Abstract
Multiple lines of evidence indicate that hypocretin/orexin (HCRT) participates in the regulation of arousal and arousal-related process. For example, HCRT axons and receptors are found within a variety of arousal-related systems. Moreover, when administered centrally, HCRT exerts robust wake-promoting actions. Finally, a dysregulation of HCRT neurotransmission is associated with the sleep/arousal disorder, narcolepsy. Combined, these observations suggested that HCRT might be a key transmitter system in the regulation of waking. Nonetheless, subsequent evidence indicates that HCRT may not play a prominent role in the initiation of normal waking. Instead HCRT may participate in a variety of processes such as consolidation of waking and/or coupling metabolic state with behavioral state. Additionally, substantial evidence suggests a potential involvement of HCRT in high-arousal conditions, including stress. Thus, HCRT neurotransmission is closely linked to high-arousal conditions, including stress, and HCRT administrations exerts a variety of stress-like physiological and behavioral effects that are superimposed on HCRT-induced increases in arousal. Combined, this evidence suggests the hypothesis that HCRT may participate in behavioral responding under high-arousal aversive conditions. Importantly, these actions of HCRT may not be limited to stress. Like stress, appetitive conditions are associated with elevated arousal levels and a stress-like activation of various physiological systems. These and other observations suggest that HCRT may, at least in part, exert affectively-neutral actions that are important under high-arousal conditions associated with elevated motivation and/or need for action.
Keywords: Hypocretin, Orexin, Arousal, Stress, Waking, Reward
1. HCRT AND WAKING
The HCRT neuropeptides, HCRT-1 and HCRT-2, are synthesized solely in the lateral hypothalamus (LH) and adjacent regions. Despite their limited number and restricted origin, HCRT neurons extend a vast projection system that innervates virtually the entire the neuraxis. Of particular relevance for the current review, HCRT-containing fibers and receptors are located within multiple brainstem and basal forebrain structures associated with the regulation of behavioral state (Sakurai et al., 1998; Peyron et al., 1998; Date et al., 1999; Taheri et al., 1999; Marcus et al., 2001). Moreover, intracerebroventricular (ICV) administration of HCRT-1 and HCRT-2 exert robust wake-promoting and sleep-suppressing actions (Hagan et al., 1999; Ida et al., 1999; Piper et al., 2000; España et al., 2001; España et al., 2002), effects similar to those seen with optogenetic stimulation of HCRT neurons (Adamantidis et al., 2007). Finally, HCRT-induced waking is associated with a variety of behaviors typical of spontaneous waking including eating, drinking, grooming, and locomotor activity (Hagan et al., 1999; España et al., 2002).
A key advance in our understanding of the neurobiology of HCRT was the identification a link between narcolepsy and a dysregulation of HCRT neurotransmission. For example, in mice, the loss of HCRT or HCRT receptors results in a narcolepsy-like phenotype, including cataplexy and disrupted sleep/wake patterns (Chemelli et al., 1999; Hara et al., 2001). Additionally, a genetic defect in the gene for the HCRT receptor, HCRTr2, has been identified in a longstanding canine model of narcolepsy (Lin et al., 1999). Finally, numerous studies demonstrate a degenerative loss of HCRT neurons in human narcolepsy (Nishino et al., 2000; Ripley et al., 2001; Peyron et al., 2000; Thannickal et al., 2000; Crocker et al., 2005).
Combined these observations suggested that HCRT neurotransmission is necessary for the initiation and maintenance of waking and normal levels of arousal. However, additional observations suggest that this hypothesis is likely untenable. For example, although HCRT knockout mice exhibit cataplexy and fragmented sleep and waking, they do not display an overall reduction in time spent awake or excessive amounts of sleep (Mochizuki et al., 2004). Thus, at least in mice, the loss of HCRT does not lead to an overall reduction in arousal/waking per se, and instead results in the fragmentation of sleep and waking. Moreover, in humans, clinical studies indicate that HCRT loss may be more closely associated with cataplexy rather than reductions in arousal/waking. For example, 90% of narcoleptics with cataplexy have virtually undetectable HCRT in the CSF, while approximately 90% of narcoleptics without cataplexy have only moderately reduced or even normal levels of CSF HCRT (Nishino et al., 2000; Ripley et al., 2001). Nonetheless, given the variability in severity of cataplexy seen across patients with undetectable HCRT, the role of HCRT in narcolepsy-related cataplexy is unclear.
In sum, although the central administration of HCRT is sufficient to promote waking, HCRT does not appear to be necessary for normal levels of waking/arousal. This work and work reviewed below suggests that, instead, HCRT may participate in the maintenance of normal sleep-wake patterns, including sleep-consolidation, coupling of metabolic state with behavior, and/or behavioral/physiological responding under high-arousal conditions.
2. NEUROCIRCUITRY OF HCRT-INDUCED AROUSAL
Locus Coeruleus-Noradrenergic System
Limited studies have examined the neural circuitry associated with the arousal-promoting actions of HCRT. One system that has received particular interest in this regard is the locus coeruleus (LC)-noradrenergic system, which exerts robust arousal-promoting actions (Berridge, 2008). Early observations demonstrated that HCRT fibers innervate the LC and that, in vitro and in vivo, HCRT activates LC neurons (Peyron et al., 1998; Hagan et al., 1999; Horvath et al., 1999; Ivanov and Aston-Jones, 2000; Bourgin et al., 2000; España et al., 2003). Moreover, when infused into the LC region of the brainstem, HCRT increases time spent awake (Bourgin et al., 2000). Combined, these observations suggest a likely involvement of the LC-noradrenergic system in the wake-promoting actions of HCRT.
Basal Forebrain
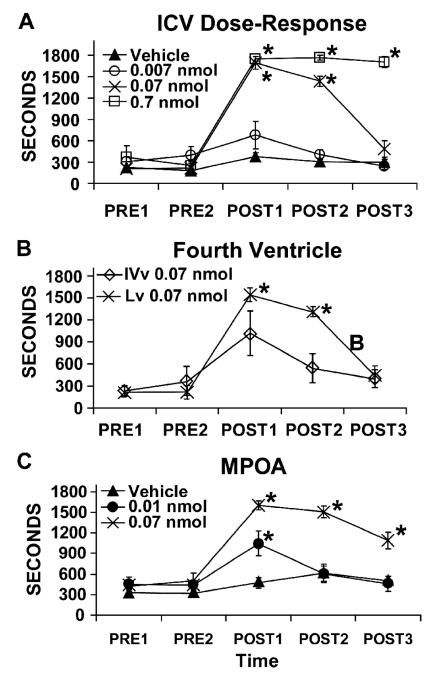
Despite the above-described observations regarding excitatory actions of HCRT on LC neurons, when infused into the fourth ventricle immediately adjacent to the LC HCRT produces weaker and longer latency wake-promoting actions relative to HCRT infusion into the lateral ventricles (España et al., 2001). This suggests that HCRT-induced waking may involve actions at sites anterior to the LC. Indeed, HCRT fibers and receptors are located within several arousal-related basal forebrain structures (for review, Sutcliffe and de Lecea, 2002). These include the general region of the medial septal area (MSA), the general region of the medial preoptic area (MPOA), and the substantia innominata (SI; Hobson et al., 1975; Buzsaki et al., 1988; Berridge and Foote, 1996; Berridge and O’Neill, 2001). Moreover, when infused directly into the MSA, MPOA or SI, but not immediately outside these regions, HCRT produces dose-dependent increases in time spent awake and decreases in slow-wave and REM sleep (España et al., 2001; Thakkar et al., 2001). Across these three regions, the largest increases in waking are observed following infusions within the MPOA (see Figure 1C).
