processes that normally govern responding for natural rewards toward drug-directed behavior (Kelley and Berridge, 2002). Drugs of abuse may produce this effect by neuroadaptively altering neural systems that regulate “normal” motivation. Evidence of drug-induced dysregulation of the Orx/Hcrt system exists with alcohol. Prepro-orexin mRNA is upregulated in the LH of inbred alcohol-preferring (iP) rats following chronic ethanol consumption (Lawrence et al., 2006).
A possibility derived from this hypothesis is that the Orx/Hcrt system may, over the course of repeated drug use, acquire a preferential role in mediating the effects of stimuli conditioned to drugs of abuse vs. natural rewards. A major Orx/Hcrt projection exists from the LH/PFA to the PVT (Parsons et al., 2006), and the PVT has been proposed to be a key relay gating Orx/Hcrt-coded reward-related communication between the LH/PFA and both the ventral and dorsal striatum (Kelley et al., 2005). Moreover, this “hypothalamic-thalamic-striatal axis” has been suggested to have evolved to prolong the central motivational state in the case of feeding beyond the fulfillment of immediate energy needs, thereby promoting the development of energy reserves for potential future food shortages (Kelley et al., 2005). Maladaptive recruitment of this system by drugs of abuse may “tilt” its function toward drug-directed behavior, which may explain the increased sensitivity of the Orx/Hcrt system to antagonist interference with drug-seeking behavior as opposed to behavior directed toward natural rewards.
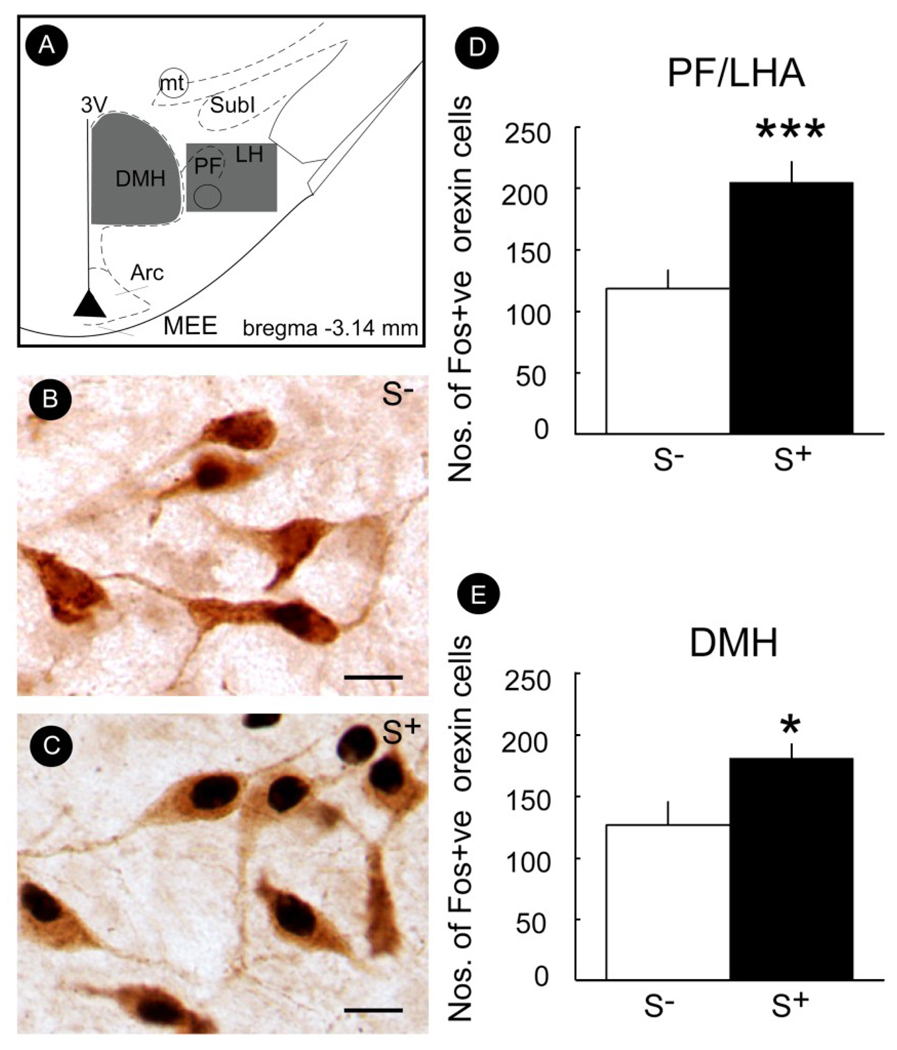
A role for Orx/Hcrt projections from the LH to PVT in drug seeking is, in fact, strongly suggested by the finding that drug-related associated contextual cues activate these neurons (Dayas et al., 2008). Significantly larger numbers of Fos-positive hypothalamic Orx/Hcrt neurons were seen in rats exposed to contextual stimuli previously associated with ethanol availability than in rats exposed to the same stimulus previously paired with non-reward (Fig. 6). Moreover, presentation of the ethanol-related stimuli also increased the number of Fos-positive PVT neurons (Fig. 7), and these neurons were closely associated with Orx/Hcrt fibers (for additional details, see Dayas et al., 2008).
