Abstract
Stress is most often associated with aversive states. It rapidly induces the release of hormones and neuropeptides including dynorphin, which activates kappa opioid receptors (KORs) in the central and peripheral nervous systems. In animal models, many aversive effects of stress are mimicked or exacerbated by stimulation of KORs in limbic brain regions. Although KOR signaling during acute stress may increase physical ability (by producing analgesia) and motivation to escape a threat (by producing aversion), prolonged KOR signaling in response to chronic or uncontrollable stress can lead to persistent expression of behavioral signs that are characteristic of human depressive disorders (i.e., “prodepressive-like” signs). Accumulating evidence suggests that KORs contribute to the progressive amplification (sensitization) of stress-induced behaviors that occurs with repeated exposure to stress. Many of the aversive effects of stress are blocked by KOR antagonists, suggesting that these agents may have potential as therapeutics for stress-related conditions such as depression and anxiety disorders. This review summarizes current data on how KOR systems contribute to the acute (rapid), delayed, and cumulative molecular and behavioral effects of stress. We focus on behavioral paradigms that provide insight on interactions between stress and KOR function within each of these temporal categories. Using a simplified model, we consider the time course and mechanism of KOR-mediated effects in stress and suggest future directions that may be useful in determining whether KOR antagonists exert their therapeutic effects by preventing the development of stress-induced behaviors, the expression of stress-induced behaviors, or both.
Keywords: stress, depression, anxiety, dynorphin, kappa opioid, model, rat, mouse
1. Introduction
1.1. Kappa Opioid Receptor (KOR) System and Depression
The endogenous opioid system is an important mediator of emotional and behavioral responses to stress. It comprises three families of neuropeptides (endorphins, enkephalins, and dynorphins) and three cognate receptor subtypes (mu [MOR], delta [DOR], and kappa [KOR]). The dynorphin family of neuropeptides (herein referred to as “dynorphin”) comprises six peptides of varying lengths that are formed from the precursor prodynorphin (PDyn; see Schwarzer, 2009) and which activate KORs located in the peripheral and central nervous systems (Chavkin et al., 1982). Although activation of all three opioid receptor subtypes produces analgesia via inhibition of ascending pain fibers, central opioid receptor signaling produces opposing effects on mood: MOR or DOR activation elevates mood (Filliol et al., 2000; Shippenberg et al., 2008) whereas KOR activation produces dysphoria (defined here as an unpleasant or aversive state) in humans (Pfeiffer et al., 1986; Wadenberg, 2003) and prodepressive-like behaviors (including those thought to reflect anhedonia, dysphoria, and anxiety) in rodents (Bals-Kubik et al., 1993; Mague et al., 2003; Carlezon et al., 2006; Carlezon et al., 2009). Even salvinorin A—a selective KOR agonist currently marketed as a safe and legal hallucinogen—produces anxiogenic and otherwise unpleasant effects in humans that deter repeated or compulsive use (Gonzalez et al., 2006).
During acute stress, KOR signaling may increase physical ability (by producing analgesia) and motivation to escape threat (by producing aversion) and thereby facilitate adaptive responses. However, prolonged KOR signaling in response to chronic or uncontrollable stress may lead to persistent changes in behavior that are characteristic of those seen in human depressive disorders (see Kessler, 1997; Nestler and Carlezon, 2006; Pittenger and Duman, 2008). Animal models have been instrumental in the study of KORs within the context of stress and depressive disorders: tests such as place conditioning and intracranial self stimulation (ICSS) are sensitive to treatments that cause aversion (dysphoria) or reduced sensitivity to rewarding stimuli (anhedonia), and the intensity of these signs can be quantified. Behavioral signs in rodents that resemble the behavioral signs that are observable in humans with depressive disorders are often qualified with the suffix “-like”, to acknowledge the imperfection inherent in models where individuals cannot articulate their symptoms.
The prodepressive-like consequences of stress in rodents are decreased by KOR antagonists or by ablation of the genes encoding KORs or PDyn (Newton et al., 2002; Mague et al., 2003; McLaughlin et al., 2003; Beardsley et al., 2005; McLaughlin et al., 2006a; Bruchas et al., 2007b). These antidepressant-like effects are often most apparent after repeated stress, suggesting that the KOR system may be especially important in mediating the amplification or sensitization of stress responses. Many other stress-responsive systems have been implicated in the etiology and pathophysiology of mood disorders, including those utilizing cortisol, corticotropin releasing factor (CRF), vasopressin, and brain derived neurotrophic factor (BDNF) (de Kloet et al., 2005; Duman and Monteggia, 2006; Zhang et al., 2007; Koob, 2008; Mathew et al., 2008). Dynorphin signaling also affects—and is affected by—these other stress-responsive systems, highlighting the coordinated role these systems play in regulating the stress response and in establishing individual vulnerability or resiliency to stress-related disorders (Nair et al., 2005; Feder et al., 2009).
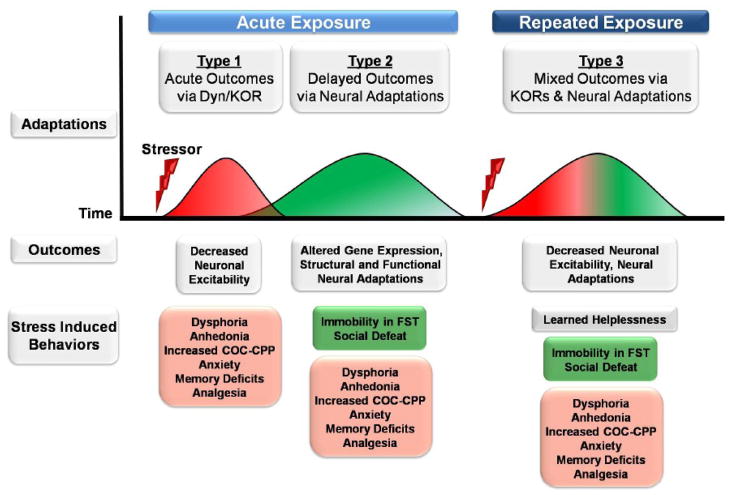
The objective of this review is to summarize currently available data on how the KOR system contributes to molecular and behavioral effects of stress. We focus on behavioral paradigms that differentiate between the role of KORs in mediating acute (rapid), delayed, and cumulative effects of stress. For the purposes of developing testable hypotheses, we divide KOR-mediated stress responses into three temporal categories: 1) acute responses to stress—such as changes in behavior thought to reflect dysphoria or anhedonia—that do not require prior stress exposure and are primarily mediated by KOR-induced changes in the activity of limbic circuits [acute expose, acute outcomes]; 2) delayed or sensitized responses to stress—such as changes in behavior thought to reflect altered coping strategies (e.g., increased immobility in the forced swim test)—that require prior stress exposure to occur and are mediated at least in part by neural adaptations that are a consequence or cause of increased KOR signaling [acute exposure, delayed outcomes]; and 3) delayed responses to stress that are produced by repeated stress exposure and likely require multiple rounds of neural adaptations to occur (e.g., changes in behavior thought to reflect learned helplessness) [repeated exposure, mixed outcomes] (Fig. 1). Using this simplified model, we consider the time course and mechanisms of KOR-mediated effects in stress. It is important to note that many studies using KOR antagonists have been designed to accommodate the slow onset of maximal antagonism (4–24h) and extended duration of action (>3 weeks) of currently available antagonists (e.g., norBNI, JDTic) (Endoh et al., 1992; Horan et al., 1992; Jones and Holtzman, 1992; Carroll et al., 2004; Beardsley et al., 2005; Bruchas et al., 2007a). The unusual pharmacology of these compounds limits some of the conclusions that can be drawn about the role of KORs in the development and expression of stress-induced behaviors. In the final section we compare the effects of KOR antagonists when they have been given before, between, or after stress exposure, and suggest future directions that may be helpful in determining whether KOR antagonists exert their therapeutic effects by preventing the development of stress-induced behaviors, the expression of stress-induced behaviors, or both.
Figure 1.
Simplified model depicting the time course of stress-induced molecular and behavioral effects. Stress activates the release of hormones and neuropeptides, including dynorphin, which activates kappa opioid receptors (KORs) in the central and peripheral nervous systems (Adaptations). Acute exposure to stress (or KOR agonist) initiates rapid changes in neuronal function (red waveform; Type 1) that are mediated in part by KOR-mediated decreases in neuronal excitability (Outcomes). These acute decreases in neuronal excitability, which may increase or decrease the activity of neural networks, are associated with the rapid expression of a number of stress-induced behaviors that do not require prior exposure to stress to occur (red box). Acute exposure to stress also initiates delayed molecular changes (green waveform; Type 2), such as altered gene expression, that produce structural and functional neural adaptations. Stress-induced KOR signaling can be a cause or consequence of these neural adaptations and can contribute to the expression of sensitized stress responses (light green box) that require prior stress exposure to occur. Finally, re-exposure to stress initiates acute KOR signaling (red portion of waveform; Type 3) and additional neural adaptations (green portion of waveform) that are associated with Type 1 and 2 behavioral responses (red and green boxes, respectively), but may also contribute to the emergence of additional stress-sensitized behaviors that require multiple stress exposures to occur (gray box). The antidepressant- and anxiolytic-like properties of KOR antagonists may result from the prevention of acute and delayed effects of KOR signaling.
1.2. Dynorphin Signaling: A Neuropeptide Brake on Neuronal Activity
The signaling mechanisms of neuropeptides and classical neurotransmitters (e.g., glutamate, GABA) differ considerably across a number of parameters, suggesting they have different roles in information processing (see Hokfelt et al., 2000; Ludwig and Leng, 2006). Classical neurotransmitters are released primarily at synaptic active zones in response to single action potentials and typically activate cognate receptors on one postsynaptic target. The high fidelity and specificity of this signal depends on several mechanisms that restrict its spatiotemporal profile, including rapid neurotransmitter degradation, reuptake mechanisms, and low (μM) receptor affinity. In contrast, neuropeptides are released at both synaptic and extrasynaptic sites in response to sustained neuronal activity. Upon release, neuropeptides are more slowly degraded by extracellular peptidases and are therefore able to diffuse much greater distances (~50–100 μm). This mode of action enables neuropeptides to more broadly activate their receptors, which have a high (nM) affinity (see Chavkin, 2000). Based on these differences, recent hypotheses suggest that classical neurotransmitters convey information between pairs of neurons whereas neuropeptides convey information and coordinate activity across broader networks of neurons (Ludwig and Leng, 2006). It is important to note that these distinctions are less apparent for neuromodulators such as dopamine (DA) or serotonin, which often signal extrasynaptically (Benfenati and Agnati, 1991; Hensler, 2006; Rice and Cragg, 2008).