Figure 1.
Wake-promoting effects of HCRT-1 infused into the lateral ventricle (Lv; Panel A), the fourth ventricle (IVv; Panel B) and the medial preoptic area (MPOA; Panel C). Symbols represent mean (± SEM) time (sec) spent awake per 30-min epoch. PRE1 and PRE2 represent pre-infusion epochs and POST1-POST3 represent post-infusion epochs. In all panels, vehicle-treated animals spent the majority of the testing period asleep. Panel A: HCRT-1 produces dose-dependent increases in waking, with significant increases observed at 0.07 and 0.7 nmol. Panel B: 0.07 nmol infusion of HCRT-1 produces a smaller magnitude in waking when infused into the fourth ventricle as compared to infusion into the lateral ventricle. Additionally, HCRT-induced waking following infusions into the fourth ventricle occurred with a longer latency than that observed following lateral ventricle infusions. Thus, latency to waking following fourth ventricular infusion of HCRT was 320 ± 40 sec from start of 120-sec infusion (Range = 216-416 sec) while the latency to waking following infusions into the lateral ventricle was 191 ± 48 sec from the start of the infusion (Range = 123-375 sec). Panel C: When infused bilaterally into the MPOA (250 nl/hemisphere), HCRT-1 produced a robust increase in waking similar to that seen with ICV infusions. Qualitatively similar effects were observed with HCRT-1 infusions into the medial septal area and substantia innominata. For all panels *P<0.01 significantly different from vehicle-treated animals. Modified from (España et al., 2001).
Additional Brain Regions
HCRT neurons project to numerous arousal-related structures beyond the LC and basal forebrain structures described above. These include dopaminergic, serotonergic, and histaminergic nuclei. Where examined, HCRT appears to exert excitatory actions on these cell populations (Bayer et al., 2001; Eriksson et al., 2001; Brown et al., 2002; Korotkova et al., 2003). Combined, these observations indicate that the wake-promoting actions of HCRT involve actions of the peptide(s) within multiple terminal fields.
Anatomical Organization of HCRT Neurons Projecting to the LC and Basal Forebrain
Retrograde tracing studies demonstrate that across the LC, MSA, MPOA and SI, HCRT projections to basal forebrain regions are largely ipsilateral (80%), whereas projections to the brainstem nucleus, LC, are more bilateral in nature (65%; España et al., 2005). In general, basal forebrain-projecting HCRT neurons are only weakly topographically organized, with a slight preference for these neurons to be located within the medial half of the HCRT neuronal field (55%-60%, depending on region). A somewhat larger proportion of LC-projecting HCRT neurons were located within the dorsal half of the HCRT field (65%). Importantly, a sizeable proportion of HCRT neurons appear to project simultaneously to multiple basal forebrain (MSA, MPOA, SI) and brainstem (i.e. LC) terminal fields, suggesting coordinated/concerted actions of HCRT across multiple anatomically-distinct, yet functionally-related regions (for details, see España et al., 2005).
3. ACTIVITY PATTERNS OF HCRT NEURONS
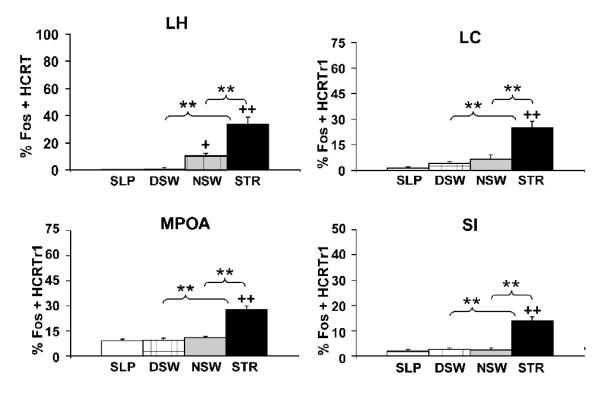
An important clue into the behavioral functions of a neurotransmitter system is the activity profile of that system. Microdialysis, c-fos expression and electrophysiological measures of HCRT neuronal activity provide strikingly similar observations: HCRT neurons are relatively quiescent during both sleep and quiet waking and display increased activity rates under conditions associated with higher arousal levels. For example, only low levels of the protein product of the c-fos gene, Fos, were observed in HCRT-synthesizing neurons in rats that were spontaneously awake either diurnally or nocturnally (Figure 2; España et al., 2003; Estabrooke et al., 2001). Nocturnal waking was associated with modestly higher Fos levels than seen with diurnal waking. A similar pattern of Fos expression was observed for neurons expressing the HCRTr1 receptor within basal forebrain regions (MS, MPOA, SI) and the LC (Figure 2; España et al., 2003). Moreover, the relatively small amount of Fos observed in HCRT-synthesizing neurons nocturnally was more strongly correlated with locomotor activity than with time spent awake (España et al., 2003). This is of interest, given the above-described relationship between HCRT dysfunction and cataplexy and other observations suggesting a link between HCRT neuronal transmission and motor activity (Torterolo et al., 2003).
Figure 2.
Effects of varying behavioral state/environmental conditions on the percentage of Fos-immunoreactive (ir) nuclei within hypocretin-synthesizing neurons (prepro-HCRT-ir) and hypocretin-1 receptor-expressing neurons (HCRTr1-ir). Shown are percentage Fos-positive HCRT neurons from diurnal sleeping (SLP), diurnal spontaneous waking (DSW), nocturnal spontaneous waking (NSW) and novelty-stress (STR) conditions. Neither diurnal sleeping nor diurnal spontaneous waking was associated with an increase in the percentage of Fos-ir within prepro-HCRT-ir neurons in LH. Nocturnal spontaneous waking was associated with a slight, yet significant, increase in the percentage of Fos-ir within prepro-HCRT-ir neurons. In contrast, novelty-stress produced a significantly higher percentage of Fos-ir within prepro-HCRT-ir relative to diurnal sleeping, diurnal spontaneous waking and nocturnal spontaneous waking. Within HCRTr1-ir neurons, only novelty-stress was associated with increased levels of Fos-ir. +P < 0.05; ++P < 0.01 significantly different from diurnal sleeping. **P < 0.01 significantly different from group indicated by brackets. Modified from (España et al., 2003).