Figure 6.
Mean (± SEM) number of Fos-positive Orx/Hcrt cells in the perifornical/lateral hypothalamus (PF/LHA) (D) or dorsomedial hypothalamus (DMH) (E) in rats exposed to either the ethanol- (S+) or non-reward-related stimulus (S−). A significantly greater number of Fos-positive Orx/Hcrt cells was observed in animals exposed to the S+ (vs. S−) in both the PF/LHA and DMH. (B & C) Photomicrographs illustrating the effects of S− or S+ exposure on the number of Fos-positive Orx/Hcrt cells in the PF/LHA. (A) Schematic illustrating the rostro-caudal level from which photomicrographs (B & C) of PF/LHA tissue, immunolabeled for Fos-protein and Orx/Hcrt, were made. *p < 0.05, ***p < 0.001, compared with S−. Scale bar = 20 µm. Taken with permission from Dayas et al. (2008).
Figure 7.
(A) Schematic illustrating the rostro-caudal level from which photomicrographs of paraventricular thalamus (PVT) tissue were made. (B) Counts (mean ± SEM) of Fos-positive PVT cells were significantly increased in animals exposed to the ethanol S+ vs. S− cue. **p < 0.001, compared with S−. (C & D) Photomicrographs illustrating the effects of S− or S+ exposure on the number of Fos-positive PVT neurons. Scale bar = 50 µm. Taken with permission from Dayas et al. (2008).
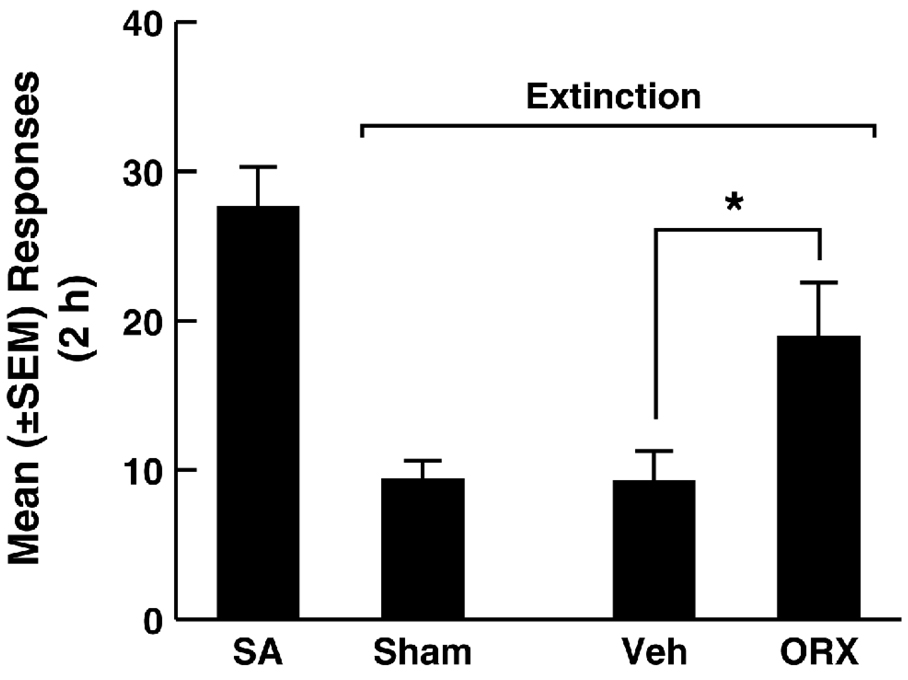
Further supporting a role for this projection in regulating drug-seeking behavior, recent data confirm that context-induced reinstatement of alcoholic beer seeking is associated with recruitment of a PVT-ventral striatum pathway (Hamlin et al., 2009). Furthermore, Orx/Hcrt injection into the PVT exerted a priming-like effect, reinstating extinguished cocaine-seeking behavior (Fig. 8).