Similar to other neuropeptides, dynorphin is released from large dense core vesicles (Cho and Basbaum, 1989; Drake et al., 1994) in response to sustained neuronal activity and activates KORs (Weisskopf et al., 1993). KORs are coupled to inhibitory Gi/o-proteins and typically decrease synaptic transmission by inhibiting adenylate cyclase, inhibiting voltage-gated Ca2+ channels (Rusin et al., 1997; Hjelmstad and Fields, 2003), and activating voltage-gated K+ channels (Simmons and Chavkin, 1996; Vaughan et al., 1997). Activation of presynaptic KORs may also decrease synaptic transmission by directly inhibiting vesicle fusion (Iremonger and Bains, 2009). In addition to rapid effects on ion channel conductance, KORs also activate signal transduction cascades, including mitogen-activated protein kinases (MAPKs), which in turn activate transcription factors and alter gene expression (see Thomas and Huganir, 2004). Growing evidence indicates that activity-dependent dynorphin release, especially from dendritic sites, may be a particularly effective mechanism by which neurons regulate their own activity (Drake et al., 1994; Brown and Bourque, 2004; Ludwig and Leng, 2006; Kreibich et al., 2008; Iremonger and Bains, 2009). Indeed, dendritic dynorphin release in the hippocampus and hypothalamus negatively regulates excitatory inputs via retrograde activation of presynaptic KORs (Drake et al., 1994; Iremonger and Bains, 2009). This inhibitory mechanism may generalize to other neuronal populations often implicated in the regulation of mood and motivation, such as the amygdala and striatum, which express dendritic dynorphin (Yakovleva et al., 2006; Reyes et al., 2007). Dendritic neuropeptide release may serve as an independent (auxiliary) mechanism of inhibition that can be engaged rapidly and broadly in response to high neuronal activity, without compromising or taxing existing feedforward and feedback inhibitory circuits. Because existing inhibitory circuits have a critical role in the computational processes of neurons (Mittmann et al., 2004), this auxiliary mechanism of inhibition may help to preserve information processing during conditions of high neuronal activity, such as during stress, which triggers dynorphin release in limbic brain regions (Schwarzer, 2009).
Although the role of KORs in the regulation of mood is not fully understood, dynorphin and KORs are expressed throughout limbic brain areas implicated in the pathophysiology of depression and anxiety disorders. Such areas include the mesocorticolimbic DA system [comprising the ventral tegmental area (VTA), nucleus accumbens (NAc), and prefrontal cortex (PFC)], the serotonergic and noradrenergic systems [comprising major cell groups in the dorsal raphe nucleus and locus coeruleus, respectively], the extended amygdala [comprising the central nucleus of the amygdala (CeA), bed nucleus of the stria terminalis (BNST), and NAc shell], the basolateral amygdala, hippocampus (HIP), and hypothalamus in humans and rodents (Fallon and Leslie, 1986; Mansour et al., 1995; Sukhov et al., 1995; Hurd, 1996; Peckys and Landwehrmeyer, 1999; Shuster et al., 2000; Alheid, 2003; Nestler and Carlezon, 2006; Koob, 2008; Schwarzer, 2009). In this review we focus primarily on interactions between KORs and dopaminergic systems because much is known about how manipulations of DA function affect motivation, which is invariably dysregulated in depressive disorders. However, it is clear that KORs are also involved in the regulation of serotonergic (Tao and Auerbach, 2002, 2005; Berger et al., 2006; Land et al., 2008; Zakharova et al., 2008) and noradrenergic (Pinnock, 1992; Berger et al., 2006; Reyes et al., 2007; Kreibich et al., 2008; Reyes et al., 2009) systems, which are the primary targets of standard antidepressant drugs (Frazer, 1997; Millan, 2004). Given the important roles of serotonin and norepinephrine in stress and behavior (Vergne and Nemeroff, 2006; Lowry et al., 2008; Smith and Aston-Jones, 2008; Valentino and Van Bockstaele, 2008), a more thorough characterization of these interactions will be essential for a complete understanding of the neurobiology of mood. Finally, dynorphin expression overlaps with that of other neuropeptide systems involved in stress and motivation, including CRF, neuropeptide Y, and vasopressin (Lin et al., 2006; Marchant et al., 2007; Reyes et al., 2008; Iremonger and Bains, 2009). Thus the KOR system is ideally positioned to produce broad effects on behavior, perhaps by serving as a braking mechanism to counteract elevations in neuronal activity induced by stress.
It is important to differentiate between the effects of KOR signaling on the activity of individual neurons, the activity of neural networks, and on behavior. Although KOR activation typically inhibits neurotransmission, depending on the circuit, this inhibitory effect might result in disinhibition of other circuits. We focus here on evidence that a key behavioral effect of KOR activation is the production of depressive-like behavioral signs, including those thought to reflect dysphoria, anhedonia, and anxiety. These behavioral signs may be mediated by KOR-induced increases or decreases in the activity of neural networks involved in mood.
2. Stress and the Time Course of KOR-Mediated Effects
Acute stress activates the hypothalamic-pituitary-adrenal (HPA) axis and releases numerous stress hormones and peptides (e.g., CRF, corticosterone, glucocorticoids, endogenous opioids). Release of these molecules has acute and delayed effects on the function of limbic circuits, including activation of the mesocorticolimbic system (Marinelli and Piazza, 2002; Sheline, 2003; Pittenger and Duman, 2008; Feder et al., 2009). These cascades contribute to adaptive behavioral responses by triggering dysphoria and anhedonia, and by the induction of active or passive coping strategies that may terminate the stress or blunt the psychological and physical impact of an inescapable stressor (see Keay and Bandler, 2001). Repeated exposure to a stressor often elicits a progressive amplification (sensitization) of stress-induced behaviors and may promote the transition from active to passive coping strategies (Keay and Bandler, 2001). Stress also causes cross-sensitization to drugs of abuse (see Kalivas and Stewart, 1991), providing a potential explanation for comorbidity of stress- and addiction-related disorders (see Koob, 2008; Sinha, 2008). Accumulating evidence suggests that KORs contribute to stress sensitization likely via a combination of acute and delayed molecular effects induced during the initial stress exposure. Here we consider the acute and delayed effects of stress in three sequential phases: (1) acute exposure and acute outcomes, (2) acute exposure and delayed outcomes, and (3) repeated exposure and mixed outcomes (Fig. 1). We highlight the consequences of two types of manipulations: 1) exposure to KOR agonist (as the most straightforward example of KOR-mediated effects) and 2) exposure to various stressors. Although KOR agonists induce a number of other physiologic effects (e.g., sedation, diuresis, neuroprotection), for the purposes of this review we focus primarily on effects most relevant to the neurobiology of depressive disorders.
2.1. Rapid Effects of Acute Stress or KOR Activation (Type 1)
Acute stress induces numerous physiologic and behavioral effects that are mediated by KOR signaling in limbic brain regions. Dynorphin release can be both a cause and consequence of stress hormone release, or may occur as a direct result of stress-induced increases in neuronal activity (Nikolarakis et al., 1987b; Przewlocki et al., 1987; Watanabe et al., 1995; Bilkei-Gorzo et al., 2008; Land et al., 2008). Evidence suggests that the acute effects of stress are caused, at least in part, by dynorphin-mediated KOR activation (Fig. 1, Type 1). Hypothetically, the predominant effect of KOR stimulation is decreased neuronal activity in cell populations that express KORs. However, if KOR stimulation quiets an inhibitory circuit, then KOR-mediated decreases in neuronal activity would disinhibit other circuits. For example, we have proposed that activation of medium spiny neurons in the NAc encodes aversive states, on the basis of converging evidence from behavioral, molecular, and electrophysiologic tests (Carlezon and Wise, 1996; Pliakas et al., 2001; Carlezon et al., 2006; Todtenkopf et al., 2006; Surmeier et al., 2007; Roitman et al., 2008). According to this model, KOR agonists would be expected to produce aversive states by disinhibiting medium spiny neurons in the NAc; to the best of our knowledge, such studies have not been conducted. In this section we describe several acute effects of KOR agonists and stress on the function of three limbic circuits implicated in depressive disorders—the mesocorticolimbic DA system, the extended amygdala, and the hippocampal system. We review evidence that KOR signaling in these circuits contributes to depressive-like effects of stress including dysphoria, anhedonia, anxiety, and memory deficits—all of which are symptoms of depressive disorders in humans. In addition, because chronic pain is often comorbid with depression (Bair et al., 2003), we also describe the role of KORs in pain circuits.
2.1.1. Stress-Induced Analgesia
Pain involves both sensory and unpleasant emotional components that are mediated by overlapping yet dissociable circuits. The absence of either component significantly impairs defensive responses to noxious stimuli, as is underscored by adverse outcomes in congenital pain disorders involving insensitivity or indifference to noxious stimuli (Nagasako et al., 2003; Cox et al., 2006; Goldberg et al., 2007). KOR signaling affects both sensory and emotional aspects of pain and therefore may be a particularly efficient integrator of defensive responses (see Ribeiro et al., 2005). Activation of KORs produces analgesia by inhibiting synaptic transmission in neural pain circuits (Pan et al., 1997; Vaughan et al., 1997; Meng et al., 2005). KORs and dynorphin are expressed at several levels of pain circuitry, including in dorsal root ganglia, the dorsal horn of the spinal cord, rostral ventromedial medulla, periaqueductal gray, sensory thalamus, and in limbic regions (Gutstein et al., 1998; Neugebauer et al., 2004; Winkler et al., 2006). Although the subcellular distribution of KORs within these circuits is not fully known, pharmacologic studies in wild-type, KOR −/−, and PDyn −/− mice suggest important roles for KORs in conveying visceral, chemical, inflammatory, and thermal pain (Simonin et al., 1998; Kieffer and Gaveriaux-Ruff, 2002); in contrast, MORs and DORs appear to preferentially convey thermal and mechanical pain (Schepers et al., 2008; Scherrer et al., 2009). Dynorphin has also been implicated in neuropathic pain via actions at KORs, as well as at non-opioid receptors, in the spinal cord (Malan et al., 2000; Lai et al., 2001; Wang et al., 2001; Xu et al., 2004; Xu et al., 2007; Gaveriaux-Ruff et al., 2008).
Numerous physical and psychological stressors produce analgesic effects (stress-induced analgesia) in rodents that are mediated by KORs (Takahashi et al., 1990; Watkins et al., 1992; Menendez et al., 1993; McLaughlin et al., 2006b). Although KOR-mediated analgesia may aid in physical responses to threat, it is possible that concurrent KOR signaling in limbic brain regions contributes to the increased incidence of depression and anxiety in individuals experiencing chronic pain or stress (Kessler, 1997; Heim and Nemeroff, 1999; Bair et al., 2003; Gureje, 2008). Chronic pain produces anhedonia-like (Pereira-DoCarmo et al., 2009) and anxiety-like (Narita et al., 2006) behaviors that are similar to those produced by stress in rodent models (see Pittenger and Duman, 2008). Chronic pain also increases the functional coupling of KORs to G-proteins in the mouse amygdala, while decreasing MOR and DOR coupling, and these changes are associated with pain-induced anxiety-like states (Narita et al., 2006). The fact that brain reward system function is reduced by both pain (Pereira-DoCarmo et al., 2009) and KOR agonists (next section) has limited the development of KOR agonists as analgesics, although there is renewed interest in the usefulness of peripherally selective KOR ligands for the management of pain in humans (Aldrich and McLaughlin, 2009).