Importantly, electrophysiological recordings of HCRT neurons demonstrate similar state-dependent fluctuations in HCRT neuronal activity (Mileykovskiy et al., 2005; Lee et al., 2005). Thus, HCRT neurons were observed to evince low discharge rates during both REM and slow-wave sleep. Moreover, HCRT neuronal discharge was not substantially elevated during quiet waking. In contrast, elevated discharge rates of HCRT neurons were associated with alert, active waking associated with motor activity. Despite this general relationship between HCRT neuronal discharge and motor activity, movement does not account entirely for the variance in HCRT neuronal activity. For example, increased HCRT neuronal discharge activity is observed in the absence of motor activity under conditions of elevated arousal and/or alertness elicited by an auditory stimulus (Mileykovskiy et al., 2005). Conversely, HCRT neurons display lower discharge rates (though still elevated relative to sleep and quiet waking) during periods of grooming and eating, behaviors associated with substantial muscle activity (Mileykovskiy et al., 2005). Finally, despite the wake-promoting actions of HCRT, presentation of a novel food, which was initially associated with aversion followed by active exploration/movement indicative of a higher arousal state, was nonetheless associated with a profound suppression of HCRT neuronal discharge rate (Mileykovskiy et al., 2005).
Finally, microdialysis studies indicate that extracellular levels of HCRT display a circadian rhythm, increasing slowly over the active period and extending into the inactive period (Yoshida et al., 2001; Deboer et al., 2004; Zeitzer et al., 2003). The circadian fluctuations in HCRT release into the ventricles is abolished by lesions of the suprachiasmatic nucleus, a key circadian nucleus (Deboer et al., 2004). Based on these observations, it has been suggested that HCRT participates in the maintenance of sustained/consolidated waking. Short-term fluctuations in HCRT neuronal discharge linked to environmental events as described above (Mileykovskiy et al., 2005) are presumably superimposed on circadian-dependent fluctuations in HCRT neuronal discharge/HCRT efflux.
Combined, these observations suggest a somewhat complex relationship between HCRT neurotransmission and arousal. Importantly, these observations provide strong evidence that HCRT is not tightly linked to waking per se.
4. CIRCADIAN-INDEPENDENT ACTIONS OF HCRT
Where examined, the behavioral actions of exogenously administered HCRT appear largely circadian-independent. Thus, ICV HCRT-1 administration in rats elicits comparable time spent awake when administered diurnally (sleep-period) vs. nocturnally (activity-period; España et al., 2002). Of course, the magnitude of the HCRT-induced shift from baseline waking is larger diurnally, given these animals display lower levels of waking during the day. Nonetheless, the amount of time spent awake following HCRT administration is similar for diurnal vs. nocturnal infusions. Similarly, diurnal vs. nocturnal administration of HCRT-1 produces comparable increases in time spent grooming, chewing of inedible material (e.g., bedding) and locomotor activity, as measured by quadrant entries and rears (España et al., 2002). It is important to note that the circadian-independent effects observed in these studies do not suggest that endogenous HCRT does not act in a circadian dependent manner. As reviewed above, ventricular levels of HCRT fluctuate in a circadian dependent fashion. Thus, HCRT may release/action may fluctuate across the circadian cycle, even if the behavioral actions of HCRT receptor stimulation are not circadian dependent.
In contrast, although diurnally-administered HCRT increases feeding and drinking in rats, when administered nocturnally, HCRT-1 has only minimal effects on eating and drinking (España et al., 2002). This largely reflects the fact that, in the early stages of the dark-phase (the first 90-minutes), vehicle-treated rats spend the majority of time awake and eat the majority of food that will be consumed during the entire dark-phase (España et al., 2002). Moreover, when multiple regression analyses were used to factor in variance in time spent awake, HCRT was observed to have minimal and statistically non-significant effects on feeding (España et al., 2002).
These observations indicate that under conditions associated with adequate access to food and minimal stress/disruption, exogenous administration of HCRT does not have potent effects on feeding. However, though outside the scope of this review, additional evidence indicates that HCRT neurons are sensitive to metabolic signals and that this sensitivity may translate into alterations in behavioral state under certain metabolic conditions. For example, HCRT knock-out mice do not display an increase in waking typically seen under fasting conditions (Yamanaka et al., 2003). These and other observations suggest that HCRT neurons may serve to couple metabolism with behavioral state, promoting appropriate behavior (e.g. food seeking) under varying metabolic conditions (e.g. food deprivation; for review, Teske et al., 2008; Adamantidis and de Lecea, 2009).
5. HCRT AND STRESS
Introduction to Stress
The concept of stress as a behavioral state elicited by challenging or threatening events arises from nearly a century of research, starting with the seminal work of Cannon (1914) and Selye (1946). In these early studies, disparate environmental events, that had in common the potential to disrupt homeostasis and threaten an animal’s well-being were observed to affect similarly various physiological systems. Initially, emphasis was placed primarily on stressor-induced activation of peripheral endocrine systems, particularly catecholamine systems (norepinephrine and epinephrine) and the hypothalamo-pituitary-adrenal (HPA) axis. Activation of the HPA axis involves increased release of corticotropin-releasing factor (CRF) from the paraventricular nucleus of the hypothalamus (PVN), stimulating adrenocorticotropin hormone (ACTH) secretion from the pituitary, which subsequently increases secretion of adrenal corticosteroids (corticosterone/cortisol). It was proposed that the activation of these systems results in an enhanced ability of the animal to physically contend with challenging situations (Cannon, 1914; Selye, 1946).
Later work began to examine the central mechanisms involved in stress-related behavior. It should be noted that the precise affective and cognitive components of stress remain unclear. However, a key aspect of stress is a heightened level of arousal and readiness for action. Indeed, sustained arousal may be a defining feature of stress. Importantly, elevated arousal does not necessarily involve increased motor activation (e.g. fear-induced freezing). Instead, stress-related elevations in arousal reflect activation of physiological systems that better prepare the animal for challenges. Superimposed on stress-related increases in arousal are alterations in a variety of state-dependent processes, including attention, memory, and sensory information processing (Arnsten et al., 1985; Berridge and Dunn, 1989; Arnsten, 1999). Although stress may also be associated with changes in affective state (i.e. negative affect), it is important to note that both positive and aversive conditions are associated with an activation of peripheral and central ‘stress’ systems (see below for further discussion). Thus, stress likely involves alterations in a variety of physiological and behavioral systems that are independent of affect.
A number of central neurotransmitter systems have been implicated in stress including, catecholamines and CRF. More recent work, reviewed below, suggests a potential involvement of HCRT in stress-related behavior and physiology.
