Abstract
The respiratory-related evoked potential (RREP) is increasingly used to study the neural processing of respiratory signals. However, little is known about the cortical origins of early (Nf, P1, N1) and later RREP components (P2, P3). By using high-density EEG, we studied cortical sources of RREP components elicited by short inspiratory occlusions in 18 healthy volunteers (6 female, mean age 20.0 ± 1.8 years). Topographical maps for Nf and P1 showed bilateral maximum EEG voltages over the frontal and centro-parietal cortex, respectively. Cortical source analyses (minimum norm estimates) in addition to topographical maps demonstrated bilateral sensorimotor cortex origins for N1 and P2 which were paralleled by an additional frontal cortex source (p’s < 0.05). The source of the P3 was located at the parietal cortex (p < 0.05). The results support previous findings on the cortical sources of early RREP components Nf , P1 and N1 and demonstrate the cortical sources of later RREP components P2 and P3.
Keywords: cortex, EEG, perception, respiratory-related evoked potential, minimum norm estimate
1. Introduction
The respiratory-related evoked potential (RREP) recorded from the electroencephalogram (EEG) is an important, increasingly used non-invasive technique for studying the neural processing of respiratory signals (Davenport et al., 1986). The RREP is a measure of cerebral cortical activity elicited by short inspiratory occlusion or breathing against inspiratory resistive loads and quantifies the initial arrival and further processing of sensory afferent respiratory information in the cortex. The RREP contains early components Nf, P1 and N1 (< 130 ms post stimulus) and later components P2 and P3 (> 150 ms post stimulus). The early components presumably reflect the initial arrival and first-order processing of afferent respiratory signals in sensory regions, whereas the later components P2 and P3 may characterize subsequent higher-order cognitive processing in other cortical areas (Chan and Davenport, in press). However, the spatio-temporal cortical dynamics underlying these scalp-recorded events are not well understood. The rare studies estimating the cortical generators of the RREP have been associated with limitations such as small numbers of EEG electrodes, focus on less informative voltage topography maps or small sample sizes. In addition, most previous work looked at the early RREP components Nf and P1 (Davenport et al., 1996; Logie et al., 1998), whereas no source space analysis is available for the components N1, P2 and P3.
In the present study, we examined the possible cortical sources of the Nf, P1, N1, P2 and P3 RREP components elicited by short inspiratory occlusions using an improved methodology of distributed source modeling, in addition to scalp topography maps. Taking advantage of a dense-array EEG montage (129 sensors), scalp voltages were converted to an estimated spherical source space using the minimum-norm estimate (MNE; Hauk 2004), a robust and conservative source estimation algorithm.
2. Method
After providing informed written consent, eighteen healthy, non-smoking volunteers without respiratory disease participated in this study (6 female, mean age 20.0 ± 1.8 years, mean forced expiratory volume in 1 s (FEV1) 3.78 ± 0.84 L), that was approved by the Institutional Review Board of the University of Florida.
The experimental protocol was divided into 4 blocks of 7 min, separated by 2min resting intervals. Each block started with a 1 min epoch of adaptation to the mouthpiece breathing during which no inspiratory occlusions were presented. A 6 min epoch with inspiratory occlusions followed while participants viewed pictures presented on a monitor. In each block, participants breathed, via a mouthpiece, through a breathing circuit consisting of a non-rebreathing two-way valve (Hans Rudolph Inc., Kansas City, USA) with the nose occluded by a clip. The inspiratory port of the valve was connected to a pressure activated occluder. Mouth pressure (Pm, in cmH2O) was continuously recorded from the center of the valve by a differential-pressure transducer (model MP-45, Validyne Engineering, Northridge, USA), connected to a PowerLab biosignal recording unit (ADInstruments, Bella Vista, Australia), and displayed on a monitor. Inspiration was interrupted by manual activation of the occluder after the onset of inspiration as indicated by the Pm signal on the monitor with a parallel marker signal being sent to the EEG recorder. Occlusions (160 ms duration) were presented every two to six breaths resulting on average in a total of 93 occlusions per participant.