Figure 8.
Intra-PVT Orexin-A (ORX) injection reinstates extinguished cocaine-seeking behavior. Rats were first trained to intravenously self-administer cocaine (0.25 mg/infusion, fixed-ratio 1, timeout 20 s, signaled by the illumination of a cue light above the active lever) for 2 weeks. Following self-administration training (SA), the animals were placed on extinction conditions for 2 weeks. Extinction sessions were identical to self-administration sessions, including the signaled 20 s TO period, but cocaine was no longer available. Under extinction conditions, microinjection of orexin A (ORX, 150 pmole) into the PVT immediately before the test reinstated extinguished cocaine-seeking behavior. *p < 0.05, compared with Vehicle (Veh; n = 6 animals/group).
Maladaptive recruitment of the Orx/Hcrt system by drugs of abuse is also suggested by findings describing neuroadaptative changes within the VTA. For example, voluntary cocaine and natural reward self-administration induces common, short-lasting, neuroadaptation in VTA dopaminergic neurons (i.e., increased glutamatergic function; Chen et al., 2008). This enhanced synaptic strength, however, is persistent and resistant to extinction only in rats self-administering cocaine and not in rats self-administering a non-drug reinforcer (Chen et al., 2008). Interestingly, several lines of evidence suggest that participation of the VTA in cocaine-induced neuronal and behavioral changes requires Orx/Hcrt inputs. For example, activation of Hcrt-r1 in the VTA is necessary for the development of cocaine-induced locomotor sensitization (Borgland et al., 2006), and Orx-A/Hcrt-1-mediated NMDA receptor plasticity in the VTA is increased in rats self-administering cocaine (Borgland et al., 2009). Additionally, short-lasting neuroadaptations in VTA dopaminergic neurons induced by high-fat chocolate food pellets have been described (Borgland et al., 2009), suggesting that the Hcrt/Orx-VTA system initially participates in the regulation of the motivation to obtain potent reinforcers in general (drug or highly palatable food). In contrast, drug-induced neuroadaptation of the Hcrt/Orx-VTA system is long-lasting, an effect that may be linked to the possible “tilting” of this system toward promoting and controlling drug-directed behavior.
5. CRF Interactions with N/OFQ and Orx/Hcrt Relevant for Drug Seeking
5.1. N/OFQ
Major interactions exist between CRF and N/OFQ in the CeA and BNST, brain regions with a pivotal role in the regulation of behavioral responses to stress. Using CRF-induced anorexia as a model, the anorectic effects of intracerebroventricular or intra-BNST CRF administration are reversed by microinjection of N/OFQ into the BNST but not the CeA or other CRF-rich brain regions, including the LC, ventromedial hypothalamus (VMH), paraventricular nucleus of the hypothalamus (PVN), or DR (Ciccocioppo et al., 2003b). Confirming this effect, anorexia induced by intra-BNST administration of CRF in food-deprived rats was reversed by microinjection of N/OFQ into the BNST but not other CRF-rich sites (Ciccocioppo et al., 2003b). These findings suggest that N/OFQ acts as a functional CRF antagonist in the BNST, although the mechanism underlying this effect remains to be determined.