Effects of Stress on c-fos Expression in HCRT-Synthesizing and HCRT-Receptive Neurons
The above-described observations suggest that HCRT systems may be activated under high-arousal conditions, including those associated with stress. Consistent with this, exposure of rats to a stressful, brightly-lit novel environment (novelty-stress; Hennessy and Foy, 1987; Berridge et al., 1999) results in an activation of HCRT-synthesizing neurons as well as HCRTr1-expressing neurons located within the MSA, MPOA, SI and LC (as measured by Fos immunoreactivity; Figure 2; España et al., 2003). This stress-related activation of HCRT neurons has been observed with a variety of stressors including, cold exposure, food deprivation, foot-shock, and immobilization stress (Sakurai et al., 1999; Ida et al., 2000; Zhu et al., 2002). Interestingly, although foot-shock increases Fos levels within HCRT neurons, a conditioned stimulus that predicted the foot-shock, a stressor itself, did not alter Fos levels within these neurons (Zhu et al., 2002). Together, these observations indicate that multiple stressors, but perhaps not all, activate HCRT neurons.
Stress-Like Behavioral and Physiological Actions of HCRT
Across multiple species, stress is associated with a variety of behavioral responses, including chewing or gnawing of inedible material, grooming, and fighting (Mason, 1968; Antelman et al., 1975; Dunn et al., 1981; Hennessy and Foy, 1987; Tsuda et al., 1988; Koob et al., 1988; Berridge and Dunn, 1989). Many of these behaviors serve to attenuate stressor-induced activation of a variety of central and peripheral physiological systems (i.e. coping/displacement behaviors). Interestingly, ICV and basal forebrain administration of HCRT elicits a majority of these behaviors, including grooming (Ida et al., 2000), chewing of inedible material (España et al., 2003), and locomotor activity (España et al., 2001; España et al., 2002; Martins et al., 2004). Moreover, HCRT knock-out mice display a reduced defensive response in the resident-intruder test (Kayaba et al., 2003).
Additionally, HCRT produces a variety of stress-like physiological actions. For example, in vitro, HCRT activates sympathetic preganglionic neurons (van den Pol et al., 2003) and neurons in the nucleus of the solitary tract, a primary brainstem autonomic nucleus (Smith et al., 2002a). Moreover, in vivo, HCRT infusion into the nucleus of the solitary tract increases blood pressure (Smith et al., 2002b) while HCRT knock-out mice display decreased cardiovascular and behavioral responses to stress as well as decreased basal arterial pressure (Kayaba et al., 2003). These observations are consistent with other observations demonstrating HCRT administration promotes a variety of autonomic responses associated with stress, including elevation of mean arterial blood pressure, heart rate, oxygen consumption and body temperature (Lubkin and Stricker-Krongrad, 1998; Samson et al., 1999; Shirasaka et al., 1999; Yoshimichi et al., 2001).
Combined, these observations indicate that HCRT may serve as an important modulator of autonomic and cardiovascular responding in stress (Kayaba et al., 2003). This hypothesis is consistent with results from additional studies demonstrating that HCRT activates CRF-containing neurons, resulting in an activation of the HPA axis (Jaszberenyi et al., 2000; Kuru et al., 2000), while an HCRT antagonist attenuates stressor induced increases in ACTH secretion (Samson et al., 2007). A role of HCRT in stress likely involves widespread actions of HCRT as moderate doses of HCRT produced a stress-like increase in c-fos expression within HCRT-receptive neurons (neurons expressing the HCRTr1 receptor) throughout the brain, including within the LC, MSA, MPOA, and SI (Figure 2; España et al., 2003).
Combined, these observations suggest that not only are HCRT neurons active in stress, but that HCRT simultaneously activates a variety of stress-related neural circuits. As such, HCRT may serve to coordinate physiological and behavioral responding in stress.
HCRT-Catecholamine Interactions and Stress
Substantial evidence suggests that catecholamine systems participate in stress-related alterations in arousal state and state-dependent behavioral processes (Berridge and Waterhouse, 2003; Dunn, 1988). For example, stress is associated with increased release of norepinephrine (NE) and dopamine (DA) in a variety of terminal fields, particularly regions implicated in higher cognitive and affective function (e.g. prefrontal cortex, amygdala; Thierry et al., 1976; Stone, 1978; Iuvone and Dunn, 1986; Dunn, 1988; Berridge et al., 1999). Moreover, NE and DA exert-arousal-enhancing actions and contribute to stress-related alterations in behavior/cognition (Robbins, 1984; Berridge and Dunn, 1989; Arnsten, 2000; Berridge and Waterhouse, 2003; Berridge, 2008). Combined, these observations suggest a prominent role of NE and DA in stress-related alterations in behavioral state and state-dependent behavior.
Previous studies indicate that HCRT produces a stress-like activation of catecholamine neurons. For example, as described above, HCRT activates LC neurons, similar to that seen in stress (Hagan et al., 1999; Horvath et al., 1999; Ivanov and Aston-Jones, 2000; Bourgin et al., 2000; Kiyashchenko et al., 2001). Moreover, when administered either into the lateral ventricles or directly into the ventral tegmental area (VTA), HCRT elicits an activation of DA systems in a stress-like pattern, with increases in DA release observed within the prefrontal cortex and the nucleus accumbens shell, but not the nucleus accumbens core (Narita et al., 2006; Vittoz and Berridge, 2006). This pattern of a differential sensitivity of prefrontal DA vs. accumbens core DA is similar to that seen in stress (Dunn, 1988; Berridge et al., 1999).
Recent observations suggest that the differential actions of HCRT on DA efflux across DA terminal fields involve, at least in part, the differential activation of a subpopulation of VTA DA neurons. Specifically, HCRT-induced activation of VTA DA neurons, as measured by Fos-immunoreactivity, is primarily restricted to a population of small-to-medium-sized, but not large, DA neurons located within the caudomedial VTA (Vittoz et al., 2008). Furthermore, within this region of the VTA, the excitatory actions of HCRT were restricted to DA neurons projecting to the prefrontal cortex and shell accumbens and not DA neurons projecting to the core accumbens (Vittoz et al., 2008). Combined, this evidence suggests that the excitatory actions of HCRT on DA neurotransmission are restricted to a subpopulation of DA neurons that project to cortical/limbic structures implicated in stress-related alterations in behavior.
Activating Actions of HCRT on CRF Neurotransmission
The neuropeptide, CRF, plays a prominent role in coordinating the constellation of behavioral and physiological responses observed in stress (Koob and Bloom, 1985; Dunn and Berridge, 1990). The activation of CRF neurons located within the PVN, leading to increases in circulating glucocorticoids (via actions of ACTH), is considered a defining feature of stress. Moreover, actions of CRF neurons outside the PVN, including the amygdala, are strongly implicated in behavioral responding in stress (Dunn and Berridge, 1990). Given this, it is of interest that HCRT fibers are located within close proximity of CRF neurons within both the PVN as well as the amygdala. Moreover, in vitro, HCRT activates PVN neurons (Shirasaka et al., 2001; Samson et al., 2002) and, in vivo, ICV HCRT increases plasma levels of corticosterone and ACTH (Malendowicz et al., 1999; Ida et al., 2000; Kuru et al., 2000; Jászberényi et al., 2000; Nowak et al., 2000; Al Barazanji et al., 2001; Samson et al., 2002). Together, these observations indicate that HCRT efferents exert excitatory actions on CRF PVN neurons. It remains to be determined whether HCRT modulates activity of extrahypothalamic CRF neurons.