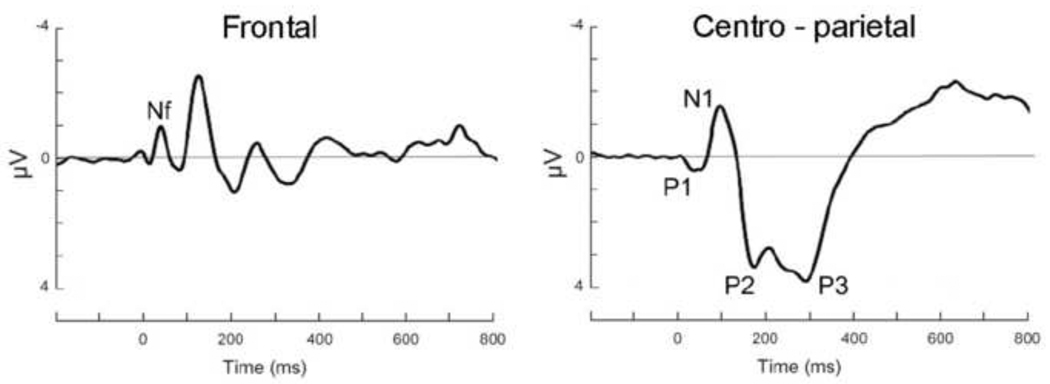
EEG data were recorded from the scalp using a 129-channel system (Electrical Geodesics Inc., Eugene, USA) with scalp impedance < 50 kΩ, sampling rate = 250 Hz, vertex sensor as reference electrode and on-line bandpass filter (0.1 to 56 Hz). All further processing was performed offline, using functions built into BESA 5.1. After low-pass filtering at 30 Hz, segments with incomplete inspiratory occlusions were removed and ocular artifacts were corrected using the algorithm implemented in BESA. Occlusion epochs were then extracted (200 ms pre- and 1300 ms post-stimulus) and averaged across the 4 blocks for each participant. Using a maximum of 200 µV as the cutoff amplitude, 72 occlusion epochs per participant were retained on average. Based on previous reports (e.g., Davenport et al., 1986; Redolfi et al., 2005; Webster and Colrain, 2000a), the RREP components were identified as follows: Nf = negative peak in the frontal region (latency: 25–50 ms), P1 = positive peak in the centro-parietal region (latency: 45–65 ms), N1 = negative peak in the centro-lateral region (latency: 85–125 ms), P2 = positive peak in the central region (latency: 160– 230 ms), and P3 = positive peak in the centro-parietal region (latency: 250–350 ms).
After calculating the grand average RREP waveform across participants, scalp topography maps of the EEG voltage were created for the RREP components. Cortical sources for each component were then estimated for each individual using a minimum norm estimation (MNE) technique as described by Hauk (2004). Briefly, the MNE is an inverse method for reconstructing the primary current that underlies an extracranially recorded time-locked brain potential such as the RREP. As it does not require a priori assumptions on the number and location of electrocortical sources, it is particularly well suited for an exploratory analysis of distributed sources in mid-latency and late brain potentials. The signal origin was estimated using 4096 model source locations, being equidistantly arranged on three concentric shells (80%, 60% and 40% of electrode radius) to approximate the brain volume. The Tikhonov–Philips approach was applied for spatial regularization with a factor of λ = 0.01 to suppress uncorrelated noise. The shell at 60% electrode radius was used, because this shell yields the best compromise between depth sensitivity and spatial resolution (Hauk, 2004). The 129 locations on this shell nearest to the electrode locations were selected for further analysis.
The statistical significance of the consistency of source topographies across the participants was examined by entering individual MNE amplitudes into separate permutation analyses for each RREP component. Specifically, it was tested whether and where the peak source strength locations (defined as those source locations with intensities of at least 60 % above the mean of a given source topography) obtained for each participant overlap more frequently than in 95% of 8000 permutation runs in which the peak location was randomly drawn from all possible locations. This permutation technique yields the critical threshold for the number of participants with overlapping source maxima that are needed to reach a p < 0.05 criterion under the null hypothesis (Keil et al., 2006).
3. Results
Figure 1 shows the observed RREP components Nf, P1, N1, P2 and P3 after inspiratory occlusions over the frontal and centro-parietal region.
Figure 1.
Group mean respiratory-related evoked potential over the frontal and centro-parietal region elicited by inspiratory occlusions.