Evidence also supports interactions between N/OFQ and CRF in the CeA. In the alcohol-preferring msP rat line, administration of N/OFQ into the CeA, a key component of the extrahypothalamic CRF system, effectively reduces ethanol intake (Economidou et al., 2008). More direct evidence for N/OFQ-CRF interactions in the CeA comes from electrophysiological studies showing that CRF in the CeA, similar to ethanol, facilitates GABA release and increases GABAA receptor-mediated inhibitory postsynaptic potentials (IPSPs), an effect blocked by N/OFQ (Roberto and Siggins, 2006; Cruz et al., 2009a; Cruz et al., 2009b). Furthermore, basal GABA release in CeA neurons of ethanol-dependent rats is elevated and N/OFQ-induced inhibition of IPSPs is increased, indicating enhanced sensitivity to N/OFQ. Additionally, the ability of CRF to increase IPSPs is augmented in CeA neurons of ethanol-dependent rats, suggesting that the interactive effects of N/OFQ and CRF on GABAergic transmission in the CeA undergo significant neuroadaptation during the development of ethanol dependence (Cruz et al., 2009a; Cruz et al., 2009b). Altogether, these findings suggest that N/OFQ reduces ethanol drinking (see section 3.3), partially via a functional CRF antagonist action observed in the extended amygdala. Although direct evidence for this possibility is still lacking, alcohol-preferring msP rats show substantial N/OFQ gene overexpression in the CeA and BNST and overexpression of the NOP receptor gene in the CeA and BLA. These findings tentatively confirm the hypothesis that msP rats exhibit an upregulation of the N/OFQ system in the CeA , BNST, and BLA. The upregulation of the N/OFQ system may represent a compensatory response to a concurrent innate upregulation of the extrahypothalamic CRF system (Hansson et al., 2006) that contributes to increased spontaneous ethanol intake in this line of rats (Economidou et al., 2008).
5.2. Orx/Hcrt
Emerging evidence suggests that the Orx/Hcrt system participates in regulating stress responses and may be an integral part of the brain stress system (Paneda et al., 2005). For example, Orx/Hcrt peptides have been shown to induce activation of the HPA axis, an effect that is prevented by α-helical CRF9–41, a nonselective CRF antagonist (Jaszberenyi et al., 2000). CRF-immunoreactive terminals make direct synaptic contacts with hypocretin-expressing neurons in the LH, and numerous Orx/Hcrt neurons express CRF1 and CRF2 receptors, providing an anatomical basis for potential modulation of these neurons by CRF (Winsky-Sommerer et al., 2004). Furthermore, CRF and Orx/Hcrt appear to interact in regulating the effects of behavioral responses to stress, considering the evidence that acute restraint stress results in upregulation of orexin/hypocretin mRNA in the LH (Reyes et al., 2003) and both restraint and footshock stress induce c-fos expression in the perifornical region of the LH, an effect that is reduced in CRF1 knockout mice (Winsky-Sommerer et al., 2004). Finally, the LH Orx/Hcrt system projects to the BNST, a CRF-rich brain region with a critical role in the control of stress responses and stress-induced drug seeking (Cummings et al., 1983; Erb et al., 2001), suggesting that CRF and Orx/Hcrt may interactively regulate behavioral responses to stress in the BNST. Consistent with this hypothesis are recent findings showing that SB334867 attenuates stress-induced reinstatement of cocaine-seeking (Boutrel et al., 2005) and that Orx-A/Hcrt-1-induced reinstatement of cocaine-seeking is reversed by intracerebroventricular administration of the CRF1/CRF2 antagonist d-Phe CRF12–41 (Boutrel et al., 2005). Although unclear is whether d-Phe CRF12–41 produced this effect in the BNST, this finding provides direct evidence for interactions between CRF and Orx/Hcrt relevant for drug-seeking behavior associated with stress. However, in a recent study (Wang et al., 2009), intra-VTA administration of the Hcrt-r1 antagonist SB408124 was not effective in reducing stress-induced reinstatement of cocaine seeking, and intra-VTA injection of α-helical CRH9–41 had no effect on either glutamate release, dopamine release, or cocaine seeking induced by intra-VTA infusion of Orx-A/Hcrt-1. These findings suggest that although CRF and Orx/Hcrt may interact to regulate drug-seeking behavior in other brain areas, such interactions do not occur in the VTA.