2.1.2. Depressive-like Effects
2.1.2.1. Mesocorticolimbic Dopamine System
In both humans and laboratory animals, KOR agonists produce dysphoria and anhedonia, which are hallmark characteristics of depressive disorders (American Psychiatric Association DSM-IV-TR, 2000). The effects appear to be mediated, at least in part, by decreased function of the mesocorticolimbic DA system, which is a central component of brain reward circuitry (Carlezon and Thomas, 2009). The aversive effects of KOR agonists have been characterized extensively in rodents using place conditioning paradigms, where they establish conditioned place aversions (CPAs) after systemic administration (Shippenberg and Herz, 1987; Suzuki et al., 1992; Zhang et al., 2005; Bruchas et al., 2007b; Land et al., 2008) or microinfusion into the mesocorticolimbic DA system or other regions (Bals-Kubik et al., 1993; Sante et al., 2000). These same agents do not produce aversions in KOR −/− mice (Simonin et al., 1998), demonstrating that the aversive effects require intact KOR signaling. Although acute administration of KOR agonists produces prodepressive-like behaviors, there is some evidence (discussed below) that even these effects may involve rapid adaptations in intracellular signaling pathways in addition to rapid changes in ion channel conductance. The aversive effects of exposure to forced swim or foot-shock stress, as reflected by the development of conditioned aversions to odors or places paired with stress, are blocked by KOR antagonist treatment and absent in PDyn −/− mice (Land et al., 2008). The aversive effects of stress are mimicked by KOR agonist treatment in unstressed mice, consistent with prior evidence that KOR agonists have aversive effects in rodents (Bals-Kubik et al., 1993; Todtenkopf et al., 2004; Bruchas et al., 2007b) and dysphoric effects in humans (Pfeiffer et al., 1986; Wadenberg, 2003), even when used recreationally (see Gonzalez et al., 2006). The conclusion that disruption of KOR function decreases the aversive effects of stress is strengthened by evidence that KOR antagonism does not affect associative learning in place or odorant conditioning paradigms (Carlezon et al., 1998; McLaughlin et al., 2003; Land et al., 2008).
Place conditioning studies involve repeated drug-environment (or stressor-environment) pairings. As such, these studies could also be considered examples of the effects of repeated drug (stress) exposure (i.e., Type 2 or 3 behaviors). We classify place aversion studies as Type 1 behaviors because evidence suggests that at least some of the dysphoric effects of KOR signaling occur rapidly (likely due to decreases in mesocorticolimbic DA), and do not require prior stress exposure to occur. For example, KOR agonists produce acute dysphoria in humans (Pfeiffer et al., 1986) and immediate decreases in the function of brain reward circuits in rodents (Todtenkopf et al., 2004; Carlezon et al., 2006; Tomasiewicz et al., 2008). Interestingly, KOR antagonists can block the anhedonic effects of KOR agonists, but do not affect ICSS thresholds when given alone, suggesting that KOR antagonists do not have intrinsic rewarding effects that would promote abuse liability. Together these data establish that KOR signaling produces acute prodepressive-like effects that contribute to the aversive effects of stress.
The prodepressive-like effects of KOR agonists appear to involve decreased DA transmission in the mesocorticolimbic system, where KORs are expressed on VTA cell bodies and on the presynaptic terminals of VTA afferents in the NAc (Svingos et al., 1999; Margolis et al., 2003). DA release in the NAc is associated with the rewarding effects of drugs of abuse and natural rewards (see Wise and Rompré, 1989). Paradoxically, mild stress also increases DA in the NAc, whereas intense or chronic stress produces substantial decreases (Di Chiara et al., 1999; Yadid et al., 2001; Jensen et al., 2003; Marinelli, 2007). Stress-induced increases in DA in the NAc may be overshadowed or perceived as aversive due to concurrent changes in the activity of other brain regions (Marowsky et al., 2005; Carlezon and Thomas, 2009). Both stress and drugs of abuse activate the transcription factor CREB (cAMP response element binding protein) and increase dynorphin in the NAc in rodents (Pliakas et al., 2001; Barrot et al., 2002; Shirayama et al., 2004; Walters et al., 2005). In particular, repeated exposure to drugs of abuse increases CREB activity and dynorphin levels with a time course that parallels the emergence of negative affective states associated with psychostimulants (e.g., withdrawal associated dysphoria and anxiety) (Cole et al., 1995; Turgeon et al., 1997; Mattson et al., 2005; Koob, 2009a), providing additional evidence that dynorphin may contribute to neural adaptations involved in stress and addiction (Cleck and Blendy, 2008). The anatomic distribution of KORs suggests that KOR activation may regulate DA transmission in the NAc and that the aversive effects of KOR agonists may result from decreased mesocorticolimbic DA (Carlezon and Thomas, 2009). Indeed, KOR agonists produce prolonged decreases in extracellular DA concentrations in the NAc after systemic administration (Di Chiara and Imperato, 1988; Carlezon et al., 2006) or microinfusions directly into the NAc (Spanagel et al., 1992) or dorsal striatum (Gehrke et al., 2008). They also inhibit excitatory inputs to the VTA (Margolis et al., 2005) and VTA afferents to the medial PFC (Margolis et al., 2006). Thus KOR-mediated decreases in DA transmission appear to contribute importantly to the acute prodepressive-like effects of KOR agonists and stress. It is important to emphasize that there are individual differences in the effects of stress and that, under some circumstances, stress can be rewarding in humans (Zuckerman, 1990; Marinelli, 2007) and rodents (Dellu et al., 1996). Indeed, rats will self-administer corticosterone (Piazza et al., 1993) and certain types of stress increase DA in the NAc, PFC, and amygdala (Abercrombie et al., 1989; Tidey and Miczek, 1996; Inglis and Moghaddam, 1999), perhaps by direct effects of CRF on the function of dopaminergic neurons in the VTA (Wanat et al., 2008). Given evidence that KORs decrease DA neurotransmission in the NAc, it is intriguing to note that administration of CRF can establish conditioned place preference-like responses in PDyn −/− mice (Land et al., 2008). These paradoxical effects likely reflect individual differences in the ways in which DA and other inputs are integrated within the NAc (Carlezon and Thomas, 2009), as well as differences in the activation of other stress-responsive systems (Kabbaj et al., 2000).
KOR systems have been implicated in stress-induced changes in the function of brain reward systems, which may in turn contribute to depressive disorders and addiction (Marinelli, 2007; Koob, 2008; Pittenger and Duman, 2008; Carlezon and Thomas, 2009). KOR antagonists prevent stress (e.g., foot-shock, forced-swim) -induced reinstatement of cocaine seeking behavior, but do not affect cocaine-primed reinstatement in rodents (Beardsley et al., 2005; Carey et al., 2007; Redila and Chavkin, 2008). Similarly, exposure to repeated forced swim or social defeat stress potentiates cocaine conditioned place preference (COC-CPP), an effect that is blocked by pretreatment with a KOR antagonist and absent in KOR −/− or PDyn −/− mice (McLaughlin et al., 2003; McLaughlin et al., 2006a; McLaughlin et al., 2006b). Disruption of KOR signaling does not affect COC-CPP in unstressed mice or rats (Carlezon et al., 1998), suggesting that there are low basal levels of KOR signaling within brain reward circuits in the absence of stress. KOR agonist treatment mimicked the effects of stress and potentiated COC-CPP when given 60 min before cocaine, but decreased COC-CPP when given 15 min before (McLaughlin et al., 2006a). One interpretation of these results is that stress-induced KOR activation produces a dysphoric effect that enhances the subsequent rewarding properties of cocaine, though the time course of these effects suggests a complicated interaction between KOR signaling, DA, and reward. As an example, KOR stimulation might trigger compensatory alterations in the sensitivity of DA receptors that can occur within this 1-hr time frame. Use of a test such as ICSS, which enables “real time” measurement of motivation (Carlezon and Chartoff, 2007), might enable a more precise characterization of the phasic nature of this effect and help to determine if the time frame is compatible with that required for neuroadaptations involving altered gene expression.
2.1.2.2. Amygdala and Extended Amygdala
In addition to dysphoria and anhedonia, some aspects of the aversive effects of KOR agonists appear to involve increased anxiety. KORs and dynorphin are expressed throughout brain areas involved in fear and anxiety, including the amygdala and extended amygdala (Fallon and Leslie, 1986; Mansour et al., 1995; Alheid, 2003). Systemic administration of KOR antagonists increases open arm exploration in the elevated plus maze (EPM) and decreases conditioned fear in the fear-potentiated startle paradigm in rats, both anxiolytic-like effects (Knoll et al., 2007). Similarly, PDyn −/− mice show an anxiolytic-like phenotype that is reversed by pretreatment with KOR agonist and mimicked in wild-type mice treated with KOR antagonist (Wittmann et al., 2009). Paradoxically, studies in other strains of PDyn −/− and KOR −/− mice have reported increases in anxiety (Bilkei-Gorzo et al., 2008) or no effect on anxiety (Simonin et al., 1998), respectively. Discrepancies among these studies may reflect lab-specific differences in basal stress levels or the stressfulness of the behavioral paradigms used, as well as strain- or mutation-related capacity for compensatory adaptations. KOR systems also regulate the expression of other stress hormones, although these interactions are complicated. For example, decreases are observed in serum corticosterone and in CRF levels in the CeA and paraventricular nucleus of the hypothalamus of PDyn −/− mice, and this effect can be reproduced in wild-type mice treated with KOR antagonist (Wittmann et al., 2009). However, there is also evidence from other strains of PDyn −/− mice that the absence of KOR signaling may have no effect (McLaughlin et al., 2006a) or may prolong the stress response (Bilkei-Gorzo et al., 2008), highlighting the complexity of interactions that likely depend upon basal stress levels.