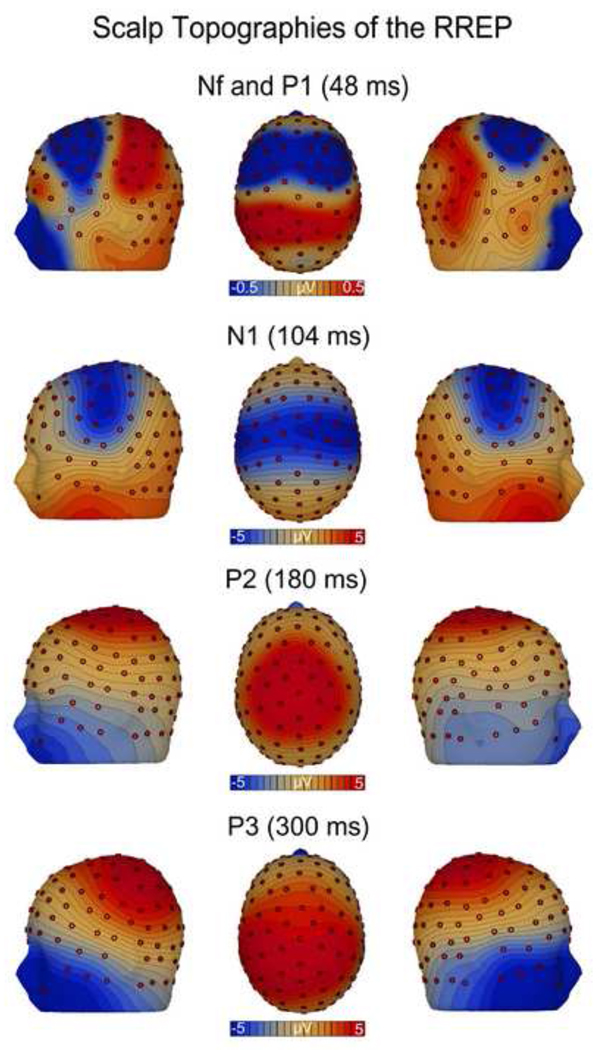
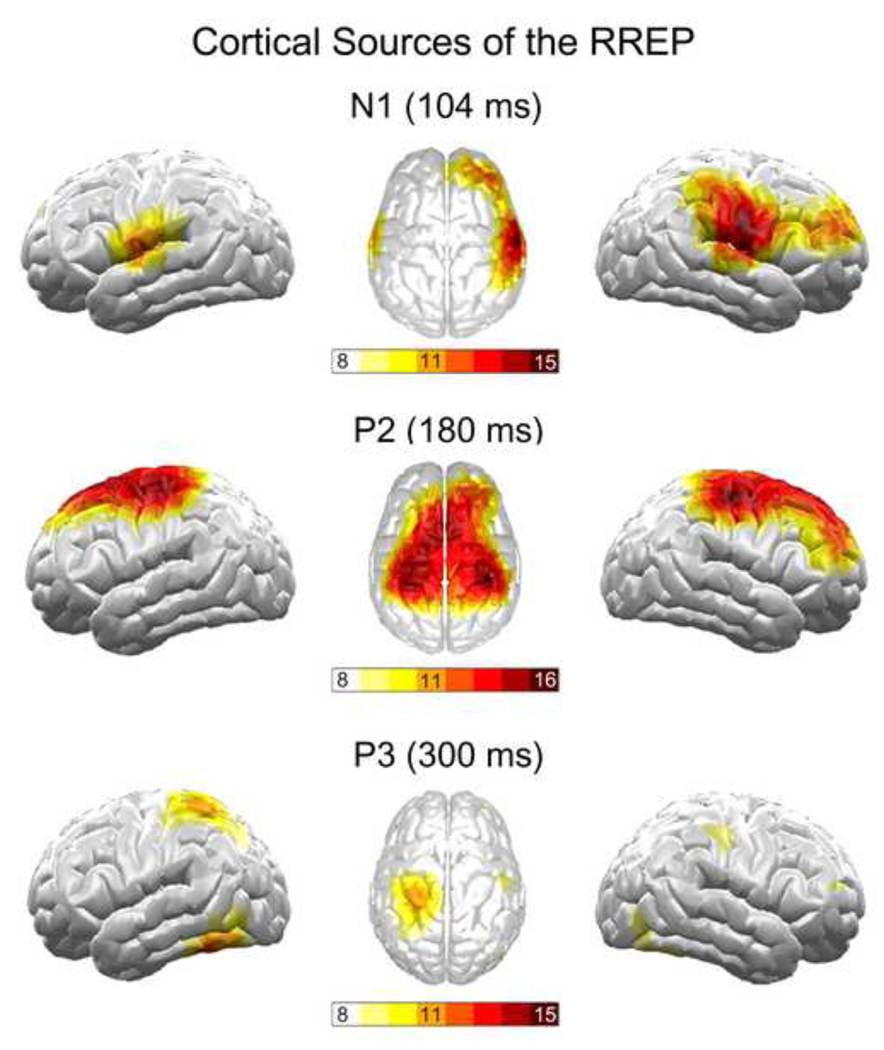
The scalp topography maps showed a bilateral negative EEG voltage over the frontal cortex for Nf and a positive EEG voltage over the centro-parietal cortex for P1 (Fig. 2). Due to small signal intensities (< 1 µV), the MNE source analyses did not yield a stable source solution for Nf and P1. The scalp topography map for N1 demonstrated a large negative band overlying wide areas of the sensorimotor strips with maximal EEG voltages at lateral sides (Fig. 2). The MNE source analyses revealed a corresponding source maximum in the lateral sensorimotor cortices (p < 0.05), but no midline source. An additional source maximum was found in the right lateral frontal cortex (p < 0.05; Fig. 3). The scalp topography map for P2 showed a positive EEG voltage over the sensorimotor cortex with its maximum around the vertex (Fig. 2). The MNE source analyses demonstrated a corresponding source in the sensorimotor cortices lateral to the vertex region (p < 0.05). An additional source peak was found in the midline frontal cortex (p < 0.05; Fig. 3). The scalp topography for P3 was characterized by widespread positive voltage over the centro-lateral parietal cortex (Fig. 2). The MNE source analyses revealed a corresponding source pattern, with maxima in the lateral parietal cortices (p < 0.05), with a left hemisphere dominance.
Figure 2.
Group mean scalp topography maps of the EEG voltage for the RREP components Nf, P1, N1, P2 and P3 which were mapped at their peak latencies.
Figure 3.
Cortical sources for the RREP components N1, P2 and P3 based on minimum norm estimation (MNE). Increasing color intensity indicates increasing overlap between individual MNE’s. For each component, a spatial overlap between 11 or more individuals indicated statistical significance at p < 0.05 which is graphically thresholded with the colors orange until dark red.
4. Discussion