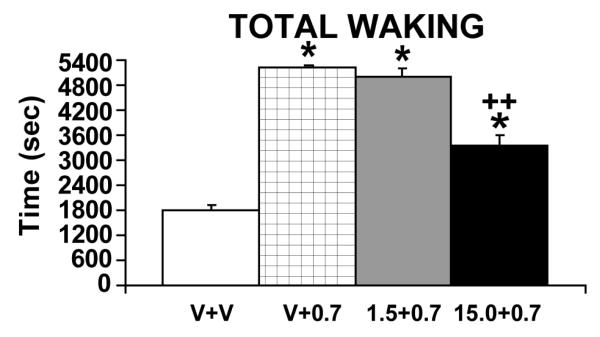
Similar to HCRT, central administration of CRF and ACTH elicit a variety of behavioral responses observed in stress, including locomotion, grooming and chewing of inedible objects (Gispen and Isaacson, 1981; Dunn et al., 1987). Combined, these observations suggest a possible interaction between HCRT and CRF in the regulation of stress-related behaviors. Consistent with this, pretreatment with a CRF antagonist (alpha-helical CRF9-41), reduced HCRT-induced grooming, face washing, and locomotor activation in rats (Ida et al., 2000). Moreover, in recently completed studies, we observed a dose-dependent decrease in HCRT-induced waking by ICV pretreatment with a CRF antagonist (alpha-helical CRF12-41; España and Berridge, unpublished observations). This effect of the CRF antagonist was most prominent at higher doses of HCRT (i.e. 0.7 nmol but not 0.07 nmol) that produce large increases in waking and a widespread, stress-like neuronal activation (Figure 3; España et al., 2003; Berridge and España, 2005). Thus, CRF may participate in HCRT-induced arousal particularly under high-arousal, stress-like conditions.
Figure 3.
Effects of pretreatment with the CRF antagonist, alpha-helical CRF12-41, on HCRT-induced waking. Shown are the effects of vehicle pretreatment followed by a vehicle infusion (V + V), vehicle pretreatment followed by a 0.7 nmol HCRT infusion (V + 0.7), 1.5 nmol alpha-helical CRF pretreatment followed by a 0.7 nmol HCRT infusion (1.5 + 0.7) and 15.0 nmol alpha-helical CRF pretreatment followed by a 0.7 nmol HCRT infusion (15.0 + 0.7). All infusions were ICV (2 μl volume over 2-minutes). HCRT-1 preceded by vehicle pretreatment, significantly increased waking relative to vehicle-vehicle treatment (V+0.7 vs V+V). Pretreatment with alpha-helical CRF, resulted in a dose-dependent attenuation of HCRT-induced waking. *P<0.05; **P<0.01 significantly different from V + V; +P < 0.01 significantly different from V + 0.7. (España and Berridge, unpublished observations).
Summary: HCRT and Stress
The above-described observations indicate an activation of HCRT neurons and HCRT receptor-expressing neurons in stress. Additional observations suggest a variety of stress-like actions of HCRT, including intense and widespread neuronal activation (as measured by Fos), elevated arousal levels, the induction of stress-like behavior, and an activation of stress-related neural systems, including NE, DA and CRF systems. It should be noted that excitatory actions of HCRT are observed in a variety of stress-related structures outside the LC and VTA, including the PVN, the bed nucleus of the stria terminalis and the central nucleus of the amygdala (Date et al., 1999; Kuru et al., 2000; Al Barazanji et al., 2001; España et al., 2003; Sakamoto et al., 2004). These observations suggest a likely role of HCRT in behavioral and physiological responding in stress. These observations may further suggest a potential involvement of HCRT in stress-related psychopathology, such as post-traumatic stress disorder and major depression.
6. HCRT AND APPETITIVE HIGH-AROUSAL STATES
Differential Sensitivity of HCRT Neurons to Rewards and Stress
The above-described observations suggest a potential involvement of HCRT in stress. However, the LH has long-been implicated in appetitive processes (for review see, Bernardis and Bellinger, 1996; Elmquist et al., 1999). Moreover, recent observations indicate that HCRT neurons are activated by conditioned rewards as measured in the conditioned place-preference test (Harris et al., 2005; Harris et al., 2007). Interestingly, this work suggests that different populations of HCRT neurons may participate in arousal/stress-related vs. appetitive/reward-seeking behavior. For example, HCRT neurons located within the LH proper were activated in animals that had developed a conditioned place-preference for food, morphine, or cocaine, while animals that had not developed a preference did not show an activation of HCRT neurons (Harris et al., 2005). An activation of HCRT neurons to conditioned-rewards was not observed in HCRT neurons located within the perifornical area/dorsomedial hypothalamus (Harris et al., 2005). In contrast, HCRT neurons in these latter regions displayed a sensitivity to footshock not seen in HCRT neurons located within the LH proper (Harris et al., 2005; Harris and Aston-Jones, 2006; Harris et al., 2007). These observations are consistent with differences in HCRT patterns of neuronal activation (perifornical/dorsomedial hypothalamus vs. LH) in response to diurnal variations in arousal and administration of anti-psychotic drugs (Estabrooke et al., 2001; Fadel et al., 2002). Combined, this evidence suggests a potential functional topography of HCRT neurons, with dorsomedial/perifornical HCRT neurons related to arousal (including stress) and LH HCRT neurons related to appetitive/reward-seeking behavior (Harris and Aston-Jones, 2006).
However, it is important to note that not all rewards/reinforcers elicit an activation of LH HCRT neurons. For example, conditioned place-preference produced by introduction of a novel object was not associated with a selective activation of LH HCRT neurons (Harris et al., 2005). Further, not all aversive conditions are associated with a topographically-defined activation of a subset of HCRT neurons. For example, in our previous studies we did not observe a differential activation of HCRT neurons across medial vs. lateral aspects of the HCRT neuronal field in animals exposed to a stressful brightly lit novel environment (Berridge et al., 1999; España et al., 2003). Specifically, novelty-stress activated 23% ± 6% of HCRT neurons in the medial aspects of the HCRT field (corresponding to the dorsomedial hypothalamus) and 22% ± 5% HCRT neurons in the lateral aspect of this field (corresponding to the LH), as measured by Fos immunoreactivity (España et al., 2003). The number of Fos-positive HCRT neurons observed in these latter studies was smaller than that observed with both rewards and footshock (Harris et al., 2005). However, in the novelty-stress studies non-stressed controls displayed minimal Fos HCRT neurons (España et al., 2003), in contrast to that reported elsewhere (Estabrooke et al., 2001; Harris et al., 2005). Lower cell counts in the novelty-stress study could reflect differences in basal arousal and/or stress levels across laboratories. In our novelty-stress studies, we took great effort to ensure low baseline arousal/stress levels in our animals. Thus, differences in the pattern or intensity of HCRT neuronal activation observed across studies with different stressors may involve differences between stressors and/or differences in baseline arousal levels.