6. Conclusion/Perspectives
Neuroadaptive changes in the extrahypothalamic CRF system induced by chronic drug use represent a major factor in the maintenance of drug and alcohol dependence. Growing evidence suggests that the N/OFQ and Orx/Hct neuropeptide systems functionally interact with and regulate the extrahypothalamic CRF system. Understanding the precise functional role of these interactions will be an important area of future research and ultimately may offer a possible avenue for restoring “normal” CRF function following chronic drug abuse. Finally, recent findings have identified interactions between the Orx/Hcrt and N/OFQ systems (Xie et al., 2008). N/OFQ potently inhibits the activity of Orx/Hcrt neurons (Xie et al., 2008), leading to the hypothesis that N/OFQ also modulates Orx/Hcrt-modulated functions, including behavioral response to stress, anxiety, reward, and addiction. Investigation of these interactions will be an important focus of future research on stress-regulatory neuropeptidergic systems.
Acknowledgments
This is publication number 20245 from The Scripps Research Institute. Supported by NIH/NIDA and NIH/NIAAA grants DA07348, DA08467, DA017097, AA014351, and AA006420. The authors thank M Arends for editorial assistance.
Abbreviations
- CRF
corticotropin-releasing factor
- Orx/Hcrt
orexin/hypocretin
- N/OFQ
nociceptin/orphanin FQ
- NOP
nociceptin/orphanin receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Aouizerate B, Ho A, Schluger JH, Perret G, Borg L, Le Moal M, Piazza PV, Kreek MJ. Glucocorticoid negative feedback in methadone-maintained former heroin addicts with ongoing cocaine dependence: dose-response to dexamethasone suppression. Addict Biol. 2006;11:84–96. doi: 10.1111/j.1369-1600.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- Aujla H, Martin-Fardon R, Sidhpura N, Zhao Y, Weiss F. Nociceptin differentially regulates anxiety-like behaviours in ethanol-dependent vs. non-dependent rats. Soc Neurosci Abstr. 2007;34:63.3. [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE. Overlapping distributions of orexin/hypocretin- and dopamine-β-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- Basso AM, Spina M, Rivier J, Vale W, Koob GF. Corticotropin-releasing factor antagonist attenuates the "anxiogenic- like" effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999;145:21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- Behan DP, De Souza EB, Lowry PJ, Potter E, Sawchenko P, Vale WW. Corticotropin releasing factor (CRF) binding protein: a novel regulator of CRF and related peptides. Front Neuroendocrinol. 1995;16:362–382. doi: 10.1006/frne.1995.1013. [DOI] [PubMed] [Google Scholar]
- Bonci A, Borgland S. Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology. 2009;56 Suppl 1:107–111. doi: 10.1016/j.neuropharm.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B. A neuropeptide-centric view of psychostimulant addiction. Br J Pharmacol. 2008;154:343–357. doi: 10.1038/bjp.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braconi S, Sidhpura N, Aujla H, Martin-Fardon R, Weiss F, Ciccocioppo R. Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2009.01119.x. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D'Souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Froldi R, Colombo G, Gessa GL, Massi M. Antidepressant-like effect of ethanol revealed in the forced swimming test in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 1999a;144:151–157. doi: 10.1007/s002130050988. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999b;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12:1145–1149. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Massi M. The nociceptin/orphanin FQ/NOP receptor system as a target for treatment of alcohol abuse: a review of recent work in alcohol-preferring rats. Physiol Behav. 2003a;79:121–128. doi: 10.1016/s0031-9384(03)00112-4. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003b;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleck JN, Blendy JA. Making a bad thing worse: adverse effects of stress on drug addiction. J Clin Invest. 2008;118:454–461. doi: 10.1172/JCI33946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Bajo M, Schweitzer P, Roberto M. Ethanol dependence induces neuroadaptation of the nociceptin and CRF systems at the GABAergic synapses in the central amygdala. Society for Neuroscience; Neuroscience Meeting Planner; Chicago, IL. 2009a. Program No. 552.17. 2009, 2009. Online. [Google Scholar]