The present study examined the cortical sources of the Nf, P1, N1, P2 and P3 RREP components by using high-density EEG and MNE source analyses in addition to scalp topography maps. Topography maps for Nf and P1 showed temporally overlapping maximum EEG voltages over the frontal and centro-parietal cortex, respectively. This supports parallel pathways for these two components with Nf related to pre-motor and P1 related to somatosensory cortices (Davenport et al., 1996; Logie et al., 1998). Results of the MNE analyses demonstrate a lateral sensorimotor cortex origin of the N1 and P2. This converges with recent findings of sensorimotor cortex activation after stimulation of respiratory pump receptors (Chan and Davenport, in press; Davenport and Vovk, 2009). The MNE analyses suggested additional sources in the right lateral frontal cortex for the N1 and in the midline frontal cortex for the P2, which were not observed with voltage topographies. For the P3, the MNE source analyses showed a dipole source in the lateral parietal cortices, which generally corresponded with the voltage map. This source in the associative cortex region is consistent with the interpretation of P3 as reflecting associative and attentional processing, rather than strictly sensory processing (Chan and Davenport, in press; von Leupoldt et al., in press).
The present results with advanced methodology such as high-density EEG and MNE source analyses are consistent with previous findings from RREP studies that exclusively used topography mapping of EEG voltages, which is vulnerable to misinterpretations of underlying cortical source generators (Michel et al., 2004). For example, Davenport et al. (1996) suggested a comparable sensorimotor cortex localization of the N1 in a sample of children which was replicated in adults by Webster and Colrain (1998; 2000a). Corresponding topographic localizations were also reported for the P2 in sensorimotor areas and for the P3 in parietal areas (Webster and Colrain, 1998, 2000a), which is further supported by the inspection of graphs for centro-parietal EEG sensors (C3, Cz, C4, P3, Pz, P4) in other RREP studies (Harver et al., 1995, Webster and Colrain, 2000b). However, the present additional frontal cortex sources for the N1 and P2 have not been reported before. As recently suggested, these sources might reflect parallel motor activation, preparation for action or cognitive processing of respiratory mechanosensation (Davenport and Vovk, 2009), but future studies are required to follow up on this hypotheses.