The aversive effects of KOR agonists and stress may also result from interactions between dynorphin and CRF that occur within the amygdala or related structures. KOR agonists increase corticosterone in rats (Laorden and Milanes, 2000) and cortisol in humans (Ur et al., 1997), both of which have been linked to KOR-mediated increases in CRF in the hypothalamus (Pfeiffer et al., 1985; Buckingham and Cooper, 1986; Nikolarakis et al., 1987a). KOR agonist-induced reinstatement of cocaine seeking was also decreased in squirrel monkeys that were treated with a CRF1 receptor antagonist, suggesting that KOR-mediated increases in CRF signaling contribute to reinstatement (Valdez et al., 2007). However, administration of a KOR antagonist or ablation of PDyn does not prevent increases in corticosterone produced by forced swim stress, indicating that stress-induced activation of the HPA axis does not depend on KORs (McLaughlin et al., 2006a). CRF triggers dynorphin release in the hypothalamus, suggesting that KOR signaling also occurs downstream of CRF (Nikolarakis et al., 1986, 1987b). The aversive effects of CRF in a place conditioning paradigm appear to involve KOR activation occurring downstream of the CRF2 receptor: these effects were prevented by pretreatment with KOR antagonist and absent in PDyn −/− mice (Land et al., 2008). Thus KOR activation may be both a cause and a consequence of increased CRF signaling. Although the site(s) of CRF and dynorphin interactions are unknown, these peptides are co-expressed in the hypothalamus (Roth et al., 1983) and CRF induces dynorphin-mediated KOR activation (phosphorylation) in brain regions involved in fear and anxiety including the basolateral amygdala (BLA), dorsal HIP, and to a lesser extent the BNST, an effect which is absent in PDyn −/− mice (Land et al., 2008). Furthermore, dynorphin and CRF are co-expressed within the lateral division of the CeA (CeL) in rats (Marchant et al., 2007), and interactions in this region may affect anxiety via projections to the locus coeruleus and BNST (Petrovich and Swanson, 1997; Morilak et al., 2005; Meloni et al., 2006; Reyes et al., 2008; Koob, 2009b). The CeL and BNST are elements of a circuit implicated in mediating long-duration fear responses (Walker and Davis, 2008), raising the possibility that KOR signaling within this circuit may play a role in responses to recurrent or prolonged stress. As such, there is considerable evidence that interactions between CRF and dynorphin within the extended amygdala are involved in the anxiogenic and aversive effects of stress (see Koob, 2009b; Rodrigues et al., 2009).
2.1.2.3. Hippocampus
The hippocampus (HIP) is another structure that is often implicated in the neurobiology of stress. Mineralocorticoid and glucocorticoid receptors are implicated in stress responsiveness and are expressed in high numbers within the HIP. Although stress-induced corticosteroid signaling in the HIP has a beneficial role in regulating the time course of the HPA axis stress response (de Kloet et al., 2005), prolonged glucocorticoid signaling can damage the HIP as measured by dendritic atrophy, decreased neurogenesis, and deficits in synaptic plasticity (McEwen and Gould, 1990; Sapolsky, 1996; McEwen, 1999; Meaney, 2001). These types of changes are often associated with prodepressive-like effects in rodents (see Pittenger and Duman, 2008), although recent theories have proposed that they might reflect adaptive processes that protect the brain from more widespread damage (McEwen, 2008). HIP volumes are reduced in individuals with posttraumatic stress disorder (Bremner et al., 1995; Woon and Hedges, 2008) and major depression (Sheline et al., 1999), and smaller HIP volumes are also predictive of vulnerability to develop stress-related disorders (Pitman et al., 2006). Although the mechanisms by which decreased HIP function contributes to vulnerability to stress are not fully known, they may involve impaired regulation of the HPA axis or downstream effects in HIP afferent regions involved in mood, such as the NAc (Kelley and Domesick, 1982; Sheline, 2003).
Opioid receptors are also expressed at moderate-to-high levels within the HIP (Clarke et al., 2001; Drake et al., 2007). Numerous stressors (restraint, forced swim, foot-shock) increase dynorphin in the HIP (Shirayama et al., 2004), and intra-HIP microinfusions of KOR antagonist have antidepressant-like effects in the learned helplessness paradigm in rats (Shirayama et al., 2004). Dynorphin is expressed in granule cell axons and dendrites, and acute dynorphin release decreases synaptic transmission and inhibits long-term potentiation at granule cell-perforant path and mossy fiber synapses (Wagner et al., 1993; Weisskopf et al., 1993; Drake et al., 1994; Terman et al., 1994; Drake et al., 2007). In behavioral assays, direct injection of KOR agonist into the CA3 region of the HIP produces memory deficits in the Morris water maze and deficits in contextual fear conditioning in mice (Daumas et al., 2007). Repeated forced swim stress also induces memory deficits in a novel object recognition (NOR) task, which were prevented by treatment with KOR antagonist and absent in PDyn −/− mice (Carey et al., 2009). Because deficits in NOR were mimicked by one injection of KOR agonist 15 min prior to testing and deficits induced by swim stress were prevented by KOR antagonist given immediately after the second swim session (1h before testing), these data suggest that stress-induced deficits in NOR may result from acute increases in KOR signaling following stress (Carey et al., 2009). Although NOR is thought to involve the perirhinal cortex, this region has reciprocal connectivity to the HIP, raising the possibility that KORs may affect the function of broad circuits implicated in learning and memory (Murray and Richmond, 2001; Carey et al., 2009). However, firm conclusions regarding the role of KOR signaling in memory are complicated with studies reporting both KOR-mediated increases and decreases in memory (Colombo et al., 1992; Hiramatsu and Hoshino, 2004). Such discrepancies may be due in part to non-specific effects of KOR ligands at other receptors (Kuzmin et al., 2006). Regardless, the ability of KORs to alter synaptic transmission in the HIP suggests that stress-induced increases in KOR signaling could contribute to changes in HIP function observed in depression. It is important to note that general disruption of KOR signaling does not appear to affect associative learning in rodents (Carlezon et al., 1998; McLaughlin et al., 2003; Bruchas et al., 2007b; Land et al., 2008), further suggesting that KORs have a modulatory role in learning and memory. These data also provide important evidence that the ability of disrupted KOR signaling to decrease the aversive effects of stress is not due to the disruption of associative learning processes that are frequently used to assess motivation in rodents (e.g., place or odorant conditioning) (Carlezon et al., 1998; Bruchas et al., 2007b; Land et al., 2008). When considered together, the majority of evidence is consistent with the hypothesis that acute KOR signaling within limbic circuits involved in reward, fear and anxiety, and memory plays a key role in the acute aversive effects of stress.
2.2. Delayed Effects of Acute Stress or KOR Activation (Type 2)
In addition to its acute effects, stress triggers delayed molecular effects (Fig. 1). Such effects likely contribute to the expression of stress-sensitized behaviors that require prior stress exposure to occur, and may reflect increased expression of passive coping strategies. Even a single exposure to numerous types of stressors (e.g., forced swim, restraint, and withdrawal from drugs of abuse) can cause neuroadaptive responses—including activation of transcription factors and immediate-early genes—that contribute to stress sensitization (Carlezon et al., 1998; Meller et al., 2003; Kreibich and Blendy, 2004; Shirayama et al., 2004; Ahmed et al., 2006; Bruchas et al., 2007b). There is some evidence to suggest that KOR signaling may be both a cause and consequence of these rapid stress-induced neural adaptations. We focus on the role of KORs in the development versus expression of stress-sensitized behavior in the forced swim test, a paradigm in which the molecular effects of stress and KOR signaling have been studied extensively.
2.2.1. KOR-Activated Intracellular Signaling Cascades
Stimulation of KORs activates all three members of the MAPK family of kinases—extracellular regulated kinase (ERK1/2), p38 stress kinase (p38), and c-Jun N-terminal kinase (JNK)—in various cell preparations, including neurons and astrocytes (Bohn et al., 2000; Kam et al., 2004; Belcheva et al., 2005; Bruchas et al., 2006; McLennan et al., 2008). KORs are coupled to inhibitory Gαi-proteins that suppress the activity of cAMP-dependent kinases and thereby alter ion channel conductances as well as intracellular signaling cascades. In addition, agonist binding to KORs triggers dissociation of βγ subunits from the G-protein complex, which participate in a variety of intracellular signaling cascades (e.g., PI3K, PLC, PKC, mobilization of intracellular Ca2+) that activate MAPKs (see Gutkind, 2000). During sustained agonist exposure, KORs are desensitized by G-protein receptor kinase 3 (GRK-3) and recruitment of β-arrestin, which promotes receptor endocytosis and recycling (McLaughlin et al., 2004). Although β-arrestin prevents continued Gαi signaling, it may also serve as a scaffold for Gβγ signaling and activation of MAPKs (McLaughlin et al., 2004; Bruchas et al., 2006; DeWire et al., 2007; McLennan et al., 2008). The mechanisms that trigger MAPK activation are diverse and can depend both on cell-type and time after KOR activation. Both in vitro and in vivo evidence suggests that whereas KOR-mediated activation of p38 is GRK3/β-arrestin-dependent, initial ERK1/2 activation occurs via a separate β-arrestin-independent pathway in the striatum (Bruchas et al., 2006; Bruchas et al., 2007b; Bruchas et al., 2008). A second β-arrestin-dependent phase of ERK1/2 activation also occurs in astrocytes and mediates the proliferative effects of KOR agonists in these cells (McLennan et al., 2008). These data suggest that KOR effects on cell function may be mediated by distinct, yet overlapping, intracellular signaling cascades. Once activated, MAPKs typically enter the nucleus and either directly or indirectly phosphorylate (activate) transcription factors including CREB, zif268, Fos, and Jun. As one example, phosphorylated CREB (pCREB) then binds to promoter regions containing cAMP response element (CRE) sites and thereby recruits transcriptional machinery that initiates gene expression (Carlezon et al., 2005). MAPKs also produce rapid effects on cellular excitability by altering ion channel conductances and AMPA receptor trafficking (Yuan et al., 2002; Kim et al., 2005; Qin et al., 2005; Lu et al., 2006). Thus KOR-mediated activation of MAPKs may encode acute effects of stress (Type 1 effects) through rapid alterations in cellular excitability, as well as delayed responses to stressful experiences (Type 2 or 3 effects) by inducing structural and functional neural adaptations (Thomas and Huganir, 2004).