To summarize, although HCRT neurons display a topographic organization in terms of sensitivity to some environmental conditions, this does not appear to extend to all conditions. Future research will need to more fully identify conditions associated with a topographically-restricted vs. widespread activation of HCRT neurons.
Is HCRT Rewarding?
The above-reviewed observations suggest reward-related actions of HCRT neurons. However, it is important to note that conditioned drug seeking reflects a complex interaction between reward-, motivation- and learning-related processes, many of which display a sensitivity to arousal (Yerkes and Dodson, 1908). Elevated arousal occurs under both appetitive and aversive conditions. Moreover, evidence indicates an activation of prototypical ‘stress’ systems under both aversive and appetitive conditions (e.g. HPA, central catecholamines; for review see, Marinelli and Piazza, 2002). Indeed, this provided the rationale for positing two forms of stress: one associated with aversive conditions (distress) and one associated with positive conditions (eustress; Selye, 1975). This suggests the working hypothesis that at least a subset of physiological indices of aversive (stress) and appetitive conditions may be independent of affective valence (pleasant vs. unpleasant) and more closely aligned with affectively-neutral processes such as arousal, motivation, learning and/or motor activation.
In this regard, it is of interest that stress is well-documented to reinstate previously extinguished drug-seeking behavior in both animals and humans (Stewart, 2000). Consistent with the above-described stress-like actions of HCRT, ICV and intra-VTA HCRT administration produces a stress-like reinstatement of drug-seeking behavior (self-administration and conditioned-place preference; Boutrel et al., 2005; Harris et al., 2005; Boutrel and de Lecea, 2008). In contrast, treatment with an HCRT R1 antagonist, SB-334867, prevents stressor-induced reinstatement of cocaine-seeking behavior (Boutrel et al., 2005). These observations suggest that HCRT participates in stressor-induced reinstatement of drug-seeking behavior.
The ability of HCRT to reinstate drug-seeking behavior is similar to that observed with NE (see above; Leri et al., 2002; Weinshenker and Schroeder, 2007), a stress-related transmitter that also exerts potent arousal-promoting actions (Berridge, 2008). Moreover, HCRT-induced reinstatement of cocaine-seeking behavior is attenuated by pharmacological inhibition of NE neurotransmission (Boutrel et al., 2005). Combined, these observations indicate an interaction between HCRT and NE in the reinstatement of drug-seeking behavior.
In contrast to the facilitatory actions of HCRT on reinstatement of reward-seeking behavior, ICV HCRT increases intracranial self-stimulation thresholds, suggesting a potential inhibitory action on reward-related systems (Boutrel et al., 2005). This argues rather strongly that when administered ICV, HCRT does not induce reward per se. This conclusion is consistent with recent observations indicating that ICV HCRT did not produce a conditioned place-preference (or conditioned place-aversion; Vittoz, Schmeichel, Berridge, unpublished observations). In contrast, when infused into the VTA, HCRT did produce a conditioned place-preference (Narita et al., 2007). Whether this latter observation reflects reward-related or memory/attention-related actions of HCRT remains unclear. It will be important for future studies to examine the effects of intra-VTA HCRT infusion on a more direct measure of reward, such as self-stimulation thresholds. Finally, the blockade of HCRT neurotransmission within the insula was observed to reduce nicotine self-administration (Hollander et al., 2008). However, it is important to note that a neurotransmitter system may play a critical role in drug-seeking behavior that is independent of reward per se (see Berridge and Robinson, 1998).
In sum, a complicated set of reward-related actions of HCRT has been observed. To date, it is not possible to synthesize these apparently discrepant observations into a unitary hypothesis regarding reward-related functions of HCRT. Nonetheless, these observations suggest that, globally, HCRT may not exert strongly rewarding effects. Instead HCRT may elicit affectively-neutral (i.e. reward-independent) alterations in arousal-, learning- and/or motivation-related circuits that are necessary for motivated behavior and/or learning about reward availability. Such an action is similar to that described for dopamine (Berridge and Robinson, 1998). Importantly, affectively neutral actions of HCRT are likely to be behaviorally significant under both appetitive and aversive conditions. Alternatively, HCRT may serve multiple reward-related and reward-independent functions that are linked to differing terminal fields. Clearly, delineating the reward-related actions of HCRT is an important area for future research.
7. SUMMARY
Substantial evidence indicates potent arousal-enhancing actions of HCRT. Additional observations suggest that HCRT neurons are activated under aversive, stress-like conditions associated with elevated arousal levels and that HCRT exerts a variety of stress-like behavioral and physiological actions. These observations suggest a potential role of HCRT in stress and, potentially, stress-related psychopathology. Importantly, many of the behavioral and physiological actions of HCRT appear to be affectively-neutral, while having an important impact on arousal and arousal-sensitive behavioral processes. Such actions could serve important functions in permitting the organism to better contend with challenging, stress-like conditions. Affect-independent behavioral actions of HCRT could also play an important role in behavioral responding in appetitive conditions. Indeed, many of the physiological effects of HCRT are associated with both appetitive and aversive conditions. Thus, HCRT may, in part, serve to modulate cognitive, motivational, motor and physiological systems under both appetitive and aversive conditions that have in common elevated arousal and a need for action. Of course, such a hypothesis does not preclude affect-related actions of HCRT. Nonetheless, evidence collected to date suggests that affect-independent actions of HCRT are likely behaviorally- and physiologically-important under varying conditions associated with elevated arousal.
Table I.
Tyrosine Hydroxylase (TH)-immunoreactive (ir) + Fos-ir Double-Labeling: Cell Counts and Percent TH-ir Neurons.