- Cruz MT, Schweitzer P, Siggins GR, Roberto M. Nociceptin opposes ethanol and CRF action on GABAergic synapses in the amygdala. Alcohol Clin Exp Res Supp. 2009b;33:149A. [Google Scholar]
- Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Dave JR, Eiden LE, Karanian JW, Eskay RL. Ethanol exposure decreases pituitary corticotropin-releasing factor binding, adenylate cyclase activity, proopiomelanocortin biosynthesis, and plasma beta-endorphin levels in the rat. Endocrinology. 1986;118:280–286. doi: 10.1210/endo-118-1-280. [DOI] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Economidou D, Fedeli A, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Effect of novel nociceptin/orphanin FQ-NOP receptor ligands on ethanol drinking in alcohol-preferring msP rats. Peptides. 2006;27:3299–3306. doi: 10.1016/j.peptides.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CM, Abusnana S, Sunter D, Murphy KG, Ghatei MA, Bloom SR. The effect of the orexins on food intake: comparison with neuropeptide Y, melanin-concentrating hormone and galanin. J Endocrinol. 1999;160:R7–R12. doi: 10.1677/joe.0.160r007. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Le AD. Prior, repeated exposure to cocaine potentiates locomotor responsivity to central injections of corticotropin-releasing factor (CRF) in rats. Psychopharmacology (Berl) 2003;170:383–389. doi: 10.1007/s00213-003-1556-1. [DOI] [PubMed] [Google Scholar]
- Erb S, Funk D, Le AD. Cocaine pre-exposure enhances CRF-induced expression of c-fos mRNA in the central nucleus of the amygdala: an effect that parallels the effects of cocaine pre-exposure on CRF-induced locomotor activity. Neurosci Lett. 2005;383:209–214. doi: 10.1016/j.neulet.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Erb S, Brown ZJ. A role for corticotropin-releasing factor in the long-term expression of behavioral sensitization to cocaine. Behav Brain Res. 2006;172:360–364. doi: 10.1016/j.bbr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Erb S, Petrovic A, Yi D, Kayyali H. Central injections of CRF reinstate cocaine seeking in rats after postinjection delays of up to 3 h: an influence of time and environmental context. Psychopharmacology (Berl) 2006;187:112–120. doi: 10.1007/s00213-006-0392-5. [DOI] [PubMed] [Google Scholar]
- Fonareva I, Spangler E, Cannella N, Sabino V, Cottone P, Ciccocioppo R, Zorrilla EP, Ryabinin AE. Increased perioculomotor urocortin 1 immunoreactivity in genetically selected alcohol preferring rats. Alcohol Clin Exp. 2009;33:1956–1965. doi: 10.1111/j.1530-0277.2009.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, Richardson HN, O'Dell LE, Zorrilla EP, Morales M, Koob GF, Sanna PP. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Pollandt S, Liu J, Krishnan B, Genzer K, Orozco-Cabal L, Gallagher JP, Shinnick-Gallagher P. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. J Neurophysiol. 2007;97:937–941. doi: 10.1152/jn.00349.2006. [DOI] [PubMed] [Google Scholar]
- Funk D, Li Z, Shaham Y, Le AD. Effect of blockade of corticotropin-releasing factor receptors in the median raphe nucleus on stress-induced c-fos mRNA in the rat brain. Neuroscience. 2003;122:1–4. doi: 10.1016/j.neuroscience.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Gurkovskaya O, Goeders NE. Effects of CP-154,526 on responding during extinction from cocaine self-administration in rats. Eur J Pharmacol. 2001;432:53–56. doi: 10.1016/s0014-2999(01)01465-0. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther. 2003;304:874–880. doi: 10.1124/jpet.102.042788. [DOI] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29:6535–6544. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, Choi EA, McNally GP. Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci. 2009;29:802–812. doi: 10.1111/j.1460-9568.2009.06623.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, Clapham JC, Arch JR. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept. 2002;104:153–159. doi: 10.1016/s0167-0115(01)00358-5. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Young B, Pletcher MT, Heilig M, Wahlestedt C. Association between the nociceptin receptor gene (OPRL1) single nucleotide polymorphisms and alcohol dependence. Addict Biol. 2008;13:88–94. doi: 10.1111/j.1369-1600.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- Jaszberenyi M, Bujdoso E, Pataki I, Telegdy G. Effects of orexins on the hypothalamic-pituitary-adrenal system. J Neuroendocrinol. 2000;12:1174–1178. doi: 10.1046/j.1365-2826.2000.00572.x. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Suda T. Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus. Endocr J. 2009;56:335–344. doi: 10.1507/endocrj.k09e-075. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska J, Wichmann J, Legowska A, Rolka K, Silberring J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav Pharmacol. 2002;13:229–235. doi: 10.1097/00008877-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Rafalski P, Biala G, Dylag T, Rolka K, Silberring J. Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur J Pharmacol. 2003;474:233–239. doi: 10.1016/s0014-2999(03)02081-8. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. The role of corticotropin-releasing factor in the median raphe nucleus in relapse to alcohol. J Neurosci. 2002;22:7844–7849. doi: 10.1523/JNEUROSCI.22-18-07844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Schmidt D, Tilders F, Cole M, Smith A, Rivier C. Prolonged exposure to intermittent alcohol vapors blunts hypothalamic responsiveness to immune and non-immune signals. Alcohol Clin Exp Res. 2000;24:110–122. [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Nociceptin/orphanin FQ tissue concentration in the rat brain. Effects of repeated ethanol administration at various post-treatment intervals. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:303–306. doi: 10.1016/s0278-5846(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Liu Z, Huang M, Zhang Z. Dopamine-dependent responses to cocaine depend on corticotropin-releasing factor receptor subtypes. J Neurochem. 2003;84:1378–1386. doi: 10.1046/j.1471-4159.2003.01635.x. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Khaliq I, Carroll FI, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–176. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Lam H, Narayanan S. Alterations in the level of OFQ/N-IR in rat brain regions by cocaine. Neuropharmacology. 2008;55:198–203. doi: 10.1016/j.neuropharm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J Pharmacol Exp Ther. 2000;294:239–247. [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Francis DM, Katz ES, Hoks MA, Serge JP. Stressor- and corticotropin releasing factor-induced reinstatement and active stress-related behavioral responses are augmented following long-access cocaine self-administration by rats. Psychopharmacology (Berl) 2008;195:591–603. doi: 10.1007/s00213-007-0950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA. Relapse prevention: introduction and overview of the model. In: Marlatt GA, Gordon JR, editors. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. London: Guilford; 1985. pp. 3–70. [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- McKay JR, Rutherford MJ, Alterman AI, Cacciola JS, Kaplan MR. An examination of the cocaine relapse process. Drug Alcohol Depend. 1995;38:35–43. doi: 10.1016/0376-8716(95)01098-j. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich E, Lorang MT, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Meyer RE. The disease called addiction: emerging evidence in a 200-year debate. Lancet. 1996;347:162–166. doi: 10.1016/s0140-6736(96)90345-1. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996a;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Zhangs G, Belknap JK, Grandy DK. Functional antagonism of μ-, δ- and κ-opioid antinociception by orphanin FQ. Neurosci Lett. 1996b;214:131–134. doi: 10.1016/0304-3940(96)12917-7. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Grisel JE, Robbins CS, Grandy DK. Antinociception mediated by the periaqueductal gray is attenuated by orphanin FQ. Neuroreport. 1997;8:3431–3434. doi: 10.1097/00001756-199711100-00003. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Maidment NT. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr., Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneda C, Winsky-Sommerer R, Boutrel B, de Lecea L. The corticotropin-releasing factor-hypocretin connection: implications in stress response and addiction. Drug News Perspect. 2005;18:250–255. doi: 10.1358/dnp.2005.18.4.908659. [DOI] [PubMed] [Google Scholar]
- Parsons MP, Li S, Kirouac GJ. The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse. 2006;59:480–490. doi: 10.1002/syn.20264. [DOI] [PubMed] [Google Scholar]