A limitation of the present study is the reduced spatial resolution provided by the MNE source analyses (~ 3.5 cm), a problem of most source estimation techniques of EEG data (Michel et al., 2004). This prevents an allocation of the obtained dipole sources for inspiratory occlusions to specific areas within the sensorimotor cortex, for example somatosensory area 3, which showed activation during respiratory muscle stimulation in animal research (for review see Davenport and Vovk, 2009). To address this issue, future studies using intracranial electrodes or imaging techniques such as fMRI are clearly needed, which might benefit from using the present cortical sources of the RREP as target areas.
In summary, this study demonstrated by using MNE cortical source analyses and topographical maps bilateral sensorimotor cortex origins for RREP components N1 and P2 which were paralleled by an additional frontal cortex source. The source of the P3 was located at the parietal cortex. The results improve our understanding of the neural processing of respiratory sensations and contribute to a more precise spatial interpretation of findings obtained with the RREP technique.
Acknowledgement
This study was supported in part by a grant from the National Institute of Mental Health (P50 MH 72850) to Peter J. Lang, and by a stipend (Heisenberg-Stipendium, DFG LE 1843/9-1) from the German Research Society (Deutsche Forschungsgemeinschaft, DFG) to Andreas von Leupoldt. The funding sources had no impact on the study design, data collection/interpretation or preparation of the present manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chan PS, Davenport PW. Respiratory Related Evoked Potential Measures of Cerebral Cortical Respiratory Information Processing. Biol. Psych. doi: 10.1016/j.biopsycho.2010.02.009. in press. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Colrain IM, Hill PM. Scalp topography of the short-latency components of the respiratory-related evoked potential in children. J. Appl. Physiol. 1996;80:1785–1791. doi: 10.1152/jappl.1996.80.5.1785. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Friedman WA, Thompson FJ, Franzen O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J. Appl. Physiol. 1986;60:1843–1848. doi: 10.1152/jappl.1986.60.6.1843. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neurobiol. 2009;167:72–86. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Harver A, Squires N, Bloch-Salisbury E, Katkin E. Event-related potentials to airway occlusion in young and old subjects. Psychophysiology. 1995;32:121–129. doi: 10.1111/j.1469-8986.1995.tb03303.x. [DOI] [PubMed] [Google Scholar]
- Hauk O. Keep it simple: A case for using classical minimum norm estimation in the analysis of eeg and meg data. Neuroimage. 2004;21:1612–1621. doi: 10.1016/j.neuroimage.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Keil A, Ihssen N, Heim S. Early cortical facilitation for emotionally arousing targets during the attentional blink. BMC Biol. 2006;4:23. doi: 10.1186/1741-7007-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logie SL, Colrain IM, Webster KE. Source localisation of the early components of the respiratory-related evoked potential. Brain Topogr. 1998;11:153–164. doi: 10.1023/a:1022210723257. [DOI] [PubMed] [Google Scholar]
- Michel CM, Murray MM, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. EEG source imaging. Clin. Neurophysiol. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Redolfi S, Raux M, Donzel-Raynaud C, Morelot-Panzini C, Zelter M, Derenne JP, Similowski T, Straus C. Effects of upper airway anaesthesia on respiratory-related evoked potentials in humans. Eur. Respir. J. 2005;26:1097–1103. doi: 10.1183/09031936.05.00139804. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Vovk A, Bradley MM, Keil A, Lang PJ, Davenport PW. The impact of emotion on respiratory-related evoked potentials. Psychophysiology. doi: 10.1111/j.1469-8986.2009.00956.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KE, Colrain IM. The relationship between respiratory-related evoked potentials and the perception of inspiratory resistive loads. Psychophysiology. 2000a;37:831–841. [PubMed] [Google Scholar]
- Webster KE, Colrain IM. The respiratory-related evoked potential: effects of attention and occlusion duration. Psychophysiology. 2000b;37:310–318. [PubMed] [Google Scholar]