2.2.2. Stress-Sensitized Behaviors: Focus on the Forced Swim Test
The forced swim test (FST) is a simple yet important procedure for studying the molecular mechanisms mediating stress-sensitized behaviors. In this test animals are forced to swim in a cylinder of water during two sessions that are typically separated by 24 h. During the first swim session (typically 15 min) animals initially struggle to escape, but eventually adopt an immobile posture in which they only make movements necessary to keep their heads above water. During the second swim session (typically 5–6 min) animals become immobile more quickly and spend a greater amount of time immobile. Sub-chronic treatment with antidepressants in the 24 h between sessions (typically at 1, 19, and 23 h after the first swim session) significantly decreases immobility, an effect correlated with antidepressant efficacy in humans (Porsolt et al., 1977; Detke et al., 1995). Evidence indicates that acute swim stress activates signal transduction cascades (e.g., CREB, MAPKs), which induce neural adaptations that facilitate immobility during the second swim session. Several lines of evidence suggest that KORs contribute to increased immobility: KOR antagonists or disruption of KOR signaling in KOR −/− and PDyn −/− mice decreases immobility in the second session, but typically does not affect behavior during the first swim session (Simonin et al., 1998; Pliakas et al., 2001; Mague et al., 2003; McLaughlin et al., 2003; Beardsley et al., 2005; McLaughlin et al., 2006a). However, even slight differences in experimental approaches may contribute to apparent discrepancies in the literature. Assuming that repeated testing under stressful conditions is necessary to trigger neuroadaptations that lead to altered behavior, it might not be surprising if the effects of KOR ablation are not detectable in versions of the FST that involve only a single exposure to swimming (Simonin et al., 1998). Similarly, modifications to the FST regimen that likely affect stress levels (e.g., use of different inter-trial intervals, colder water) (Wittmann et al., 2009) can complicate comparisons among studies. Administration of KOR agonists increases immobility when given repeatedly between swim sessions (Mague et al., 2003; Carlezon et al., 2006), providing further evidence that stimulation of KOR receptors increases depressive-like behavior. When considered together, most evidence suggests that immobility behavior becomes KOR-mediated as a result of neural adaptations induced by the first swim stress experience. As discussed below, similar KOR-dependence has been observed in other paradigms involving repeated exposure to an inescapable stressor (social defeat, learned helplessness).
2.2.2.1. Development of Stress-Sensitized Behaviors
Recent studies have identified activation of MAPKs—a key consequence of KOR stimulation—as one of the molecular consequences of stress exposure that contributes to the development of stress sensitization. Initial exposure to swim stress activates JNK, and in some studies ERK1/2, within limbic brain regions (Liu et al., 2004; Shen et al., 2004). Repeated exposure to swim stress produces KOR-dependent activation of p38 and ERK1/2 in the NAc and caudate putamen (Bruchas et al., 2007b; Bruchas et al., 2008). Administration of a p38 antagonist prior to each swim session is sufficient to decrease immobility during the second session, without affecting immobility in the first swim session. A similar pattern of effects on immobility have been found in KOR −/−, PDyn −/−, and even GRK3 −/− mice, further suggesting that KOR signaling mediates neural adaptations that contribute directly to the facilitated immobility behavior that develops with repeated testing (McLaughlin et al., 2003; McLaughlin et al., 2006a; Bruchas et al., 2007b). As discussed above, activation of p38 also contributes to the development of CPAs to KOR agonists without affecting other forms of associative or aversive learning in mice (Bruchas et al., 2007b). A role for p38 in KOR-induced CPA suggests that neural adaptations may also contribute to the aversive effects of KOR signaling. The mechanisms by which increased p38 signaling contributes to immobility (and aversion) are not known, but may involve changes in synaptic plasticity as a result of activation of immediate early genes, such as zif268 (Erg-1), which is upregulated by repeated swim stress in a p38-dependent manner (Thomas and Huganir, 2004; Bruchas et al., 2007b). Although p38 antagonist was administered prior to both the first and second swim sessions, there is evidence in this study and others that p38 is not activated until the second swim session in rodents (Liu et al., 2004; Shen et al., 2004; Bruchas et al., 2007b). If this is the case, a more rapid mechanism than changes in gene expression, such as p38-mediated phosphorylation of a substrate (e.g., ion channel, transporter protein), likely mediates p38 effects during the second swim session. Regardless, these data are consistent with a role for KOR/GRK3-mediated activation of p38 in contributing to the prodepressive-like and aversive effects of stress (Bruchas et al., 2006; Bruchas et al., 2007b).
2.2.2.2. Expression of Stress-Sensitized Behaviors
The expression of stress-sensitized behaviors is dependent on stress-induced changes in neuronal function that are revealed during subsequent exposure to stress. Several lines of evidence suggest that CREB-mediated increases in dynorphin contribute to the expression of stress-sensitized behavior. Specifically, elevating CREB activity in the NAc (via viral mediated gene transfer) elevates dynorphin gene expression (Carlezon et al., 1998) and increases immobility during the second swim session, a prodepressive-like effect that is blocked by KOR antagonists (Pliakas et al., 2001). In contrast, reducing CREB activity (via viral-mediated transfer of a dominant negative form of CREB) reduces dynorphin mRNA expression and decreases immobility, an antidepressant-like effect (Carlezon et al., 1998; Pliakas et al., 2001). Swim stress itself increases pCREB in the NAc (Pliakas et al., 2001; Bruchas et al., 2007b) and increases dynorphin in the NAc and HIP (Shirayama et al., 2004; Chartoff et al., 2009), suggesting that this molecular cascade plays a role in stress-induced behavioral adaptations under normal (physiologic) conditions. Together these findings suggest that increased KOR signaling during the second swim session contributes to immobility (Pliakas et al., 2001; Mague et al., 2003; McLaughlin et al., 2003; Beardsley et al., 2005; McLaughlin et al., 2006a; Carey et al., 2009). Interestingly, sub-chronic administration of the antidepressant desipramine beginning 1 h after exposure to forced swimming decreases immobility behavior when rats are re-tested 24 h later (Carlezon et al., 2002), and prevents increases in PDyn mRNA in the NAc after swim stress (Chartoff et al., 2009). This raises the possibility that drugs with antidepressant-like effects may share the ability to decrease dynorphin signaling in the NAc. These findings may also provide more general insight on the mechanisms by which the FST rapidly detects substances with antidepressant effects in humans: intervention with antidepressants within a narrow window after stress exposure might block the induction of neuroadaptations (including increased dynorphin expression) that lead to the development and expression of depressive-like behavior.
It is important to note that elevated CREB activity in the NAc is associated with other depressive-like signs that are detectable with “acute” testing, including anhedonia, dysphoria, and anxiety (Carlezon et al., 1998; Pliakas et al., 2001; Barrot et al., 2002; Shaw-Lutchman et al., 2002; Valverde et al., 2004; Barrot et al., 2005; Pandey et al., 2005). One possible explanation is that the effects of elevated CREB function are mediated by increased dynorphin release during testing, which is consistent with our designation of these stress-induced behaviors as “acute” effects of KOR signaling that do not require stress-induced neural adaptations. Direct manipulation of CREB function may simply amplify dynorphin signaling, although it might also induce other neural adaptations that can modify behavior.
Similar mechanisms may mediate the development and expression of other stress-sensitized behaviors. One example is increased submissive behavior in the social defeat paradigm, which is reduced by KOR antagonists and in PDyn −/− mice (McLaughlin et al., 2006b). Disruption of KOR signaling does not affect social defeat behavior during initial trials, which is consistent with growing evidence that KORs facilitate the expression of passive coping strategies (such as immobility and defeat) that are triggered in response to repeated stress. As discussed in the following section, KORs may have a preferential role in mediating behavioral responses to repeated exposure to inescapable and unpredictable stress. Regardless, current evidence indicates that acute exposure to stress or KOR agonists has enduring effects on the brain, and that the behavioral consequences of these effects are blocked by KOR antagonists.
2.3. Cumulative Effects of Repeated Stress or KOR Activation (Type 3)
Depressive disorders are chronic illnesses that develop over time and whose onset and development can be exacerbated by exposure to repeated stress, especially stressors that are inescapable and unpredictable (see Feder et al., 2009). Because the etiology and pathophysiology of depressive disorders are likely characterized by neural adaptations that occur over extended period of times, the underlying molecular and neural mechanisms may be best modeled in behavioral paradigms that involve repeated exposure to inescapable and unpredictable stressors, such as occurs in the learned helplessness and chronic mild stress paradigms that are used to study depression in laboratory animals. These paradigms are characterized by repeated exposure to stress, so they likely produce multiple rounds (waves) of acute and delayed molecular effects that accumulate. We focus on the role of KORs in the learned helplessness paradigm because, to our knowledge, the significance of KORs in chronic mild stress paradigms has not yet been thoroughly evaluated.
In the learned helplessness (LH) paradigm, animals develop a phenotype in which they fail to show escape responses to avoidable foot-shocks after they have been exposed repeatedly to inescapable foot-shock stress (IES) (see Maier and Watkins, 2005). This helpless phenotype can be attenuated by chronic antidepressant treatment (Shirayama et al., 2002; Valentine et al., 2008), suggesting that it reflects a prodepressive-like behavioral adaptation. Importantly, helplessness does not develop in animals that are exposed to escapable foot-shocks of the same quantity, duration, and intensity, and helplessness can be enhanced if animals receive IES and active avoidance tests in the same context (see Maier and Watkins, 2005; Valentine et al., 2008). Thus stressor uncontrollability and contextual fear conditioning appear to have additive effects, which may make the LH paradigm ideal for studying neural mechanisms that contribute to stress-induced anxiety and depressive behaviors.
Evidence suggests that CREB-mediated neural adaptations contribute to the development of LH. Transgenic mice with increased CREB activity in forebrain regions show increased escape failures, a prodepressive-like effect, whereas mice with decreased CREB activity show reduced escape failures; importantly, altered CREB activity does not affect the acquisition of active avoidance behavior in mice that have not received IES (Newton et al., 2002). The mechanisms of CREB-mediated effects in this paradigm are complicated and may be mediated by different CREB target genes in brain regions including the NAc, HIP, and amygdala (Chen et al., 2001; Newton et al., 2002; Wallace et al., 2004; Carlezon et al., 2005). Dynorphin has been implicated in mediating LH: IES increases dynorphin expression in the NAc and HIP (Shirayama et al., 2004) and intra-NAc or intra-HIP administration of KOR antagonist decreases escape failures in rodents (Newton et al., 2002; Shirayama et al., 2004). In both of these studies, administration of KOR antagonists 24 h after IES and 3 d before active avoidance testing was sufficient to produce an antidepressant-like effect. These findings might provide important insight on the key question of whether KOR antagonists prevent the development or expression of stress-induced behaviors: the delay between the exposure of rats to repeated IES and the administration of KOR antagonists (24 h) suggests that KORs are primarily involved in the expression of LH, and that it is not critical to block KORs before or during the stress exposure. However, because KOR antagonists have an extended duration of action (> 3 weeks) after a single administration (Horan et al., 1992; Jones and Holtzman, 1992; Beardsley et al., 2005), the long-duration of KOR blockade (3 d) before testing may reverse neural adaptations induced by stress. Additional studies are needed to determine if blocking KORs immediately before testing is sufficient to produce antidepressant-like effects, and if administration of a KOR agonist facilitates the development of a helpless phenotype.