- Perry SJ, Junger S, Kohout TA, Hoare SR, Struthers RS, Grigoriadis DE, Maki RA. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem. 2005;280:11560–11568. doi: 10.1074/jbc.M412914200. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Filip M, Frankowska M, Zaniewska M, Papla I. Effects of CP 154,526, a CRF1 receptor antagonist, on behavioral responses to cocaine in rats. Neuropeptides. 2005;39:525–533. doi: 10.1016/j.npep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamo-pituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24:1836–1849. [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999;32:254–261. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Richter RM, Zorrilla EP, Basso AM, Koob GF, Weiss F. Altered amygdalar CRF release and increased anxiety-like behavior in Sardinian alcohol-preferring rats: a microdialysis and behavioral study. Alcohol Clin Exp Res. 2000;24:1765–1772. [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. Proc Natl Acad Sci U S A. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl) 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Carrera MRA, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Rosin A, Kitchen I, Georgieva J. Effects of single and dual administration of cocaine and ethanol on opioid and ORL1 receptor expression in rat CNS: an autoradiographic study. Brain Res. 2003;978:1–13. doi: 10.1016/s0006-8993(03)02674-x. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Central administration of nociceptin/orphanin FQ blocks the acquisition of conditioned place preference to morphine and cocaine, but not conditioned place aversion to naloxone in mice. Psychopharmacology (Berl) 2004;172:129–136. doi: 10.1007/s00213-003-1643-3. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Endogenous nociceptin (orphanin FQ) suppresses basal hedonic state and acute reward responses to methamphetamine and ethanol, but facilitates chronic responses. Neuropsychopharmacology. 2008;33:877–891. doi: 10.1038/sj.npp.1301459. [DOI] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Enhanced nicotine sensitivity in nociceptin/orphanin FQ receptor knockout mice. Neuropharmacology. 2009;56:896–904. doi: 10.1016/j.neuropharm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Hohn J, Szabo G, Penke B. Critical role of endogenous corticotropin-releasing factor (CRF) in the mediation of the behavioral action of cocaine in rats. Life Sci. 1992;51:2019–2024. doi: 10.1016/0024-3205(92)90151-e. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Gardi J, Vecsernyes M, Julesz J, Telegdy G. Brain corticotropin-releasing factor mediates 'anxiety-like' behavior induced by cocaine withdrawal in rats. Brain Res. 1995;675:89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z. Neurobiology of stress and cocaine addiction: studies on corticotropin-releasing factor in rats, monkeys, and humans. Ann NY Acad Sci. 1998;851:371–387. doi: 10.1111/j.1749-6632.1998.tb09011.x. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O'Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF1 receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, Braconi S, Economidou D, Cippitelli A, Ubaldi M, Clementi S, Massi M, Ciccocioppo R. Activation of central nociceptin/orphanin FQ receptors reduced alcohiol drinking and alcohol withdrawal associated symptoms. Behav Pharmacol. 2009;20:S32. [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and β-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. J Subst Abuse Treat. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5496. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wisor JP, Hara J, Crowder TL, LeWinter R, Khroyan TV, Yamanaka A, Diano S, Horvath TL, Sakurai T, Toll L, Kilduff TS. Hypocretin/orexin and nociceptin/orphanin FQ coordinately regulate analgesia in a mouse model of stress-induced analgesia. J Clin Invest. 2008;118:2471–2481. doi: 10.1172/JCI35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuei X, Flury-Wetherill L, Almasy L, Bierut L, Tischfield J, Schuckit M, Nurnberger JI, Jr., Foroud T, Edenberg HJ. Association analysis of genes encoding the nociceptin receptor (OPRL1) and its endogenous ligand (PNOC) with alcohol or illicit drug dependence. Addict Biol. 2008;13:80–87. doi: 10.1111/j.1369-1600.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport. 2003;14:2383–2385. doi: 10.1097/00001756-200312190-00019. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The roles of urocorins 1, 2 and 3 in the brain. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of Stress and the Brain (series title: Techniques in the Behavioral and Neural Sciences, vol. 15) New York: Elsevier Science; 2005. pp. 179–203. [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]