| Cell Counts: TH-ir + Fos-ir | |||||||
| LG FOS+TH | |||||||
| TREAT | VTA | ROSTRAL VS. CAUDAL | VTA QUADRANT | ||||
| RT | CD | M RT | L RT | M CD | L CD | ||
| AECF | 3.1 ± 1.3 | 0.2 ± 0.2 | 3.8 ± 1.5 | 0.0 ± 0.0 | 0.2 ± 0.2 | 3.5 ±1.4 | 0.2 ± 0.2 |
| HCRT | 8.6 ± 1.9 | 1.7 ± 0.6 | 4.4 ± 0.9 | 1.5 ±0.5 | 0.2 ± 0.1 | 3.8 ± 0.8 | 0.5 ± 0.2 |
| SM FOS+TH | |||||||
| TREAT | VTA | ROSTRAL VS. CAUDAL | VTA QUADRANT | ||||
| RT | CD | M RT | L RT | M CD | L CD | ||
| AECF | 6.9 ± 1.6 | 1.0 ± 0.4 | 5.4 ± 1.7 | 0.7 ± 0.2 | 0.2 ± 0.2 | 5.1 ± 1.7 | 0.2 ± 0.2 |
| HCRT | 38.4 ± 5.4** | 4.3 ± 1.0* | 24.6 ± 3.1** | 3.6 ± 0.9* | 0.7 ± 0.3 | 22.6 ± 2.8** | 1.8 ± 0.7 |
| Percent TH-ir with Fos-ir | |||||||
| LG FOS+TH | |||||||
| TREAT | VTA | ROSTRAL VS. CAUDAL | VTA QUADRANT | ||||
| RT | CD | M RT | L RT | M CD | L CD | ||
| AECF | 1.5% ± 0.6% | 0.2% ± 0.2% | 4.3% ± 1.8% | 0.0% ± 0.0% | 0.5% ± 0.5% | 9.4% ± 4.2% | 0.4% ± 0.4% |
| HCRT | 3.8% ± 0.5%* | 2.1% ± 0.7% | 5.0% ± 0.8% | 5.6% ± 2.2% | 0.6% ± 0.5% | 9.6% ± 1.3% | 1.4% ± 0.7% |
| SM FOS+TH | |||||||
| TREAT | VTA | ROSTRAL VS. CAUDAL | VTA QUADRANT | ||||
| RT | CD | M RT | L RT | M CD | L CD | ||
| AECF | 2.0% ± 0.5% | 1.3% ± 0.6% | 2.1% ± 0.7% | 2.2% ± 0.8% | 0.3% ± 0.3% | 3.0% ± 1.0% | 0.3% ± 0.3% |
| HCRT | 7.6% ± 0.8%** | 3.2% ± 0.7% | 8.8% ± 1.0%** | 6.5% ± 1.4% | 1.5% ± 0.5% | 13.0% ± 2.1%* | 2.4% ± 0.9% |
The number (top) and percentage (bottom) of large (upper rows of each table) and small (lower rows of each table) TH-ir neurons with Fos-ir nuclei (mean ± SEM) within rat VTA following ICV vehicle (AECF, n=6) and HCRT (0.5 nmol; n= 13). Data are shown for the full rostralcaudal extent of the VTA (VTA OVERALL) as well as for within rostral (RT) and caudal (CD) divisions. Data are also presented for each of four quadrants within the VTA: medial rostral (M RT), lateral rostral (L RT), medial caudal (M CD) and lateral caudal (L CD). HCRT increased the number and percentage of TH-ir + Fos-ir double labeled neurons. However this effect was not due to a global activation of VTA DA neurons. Instead, HCRT primarily activated DA neurons located within the caudomedial quadrant of the VTA. Within this quadrant, HCRT activated primarily small TH-ir cells. Subsequent retrograde tracing studies demonstrated that within the caudomedial VTA, TH-ir neurons project primarily to the medial PFC and shell accumbens, but not the core accumbens (data not shown; see Vittoz and Berridge, 2008). No Fos-ir + TH-ir cells were observed within the substantia nigra pars compacta of HCRT-treated animals.
P<0.05
P<0.01 vs. vehicle-treatment. From Vittoz and Berridge, 2008.
Abbreviations
- ACTH
adrenocorticotropin hormone
- CRF
corticotropin-releasing factor
- DA
dopamine
- HCRT
hypocretin/orexin
- HPA
hypothalamo-pituitary-adrenal
- ICV
intracerebroventricular
- LC
locus coeruleus
- LH
lateral hypothalamus
- MSA
medial septal area
- MPOA
medial preoptic area
- NE
norepinephrine
- PVN
paraventricular nucleus of the hypothalamus
- SI
substantia innominata
- VTA
ventral tegmental area.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: 3. Neurophysiology, Neuropharmacology and other forms of Intercellular Communication
References
- Adamantidis A, de LL. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de LL. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Barazanji KA, Wilson S, Baker J, Jessop DS, Harbuz MS. Central orexin-A activates hHypothalamic-pituitary-adrenal axis and stimulates hypothalamic corticotropin releasing factor and arginine vasopressin neurones in conscious rats. J. Neuroendocrinol. 2001;13:421–424. doi: 10.1046/j.1365-2826.2001.00655.x. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Szechtman H, Chin P, Fisher AE. Tail pinch-induced eating, gnawing and licking behavior in rats: dependence on the nigrostriatal dopamine system. Brain Res. 1975;99:319–337. doi: 10.1016/0006-8993(75)90032-3. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Development of the cerebral cortex: XIV. Stress impairs prefrontal cortical function. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:220–222. doi: 10.1097/00004583-199902000-00024. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog. Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Berridge C, Segal DS. Stress produces opioid-like effects on investigatory behavior. Pharmacol. Biochem. Behav. 1985;22:803–809. doi: 10.1016/0091-3057(85)90531-3. [DOI] [PubMed] [Google Scholar]
- Bayer L, Eggermann E, Serafin M, Saint-Mleux B, Machard D, Jones B, Muhlethaler M. Orexins (hypocretins) directly excite tuberomammillary neurons. Eur. J. Neurosci. 2001;14:1571–1575. doi: 10.1046/j.0953-816x.2001.01777.x. [DOI] [PubMed] [Google Scholar]
- Bernardis LL, Bellinger LL. The lateral hypothalamic area revisited: ingestive behavior. Neurosci. Biobehav. Rev. 1996;20:189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- Berridge CW, España RA. Hypocretin/orexin in stress and arousal. In: de Lecea L, Sutcliffe JG, editors. Hypocretins: Integrators of Physiological Functions. Springer; Singapore: 2005. pp. 351–368. [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Dunn AJ. Restraint-stress-induced changes in exploratory behavior appear to be mediated by norepinephrine-stimulated release of CRF. J. Neurosci. 1989;9:3513–3521. doi: 10.1523/JNEUROSCI.09-10-03513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Enhancement of behavioral and electroencephalographic indices of waking following stimulation of noradrenergic beta-receptors within the medial septal region of the basal forebrain. J. Neurosci. 1996;16:6999–7009. doi: 10.1523/JNEUROSCI.16-21-06999.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Mitton E, Clark W, Roth RH. Engagement in a non-escape (displacement) behavior elicits a selective and lateralized suppression of frontal cortical dopaminergic utilization in stress. Synapse. 1999;32:187–197. doi: 10.1002/(SICI)1098-2396(19990601)32:3<187::AID-SYN5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Berridge CW, O’Neill J. Differential sensitivity to the wake-promoting actions of norepinephrine within the medial preoptic area and the substantia innominata. Behav. Neurosci. 2001;115:165–174. doi: 10.1037/0735-7044.115.1.165. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J. Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, de Lecea LL. Addiction and arousal: the hypocretin connection. Physiol Behav. 2008;93:947–951. doi: 10.1016/j.physbeh.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de LL. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc. Natl. Acad. Sci. U. S. A. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Sergeeva OA, Eriksson KS, Haas HL. Convergent excitation of dorsal raphe serotonin neurons by multiple arousal systems (orexin/hypocretin, histamine and noradrenaline) J. Neurosci. 2002;22:8850–8859. doi: 10.1523/JNEUROSCI.22-20-08850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J. Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. The emergency function of the adrenal medulla in pain and the major emotions. Am. J. Physiol. 1914;33:356–372. [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Crocker A, Espana RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, Honda M, Mignot E, Scammell TE. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T, Overeem S, Visser NA, Duindam H, Frolich M, Lammers GJ, Meijer JH. Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience. 2004;129:727–732. doi: 10.1016/j.neuroscience.2004.07.049. [DOI] [PubMed] [Google Scholar]