3. Conclusions and Implications
Stimulation of KORs mimics or exacerbates many of the acute and delayed behavioral effects of stress. Disruption of KOR function tends to block these same effects. The fact that KOR function appears to have a profound influence on behaviors that are thought to reflect motivation and emotion in animal models suggests that KORs might represent a viable target for psychiatric medications. An obvious indication for KOR antagonists is in the treatment of depressive and anxiety-related disorders, both of which are triggered or exacerbated by stress. Unfortunately, it is not entirely clear whether KOR antagonists prevent or reverse stress-induced effects, and it is even less clear when they should be given to have these very desirable effects. There is evidence that KOR antagonists can have prophylactic effects when given before stress (Table 1, before stressors). A drug of this type would be particularly useful in cases where it is possible to predict exposure to a stressor (e.g., first responders, soldiers). There is also evidence that KOR antagonists have useful effects when given after the initial stressor (Table 1, between stressors), perhaps within a narrow window during which stress induced neural adaptations are labile and still sensitive to intervention. Moreover, in some circumstances KOR antagonists may even be effective when given after repeated stressors (Table 1, after stressors). A drug of this type would have a much broader range of therapeutic uses. Determining if KOR antagonist effects are due to the reversal or prevention of stress-induced neural adaptations (development) or the blockade of acute KOR signaling (expression) is complicated by the long duration of action of currently available KOR antagonists. In most studies, KOR antagonists have been administered at time points that could affect both the development and expression of stress-induced behaviors. Differentiating between these mechanisms requires disruption of KOR signaling during more restricted time periods, such as immediately prior to the second exposure to stress, in order to test the role of KORs in the expression of stress-induced behaviors. It is conceivable that KOR antagonists may be particularly useful in treating specific signs and symptoms of depressive disorders depending on the time point at which they are administered, or that the pharmacokinetics of currently available KOR antagonists plays an essential role in their efficacy in animal models. Many of these questions are difficult to address because the extraordinarily long time course of currently available KOR antagonists hinders their study in both laboratory and clinical settings. An improved understanding of the unique ways in which stress and KOR systems interact may provide an impetus for the discovery of KOR ligands with more favorable properties, and ultimately the development of medications that are based upon an improved understanding of the brain.
Table 1.
Time Point of KOR Antagonist Administration or Gene Ablation Relative to Exposure to Stressors
| Stressor | Treatment (Method) | Treatment Time Relative to 1st Stressor | Behavioral Effect | Species, Strain | References | |
|---|---|---|---|---|---|---|
| TEST 1 | TEST 2 | |||||
| BEFORE STRESSORS | ||||||
| Forced Swim | NorBNI, GNTI (ICV) | 3d before | Not Determined | Decreased Immobility | Rats, CD |
Pliakas et al., 2001 Mague et al., 2003 |
| NorBNI (IP) | 1h before daily stress | No Effect | Decreased Immobility | Mice, C57Bl/6 | McLaughlin et al., 2003; 2006a | |
| Decreased Immobility | Carey et al., 2009 | |||||
| KOR −/− | N/A | No Effect | Decreased Immobility | Mice, C57Bl/6 | McLaughlin et al., 2003; 2006a | |
| Not Determined | Mice, Hybrid 129SV/C57Bl/6 | Filliol et al., 2003 | ||||
| PDyn −/− | N/A | No Effect | Decreased Immobility | Mice, C57Bl/6 | McLaughlin et al., 2003 | |
| PDyn −/− | N/A | No Effect | Increased Immobility* | Mice, C57Bl/6 | Wittmann et al., 2009 | |
| Social Defeat | NorBNI (IP) | 1h before daily stress | No Effect | Decreased Social Defeat | Mice, C57Bl/6 | McLaughlin et al., 2006b |
| PDyn −/− | N/A | |||||
| Stress-Induced Potentiation Cocaine-CPP | NorBNI (IP) | 1h before daily stress | Blocked Stress-Induced Potentiation of Cocaine-CPP, No Effect Unstressed Mice | Mice, C57Bl/6 | McLaughlin et al., 2003; 2006a; 2006b | |
| KOR −/− | N/A | |||||
| PDyn −/− | N/A | |||||
| Stress-Induced Reinstatement of Cocaine-Seeking | JDTic (IG) | 24h before stress | Decreased Stress-Induced Reinstatement, No Effect on Cocaine-Primed Reinstatement | Rats, Long-Evans | Beardsley et al., 2005 | |
| Arodyn (ICV) | 1h before stress | Mice, C57Bl/6 | Carey et al., 2007 | |||
| NorBNI (IP) | 1h before stress | Redila & Chavkin, 2008 | ||||
| KOR −/− PDyn −/− |
N/A | |||||
| Stress-Induced | NorBNI (IP) | 1h before daily stress | Decreased Deficit in NOR | Mice, C57Bl/6 | Carey et al., 2009 | |
| Deficit Novel Object Recognition | PDyn −/− | N/A | ||||
| Elevated Plus Maze | NorBNI, JDTic (IP) | 48h before | Increased Open Arm Exploration | Rats, CD | Knoll et al., 2007 | |
| PDyn −/− | N/A | Mice, C57Bl/6 | Wittmann et al., 2009 | |||
| KOR −/− | N/A | No Effect | Mice, Hybrid 129SV/C57Bl/6 | Simonin et al., 1998 | ||
| Zero Maze | KOR −/− | N/A | No Effect; Also No Effect Y maze | Mice, Hybrid 129SV/C57Bl/6 | Simonin et al., 1998 | |
| PDyn −/− | N/A | Decreased Exploration | Mice, C57Bl/6 | Bilkei-Gorzo et al., 2008 | ||
| NorBNI (IP) | 3d before | No Effect | Rats, CD | Knoll et al., 2007 | ||
| Open Field | NorBNI (IP) GNTI (IC) | 48h before 20h before |
Increased Center Exploration | Mice, C57Bl/6 | Wittmann et al., 2009 | |
| PDyn −/− | N/A | |||||
| KOR −/− | N/A | No Effect | Mice, Hybrid 129SV/C57Bl/6 | Simonin et al., 1998 | ||
| Light-Dark Test | PDyn −/− | N/A | No Effect | Mice, C57Bl/6 | Bilkei-Gorzo et al., 2008 | |
| PDyn −/− | N/A | Increased Time in Lit Area | Mice, C57Bl/6 | Wittmann et al., 2009 | ||
| Conditioned Fear | NorBNI, JDTic (IP) | 6d before training; 8d before testing | Decreased Conditioned Fear | Rats, CD | Knoll et al., 2007 | |
| Stress-Induced Place Aversion | NorBNI | 1h before stress | Blocked CPA | Mice, C57Bl/6 | Land et al., 2008 | |
| PDyn −/− | N/A | |||||
| BETWEEN STRESSORS | ||||||
| Forced Swim | ANTI (IP) | 1, 19, 23h after | Decreased Immobility | Rats, CD | Mague et al., 2003 | |
| NorBNI, JDTic (SC) | After | Beardsley et al., 2005 | ||||
| Learned Helplessness | NorBNI (ICV, NAc, HIP) | 1d after training, 3d before testing | Decreased Escape Failures (ICV, NAc) | Rats, CD | Newton et al., 2000 | |
| NorBNI (NAc, HIP) | Decreased Escape Failures (NAc, HIP) | Rats, CD | Shirayama et al., 2004 | |||
| AFTER STRESSORS | ||||||
| Stress-Induced Deficit in Novel Object Recognition | NorBNI (IP) | Immediately after 2nd swim session | Decreased Deficit in NOR | Mice, C57Bl/6 | Carey et al., 2009 | |
NorBNI, GNTI and JDTic, KOR Antagonists; PDyn −/−, prodynorphin knockout mice; KOR −/−, kappa opioid receptor knockout mice; NAc, nucleus accumbens; HIP, hippocampus; ICV, intracerebroventricular; IP, intraperitoneal; IC, intracisternal; CD, Sprague-Dawley; CPA, conditioned place aversion; NOR, novel object recognition;
, swim session parameters differed from those typically used to study KOR antagonists, see text for details.
Acknowledgments
This work was supported by the National Institutes of Health (MH063266, to WAC and MH078473, to ATK).
Footnotes
Disclosures
Dr. Carlezon has a US patent covering the use of kappa antagonists in the treatment of depression (Assignee: McLean Hospital) and is a member of a collaborative group that has submitted a patent application covering the synthesis and use of salvinorin derivatives (Assignees: McLean Hospital and Temple University). Ms. Knoll has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Frey JU, Korz V. Long-term effects of brief acute stress on cellular signaling and hippocampal LTP. J Neurosci. 2006;26:3951–3958. doi: 10.1523/JNEUROSCI.4901-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich JV, McLaughlin JP. Peptide kappa opioid receptor ligands: potential for drug development. Aaps J. 2009;11:312–322. doi: 10.1208/s12248-009-9105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association DSM-IV-TR. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. text revision Edition. [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Barrot M, Wallace DL, Bolanos CA, Graham DL, Perrotti LI, Neve RL, Chambliss H, Yin JC, Nestler EJ. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102:8357–8362. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati F, Agnati LF. Communication and computation in the central nervous system. Funct Neurol. 1991;6:202–209. [PubMed] [Google Scholar]
- Berger B, Rothmaier AK, Wedekind F, Zentner J, Feuerstein TJ, Jackisch R. Presynaptic opioid receptors on noradrenergic and serotonergic neurons in the human as compared to the rat neocortex. Br J Pharmacol. 2006;148:795–806. doi: 10.1038/sj.bjp.0706782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D, Zimmer A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33:425–436. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Belcheva MM, Coscia CJ. Mitogenic signaling via endogenous kappa-opioid receptors in C6 glioma cells: evidence for the involvement of protein kinase C and the mitogen-activated protein kinase signaling cascade. J Neurochem. 2000;74:564–573. doi: 10.1046/j.1471-4159.2000.740564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Bourque CW. Autocrine feedback inhibition of plateau potentials terminates phasic bursts in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 2004;557:949–960. doi: 10.1113/jphysiol.2004.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007a;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007b;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham JC, Cooper TA. Pharmacological characterization of opioid receptors influencing the secretion of corticotrophin releasing factor in the rat. Neuroendocrinology. 1986;44:36–40. doi: 10.1159/000124618. [DOI] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AN, Lyons AM, Shay CF, Dunton O, McLaughlin JP. Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. J Neurosci. 2009;29:4293–4300. doi: 10.1523/JNEUROSCI.6146-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Pliakas AM, Parow AM, Detke MJ, Cohen BM, Renshaw PF. Antidepressant-like effects of cytidine in the forced swim test in rats. Biol Psychiatry. 2002;51:882–889. doi: 10.1016/s0006-3223(01)01344-0. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology (Berl) 1996;128:413–420. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, Knoll AT, Cohen BM. Kappa-opioid ligands in the study and treatment of mood disorders. Pharmacol Ther. 2009 doi: 10.1016/j.pharmthera.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carroll I, Thomas JB, Dykstra LA, Granger AL, Allen RM, Howard JL, Pollard GT, Aceto MD, Harris LS. Pharmacological properties of JDTic: a novel kappa-opioid receptor antagonist. Eur J Pharmacol. 2004;501:111–119. doi: 10.1016/j.ejphar.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C, Carlezon WA., Jr Desipramine reduces stress-activated dynorphin expression and CREB phosphorylation in NAc tissue. Mol Pharmacol. 2009;75:704–712. doi: 10.1124/mol.108.051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C. Dynorphins are endogenous opioid peptides released from granule cells to act neurohumorly and inhibit excitatory neurotransmission in the hippocampus. Prog Brain Res. 2000;125:363–367. doi: 10.1016/S0079-6123(00)25025-5. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49:753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Basbaum AI. Ultrastructural analysis of dynorphin B-immunoreactive cells and terminals in the superficial dorsal horn of the deafferented spinal cord of the rat. J Comp Neurol. 1989;281:193–205. doi: 10.1002/cne.902810204. [DOI] [PubMed] [Google Scholar]
- Clarke S, Chen Z, Hsu MS, Pintar J, Hill R, Kitchen I. Quantitative autoradiographic mapping of the ORL1, mu-, delta- and kappa-receptors in the brains of knockout mice lacking the ORL1 receptor gene. Brain Res. 2001;906:13–24. doi: 10.1016/s0006-8993(01)02531-8. [DOI] [PubMed] [Google Scholar]
- Cleck JN, Blendy JA. Making a bad thing worse: adverse effects of stress on drug addiction. J Clin Invest. 2008;118:454–461. doi: 10.1172/JCI33946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14:813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo PJ, Martinez JL, Jr, Bennett EL, Rosenzweig MR. Kappa opioid receptor activity modulates memory for peck-avoidance training in the 2-day-old chick. Psychopharmacology (Berl) 1992;108:235–240. doi: 10.1007/BF02245314. [DOI] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, Al-Gazali L, Hamamy H, Valente EM, Gorman S, Williams R, McHale DP, Wood JN, Gribble FM, Woods CG. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S, Betourne A, Halley H, Wolfer DP, Lipp HP, Lassalle JM, Frances B. Transient activation of the CA3 Kappa opioid system in the dorsal hippocampus modulates complex memory processing in mice. Neurobiol Learn Mem. 2007;88:94–103. doi: 10.1016/j.nlm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999;46:1624–1633. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–263. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, Chavkin C. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J Neurosci. 1994;14:3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tanaka C, Nagase H. Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther. 1992;316:30–42. [PubMed] [Google Scholar]
- Fallon JH, Leslie FM. Distribution of dynorphin and enkephalin peptides in the rat brain. J Comp Neurol. 1986;249:293–336. doi: 10.1002/cne.902490302. [DOI] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Frazer A. Pharmacology of antidepressants. J Clin Psychopharmacol. 1997;17(Suppl 1):2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Karchewski LA, Hever X, Matifas A, Kieffer BL. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 2008;27:2558–2567. doi: 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrke BJ, Chefer VI, Shippenberg TS. Effects of acute and repeated administration of salvinorin A on dopamine function in the rat dorsal striatum. Psychopharmacology (Berl) 2008;197:509–517. doi: 10.1007/s00213-007-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg YP, MacFarlane J, MacDonald ML, Thompson J, Dube MP, Mattice M, Fraser R, Young C, Hossain S, Pape T, Payne B, Radomski C, Donaldson G, Ives E, Cox J, Younghusband HB, Green R, Duff A, Boltshauser E, Grinspan GA, Dimon JH, Sibley BG, Andria G, Toscano E, Kerdraon J, Bowsher D, Pimstone SN, Samuels ME, Sherrington R, Hayden MR. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet. 2007;71:311–319. doi: 10.1111/j.1399-0004.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez D, Riba J, Bouso JC, Gomez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Gureje O. Comorbidity of pain and anxiety disorders. Curr Psychiatry Rep. 2008;10:318–322. doi: 10.1007/s11920-008-0051-0. [DOI] [PubMed] [Google Scholar]
- Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci STKE 2000. 2000:RE1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Mansour A, Watson SJ, Akil H, Fields HL. Mu and kappa opioid receptors in periaqueductal gray and rostral ventromedial medulla. Neuroreport. 1998;9:1777–1781. doi: 10.1097/00001756-199806010-00019. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- Hensler JG. Serotonergic modulation of the limbic system. Neurosci Biobehav Rev. 2006;30:203–214. doi: 10.1016/j.neubiorev.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Hoshino T. Involvement of kappa-opioid receptors and sigma receptors in memory function demonstrated using an antisense strategy. Brain Res. 2004;1030:247–255. doi: 10.1016/j.brainres.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M. Neuropeptides--an overview. Neuropharmacology. 2000;39:1337–1356. doi: 10.1016/s0028-3908(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- Hurd YL. Differential messenger RNA expression of prodynorphin and proenkephalin in the human brain. Neuroscience. 1996;72:767–783. doi: 10.1016/0306-4522(96)00002-4. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Bains JS. Retrograde opioid signaling regulates glutamatergic transmission in the hypothalamus. J Neurosci. 2009;29:7349–7358. doi: 10.1523/JNEUROSCI.0381-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Jones DN, Holtzman SG. Long term kappa-opioid receptor blockade following nor-binaltorphimine. Eur J Pharmacol. 1992;215:345–348. doi: 10.1016/0014-2999(92)90055-9. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kam AY, Chan AS, Wong YH. Kappa-opioid receptor signals through Src and focal adhesion kinase to stimulate c-Jun N-terminal kinases in transfected COS-7 cells and human monocytic THP-1 cells. J Pharmacol Exp Ther. 2004;310:301–310. doi: 10.1124/jpet.104.065078. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009a;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009b doi: 10.1016/j.brainres.2009.03.038. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ, Valentino RJ. Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci. 2008;28:6516–6525. doi: 10.1523/JNEUROSCI.0390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Madjid N, Terenius L, Ogren SO, Bakalkin G. Big dynorphin, a prodynorphin-derived peptide produces NMDA receptor-mediated effects on memory, anxiolytic-like and locomotor behavior in mice. Neuropsychopharmacology. 2006;31:1928–1937. doi: 10.1038/sj.npp.1300959. [DOI] [PubMed] [Google Scholar]
- Lai J, Ossipov MH, Vanderah TW, Malan TP, Jr, Porreca F. Neuropathic pain: the paradox of dynorphin. Mol Interv. 2001;1:160–167. [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laorden ML, Milanes MV. Effects of U-50,488H and U-50,488H withdrawal on catecholaminergic neurons of the rat hypothalamus. Life Sci. 2000;66:803–815. doi: 10.1016/s0024-3205(99)00653-0. [DOI] [PubMed] [Google Scholar]
- Lin S, Boey D, Lee N, Schwarzer C, Sainsbury A, Herzog H. Distribution of prodynorphin mRNA and its interaction with the NPY system in the mouse brain. Neuropeptides. 2006;40:115–123. doi: 10.1016/j.npep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Liu YF, Bertram K, Perides G, McEwen BS, Wang D. Stress induces activation of stress-activated kinases in the mouse brain. J Neurochem. 2004;89:1034–1043. doi: 10.1111/j.1471-4159.2004.02391.x. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Malan TP, Ossipov MH, Gardell LR, Ibrahim M, Bian D, Lai J, Porreca F. Extraterritorial neuropathic pain correlates with multisegmental elevation of spinal dynorphin in nerve-injured rats. Pain. 2000;86:185–194. doi: 10.1016/s0304-3959(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Densmore VS, Osborne PB. Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol. 2007;504:702–715. doi: 10.1002/cne.21464. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Both kappa and mu opioid agonists inhibit glutamatergic input to ventral tegmental area neurons. J Neurophysiol. 2005;93:3086–3093. doi: 10.1152/jn.00855.2004. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M. Dopaminergic reward pathways and effects of stress. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. Elsevier, Ltd; 2007. [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Price RB, Charney DS. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genet C Semin Med Genet. 2008;148C:89–98. doi: 10.1002/ajmg.c.30172. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583:174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gould E. Adrenal steroid influences on the survival of hippocampal neurons. Biochem Pharmacol. 1990;40:2393–2402. doi: 10.1016/0006-2952(90)90079-z. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006a;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006b;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Myers LC, Zarek PE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Chavkin C. Prolonged kappa opioid receptor phosphorylation mediated by G-protein receptor kinase underlies sustained analgesic tolerance. J Biol Chem. 2004;279:1810–1818. doi: 10.1074/jbc.M305796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J Neurochem. 2008;107:1753–1765. doi: 10.1111/j.1471-4159.2008.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meller E, Shen C, Nikolao TA, Jensen C, Tsimberg Y, Chen J, Gruen RJ. Region-specific effects of acute and repeated restraint stress on the phosphorylation of mitogen-activated protein kinases. Brain Res. 2003;979:57–64. doi: 10.1016/s0006-8993(03)02866-x. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Gerety LP, Knoll AT, Cohen BM, Carlezon WA., Jr Behavioral and anatomical interactions between dopamine and corticotropin-releasing factor in the rat. J Neurosci. 2006;26:3855–3863. doi: 10.1523/JNEUROSCI.4957-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez L, Andres-Trelles F, Hidalgo A, Baamonde A. Involvement of spinal kappa opioid receptors in a type of footshock induced analgesia in mice. Brain Res. 1993;611:264–271. doi: 10.1016/0006-8993(93)90512-l. [DOI] [PubMed] [Google Scholar]
- Meng ID, Johansen JP, Harasawa I, Fields HL. Kappa opioids inhibit physiologically identified medullary pain modulating neurons and reduce morphine antinociception. J Neurophysiol. 2005;93:1138–1144. doi: 10.1152/jn.00320.2004. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The role of monoamines in the actions of established and "novel" antidepressant agents: a critical review. Eur J Pharmacol. 2004;500:371–384. doi: 10.1016/j.ejphar.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Mittmann W, Chadderton P, Hausser M. Neuronal microcircuits: frequency-dependent flow of inhibition. Curr Biol. 2004;14:R837–839. doi: 10.1016/j.cub.2004.09.036. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Murray EA, Richmond BJ. Role of perirhinal cortex in object perception, memory, and associations. Curr Opin Neurobiol. 2001;11:188–193. doi: 10.1016/s0959-4388(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Nagasako EM, Oaklander AL, Dworkin RH. Congenital insensitivity to pain: an update. Pain. 2003;101:213–219. doi: 10.1016/S0304-3959(02)00482-7. [DOI] [PubMed] [Google Scholar]
- Nair HP, Gutman AR, Davis M, Young LJ. Central oxytocin, vasopressin, and corticotropin-releasing factor receptor densities in the basal forebrain predict isolation potentiated startle in rats. J Neurosci. 2005;25:11479–11488. doi: 10.1523/JNEUROSCI.2524-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Kaneko C, Miyoshi K, Nagumo Y, Kuzumaki N, Nakajima M, Nanjo K, Matsuzawa K, Yamazaki M, Suzuki T. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolarakis K, Pfeiffer A, Stalla GK, Herz A. The role of CRF in the release of ACTH by opiate agonists and antagonists in rats. Brain Res. 1987a;421:373–376. doi: 10.1016/0006-8993(87)91311-4. [DOI] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic beta-endorphin and dynorphin release by corticotropin-releasing factor (in vitro) Brain Res. 1986;399:152–155. doi: 10.1016/0006-8993(86)90610-4. [DOI] [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Feedback inhibition of opioid peptide release in the hypothalamus of the rat. Neuroscience. 1987b;23:143–148. doi: 10.1016/0306-4522(87)90278-8. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Tershner SA, Fields HL. Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature. 1997;389:382–385. doi: 10.1038/38730. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Chartoff EH, Carlezon WA, Jr, Zou J, Zhang H, Kreibich AS, Blendy JA, Crews FT. CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcohol Clin Exp Res. 2005;29:176–184. doi: 10.1097/01.alc.0000153550.31168.1d. [DOI] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Pereira-DoCarmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Swanson LW. Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 1997;763:247–254. doi: 10.1016/s0006-8993(96)01361-3. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Herz A, Loriaux DL, Pfeiffer DG. Central kappa- and mu-opiate receptors mediate ACTH-release in rats. Endocrinology. 1985;116:2688–2690. doi: 10.1210/endo-116-6-2688. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock RD. A highly selective kappa-opioid receptor agonist, CI-977, reduces excitatory synaptic potentials in the rat locus coeruleus in vitro. Neuroscience. 1992;47:87–94. doi: 10.1016/0306-4522(92)90123-j. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Lason W, Hollt V, Silberring J, Herz A. The influence of chronic stress on multiple opioid peptide systems in the rat: pronounced effects upon dynorphin in spinal cord. Brain Res. 1987;413:213–219. doi: 10.1016/0006-8993(87)91012-2. [DOI] [PubMed] [Google Scholar]
- Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signaling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Drolet G, Van Bockstaele EJ. Dynorphin and stress-related peptides in rat locus coeruleus: contribution of amygdalar efferents. J Comp Neurol. 2008;508:663–675. doi: 10.1002/cne.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Chavkin C, van Bockstaele EJ. Subcellular targeting of kappa-opioid receptors in the rat nucleus locus coeruleus. J Comp Neurol. 2009;512:419–431. doi: 10.1002/cne.21880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Johnson AD, Glaser JD, Commons KG, Van Bockstaele EJ. Dynorphin-containing axons directly innervate noradrenergic neurons in the rat nucleus locus coeruleus. Neuroscience. 2007;145:1077–1086. doi: 10.1016/j.neuroscience.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. Interface of physical and emotional stress regulation through the endogenous opioid system and mu-opioid receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1264–1280. doi: 10.1016/j.pnpbp.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Dopamine spillover after quantal release: rethinking dopamine transmission in the nigrostriatal pathway. Brain Res Rev. 2008;58:303–313. doi: 10.1016/j.brainresrev.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Ledoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth KA, Weber E, Barchas JD, Chang D, Chang JK. Immunoreactive dynorphin-(1–8) and corticotropin- releasing factor in subpopulation of hypothalamic neurons. Science. 1983;219:189–191. doi: 10.1126/science.6129700. [DOI] [PubMed] [Google Scholar]
- Rusin KI, Giovannucci DR, Stuenkel EL, Moises HC. Kappa-opioid receptor activation modulates Ca2+ currents and secretion in isolated neuroendocrine nerve terminals. J Neurosci. 1997;17:6565–6574. doi: 10.1523/JNEUROSCI.17-17-06565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sante AB, Nobre MJ, Brandao ML. Place aversion induced by blockade of mu or activation of kappa opioid receptors in the dorsal periaqueductal gray matter. Behav Pharmacol. 2000;11:583–589. doi: 10.1097/00008877-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Gehrke BJ, Shippenberg TS. Endogenous kappa-opioid receptor systems inhibit hyperalgesia associated with localized peripheral inflammation. Pain. 2008;138:423–439. doi: 10.1016/j.pain.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C. 30 years of dynorphins - new insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009:353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Lutchman TZ, Barrot M, Wallace T, Gilden L, Zachariou V, Impey S, Duman RS, Storm D, Nestler EJ. Regional and cellular mapping of cAMP response element-mediated transcription during naltrexone-precipitated morphine withdrawal. J Neurosci. 2002;22:3663–3672. doi: 10.1523/JNEUROSCI.22-09-03663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen CP, Tsimberg Y, Salvadore C, Meller E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Chefer VI. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol Disord Drug Targets. 2008;7:442–453. doi: 10.2174/187152708786927813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Shuster SJ, Riedl M, Li X, Vulchanova L, Elde R. The kappa opioid receptor and dynorphin co-localize in vasopressin magnocellular neurosecretory neurons in guinea-pig hypothalamus. Neuroscience. 2000;96:373–383. doi: 10.1016/s0306-4522(99)00472-8. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. k-Opioid receptor activation of a dendrotoxin-sensitive potassium channel mediates presynaptic inhibition of mossy fiber neurotransmitter release. Mol Pharmacol. 1996;50:80–85. [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. Embo J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhov RR, Walker LC, Rance NE, Price DL, Young WS., 3rd Opioid precursor gene expression in the human hypothalamus. J Comp Neurol. 1995;353:604–622. doi: 10.1002/cne.903530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, Pickel VM. Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Senda T, Tokuyama S, Kaneto H. Further evidence for the implication of a kappa-opioid receptor mechanism in the production of psychological stress-induced analgesia. Jpn J Pharmacol. 1990;53:487–494. doi: 10.1254/jjp.53.487. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. Opioid receptor subtypes differentially modulate serotonin efflux in the rat central nervous system. J Pharmacol Exp Ther. 2002;303:549–556. doi: 10.1124/jpet.102.037861. [DOI] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. mu-Opioids disinhibit and kappa-opioids inhibit serotonin efflux in the dorsal raphe nucleus. Brain Res. 2005;1049:70–79. doi: 10.1016/j.brainres.2005.04.076. [DOI] [PubMed] [Google Scholar]
- Terman GW, Wagner JJ, Chavkin C. Kappa opioids inhibit induction of long-term potentiation in the dentate gyrus of the guinea pig hippocampus. J Neurosci. 1994;14:4740–4747. doi: 10.1523/JNEUROSCI.14-08-04740.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Parsegian A, Naydenov A, Neve RL, Konradi C, Carlezon WA., Jr Brain reward regulated by AMPA receptor subunits in nucleus accumbens shell. J Neurosci. 2006;26:11665–11669. doi: 10.1523/JNEUROSCI.3070-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon SM, Pollack AE, Fink JS. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 1997;749:120–126. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- Ur E, Wright DM, Bouloux PM, Grossman A. The effects of spiradoline (U-62066E), a kappa-opioid receptor agonist, on neuroendocrine function in man. Br J Pharmacol. 1997;120:781–784. doi: 10.1038/sj.bjp.0700971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Valentine G, Dow A, Banasr M, Pittman B, Duman R. Differential effects of chronic antidepressant treatment on shuttle box escape deficits induced by uncontrollable stress. Psychopharmacology (Berl) 2008;200:585–596. doi: 10.1007/s00213-008-1239-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Mantamadiotis T, Torrecilla M, Ugedo L, Pineda J, Bleckmann S, Gass P, Kretz O, Mitchell JM, Schutz G, Maldonado R. Modulation of anxiety-like behavior and morphine dependence in CREB-deficient mice. Neuropsychopharmacology. 2004;29:1122–1133. doi: 10.1038/sj.npp.1300416. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- Vergne DE, Nemeroff CB. The interaction of serotonin transporter gene polymorphisms and early adverse life events on vulnerability for major depression. Curr Psychiatry Rep. 2006;8:452–457. doi: 10.1007/s11920-006-0050-y. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML. A review of the properties of spiradoline: a potent and selective kappa-opioid receptor agonist. CNS Drug Rev. 2003;9:187–198. doi: 10.1111/j.1527-3458.2003.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Stellitano KE, Neve RL, Duman RS. Effects of cyclic adenosine monophosphate response element binding protein overexpression in the basolateral amygdala on behavioral models of depression and anxiety. Biol Psychiatry. 2004;56:151–160. doi: 10.1016/j.biopsych.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Walters CL, Cleck JN, Kuo YC, Blendy JA. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron. 2005;46:933–943. doi: 10.1016/j.neuron.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Weiland NG, McEwen BS. Effects of adrenal steroid manipulations and repeated restraint stress on dynorphin mRNA levels and excitatory amino acid receptor binding in hippocampus. Brain Res. 1995;680:217–225. doi: 10.1016/0006-8993(95)00235-i. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Maier SF. Kappa opiate receptors mediate tail-shock induced antinociception at spinal levels. Brain Res. 1992;582:1–9. doi: 10.1016/0006-8993(92)90310-6. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;362:423–427. doi: 10.1038/362423a0. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J Neurophysiol. 2006;96:3465–3473. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompré PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Xu M, Bruchas MR, Ippolito DL, Gendron L, Chavkin C. Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase. J Neurosci. 2007;27:2570–2581. doi: 10.1523/JNEUROSCI.3728-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Petraschka M, McLaughlin JP, Westenbroek RE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Terman GW, Chavkin C. Neuropathic pain activates the endogenous kappa opioid system in mouse spinal cord and induces opioid receptor tolerance. J Neurosci. 2004;24:4576–4584. doi: 10.1523/JNEUROSCI.5552-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadid G, Overstreet DH, Zangen A. Limbic dopaminergic adaptation to a stressful stimulus in a rat model of depression. Brain Res. 2001;896:43–47. doi: 10.1016/s0006-8993(00)03248-0. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Cebers G, Marinova Z, Hara Y, Ahmed A, Vlaskovska M, Johansson B, Hochgeschwender U, Singh IN, Bruce-Keller AJ, Hurd YL, Kaneko T, Terenius L, Ekstrom TJ, Hauser KF, Pickel VM, Bakalkin G. Prodynorphin storage and processing in axon terminals and dendrites. Faseb J. 2006;20:2124–2126. doi: 10.1096/fj.06-6174fje. [DOI] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Collins SL, Aberg M, Kumar A, Fernandez JB, Izenwasser S. Depletion of serotonin decreases the effects of the kappa-opioid receptor agonist U-69593 on cocaine-stimulated activity. Eur J Pharmacol. 2008;586:123–129. doi: 10.1016/j.ejphar.2008.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Shi YG, Woods JH, Watson SJ, Ko MC. Central kappa-opioid receptor-mediated antidepressant-like effects of nor-Binaltorphimine: behavioral and BDNF mRNA expression studies. Eur J Pharmacol. 2007;570:89–96. doi: 10.1016/j.ejphar.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. The psychophysiology of sensation seeking. J Pers. 1990;58:313–345. doi: 10.1111/j.1467-6494.1990.tb00918.x. [DOI] [PubMed] [Google Scholar]