- Dunn AJ. Stress-related activation of cerebral dopaminergic systems. Ann. N. Y. Acad. Sci. 1988;537:188–205. doi: 10.1111/j.1749-6632.1988.tb42106.x. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW, Lai YI, Yachabach TL. CRF-induced excessive grooming behavior in rats and mice. Peptides. 1987;8:841–844. doi: 10.1016/0196-9781(87)90069-6. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Guild AL, Kramarcy NR, Ware MD. Benzodiazepines decrease grooming in response to novelty but not ACTH or beta-endorphin. Pharmacol. Biochem. Behav. 1981;15:605–608. doi: 10.1016/0091-3057(81)90217-3. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Eriksson KS, Sergeeva O, Brown RE, Haas HL. Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J. Neurosci. 2001;21:9273–9279. doi: 10.1523/JNEUROSCI.21-23-09273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): Basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- España RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- España RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943:224–236. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- España RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J. Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J. Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen WH, Isaacson RL. ACTH-induced excessive grooming in the rat. Pharmacol. Ther. 1981;12:209–246. doi: 10.1016/0163-7258(81)90081-4. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav. Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Foy T. Nonedible material elicits chewing and reduces the plasma corticosterone response during novelty exposure in mice. Behav. Neurosci. 1987;101:237–245. doi: 10.1037//0735-7044.101.2.237. [DOI] [PubMed] [Google Scholar]
- Hobson JA, McCarley RW, Wyzinski PW. Sleep cycle oscillation: reciprocal discharge by two brainstem neuronal groups. Science. 1975;189:55–58. doi: 10.1126/science.1094539. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J. Comp Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite- stimulating neuropeptide, orexin and neuropeptide Y, on the various behavioral activities of rats. Brain Res. 1999;821:526–529. doi: 10.1016/s0006-8993(99)01131-2. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem. Biophys. Res. Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Dunn AJ. Tyrosine hydroxylase activation in mesocortical 3,4-dihydroxyphenylethylamine neurons following footshock. J. Neurochem. 1986;47:837–844. doi: 10.1111/j.1471-4159.1986.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Aston-Jones G. Hypocretin/orexin depolarizes and decreases potassium conductance in locus coeruleus neurons. Neuroreport. 2000;11:1755–1758. doi: 10.1097/00001756-200006050-00031. [DOI] [PubMed] [Google Scholar]
- Jászberényi M, Bujdosó E, Pataki I, Telegdy G. Effects of orexins on the hypothalamic-pituitary-adrenal system. J. Neuroendocrinol. 2000;12:1174–1178. doi: 10.1046/j.1365-2826.2000.00572.x. [DOI] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am. J. Physiol Regul. Integr. Comp Physiol. 2003 doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Lai YY, Siegel JM. Increased and decreased muscle tone with orexin (hypocretin) microinjections in the locus coeruleus and pontine inhibitory area. J. Neurophysiol. 2001;85:2008–2016. doi: 10.1152/jn.2001.85.5.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Corticotropin-releasing factor and behavior. Fed. Proc. 1985;44:259–263. [PubMed] [Google Scholar]
- Koob GF, Thatcher-Briton K, Tazi A, Le Moal M. Behavioral pharmacology of stress: Focus on CNS corticotropin-releasing factor. Adv. Exp. Med. Biol. 1988;245:25–34. doi: 10.1007/978-1-4899-2064-5_3. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J. Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport. 2000;11:1977–1980. doi: 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J. Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem. Biophys. Res. Commun. 1998;253:241–245. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Tortorella C, Nussdorfer GG. Orexins stimulate corticosterone secretion of rat adrenocortical cells, through the activation of the adenylate cyclase-dependent signaling cascade. J. Steroid Biochem. Mol. Biol. 1999;70:185–188. doi: 10.1016/s0960-0760(99)00110-7. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur. J. Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Martins PJ, D’Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul. Pept. 2004;117:155–158. doi: 10.1016/j.regpep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Mason JW. The scope of psychoendocrine research. Psychosom. Med. 1968:565–575. doi: 10.1097/00006842-196809000-00019. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocreting (orexin) neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Crocker A, McCormack S, Yanagisawa M, Sakurai T, Scammell TE. Behavioral state instability in orexin knock-out mice. J. Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Miyatake M, Ikegami D, Kurashi K, Suzuki T. Implication of protein kinase C in the orexin-induced elevation of extracellular dopamine levels and its rewarding effect. Eur. J. Neurosci. 2007;25:1537–1545. doi: 10.1111/j.1460-9568.2007.05403.x. [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- Nowak KW, Mackowiak P, Switonska MM, Fabis M, Malendowicz LK. Acute orexin effects on insulin secretion in the rat: in vivo and in vitro studies. Life Sci. 2000;66:449–454. doi: 10.1016/s0024-3205(99)00611-6. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van Den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur. J. Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Ripley B, Overeem S, Fujiki N, Nevsimalova S, Uchino M, Yesavage J, Di Monte D, Dohi K, Melberg A, Lammers GJ, Nishida Y, Roelandse FW, Hungs M, Mignot E, Nishino S. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology. 2001;57:2253–2258. doi: 10.1212/wnl.57.12.2253. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Cortical noradrenaline, attention and arousal. Psychol. Med. 1984;14:13–21. doi: 10.1017/s0033291700003032. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul. Pept. 2004;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Moriguchi T, Furuya K, Kajiwara N, Nakamura T, Yanagisawa M, Goto K. Structure and function of human prepro-orexin gene. J. Biol. Chem. 1999;274:17771–17776. doi: 10.1074/jbc.274.25.17771. [DOI] [PubMed] [Google Scholar]
- Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am. J Physiol Regul. Integr. Comp Physiol. 2007;292:R382–R387. doi: 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 1999;831:248–253. doi: 10.1016/s0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Follwell M, Ferguson AV. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul. Pept. 2002;104:97–103. doi: 10.1016/s0167-0115(01)00353-6. [DOI] [PubMed] [Google Scholar]
- Selye H. The general adaptation syndrome and the diseases of adaptation. J. Clin. Endocrinol. Metab. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- Selye H. Confusion and controversy in the stress field. J Human Stress. 1975;1:37–44. doi: 10.1080/0097840X.1975.9940406. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am. J. Physiol Regul. Integr. Comp Physiol. 2001;281:R1114–R1118. doi: 10.1152/ajpregu.2001.281.4.R1114. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am. J. Physiol. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- Smith BN, Davis SF, van Den Pol AN, Xu W. Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience. 2002a;115:707–714. doi: 10.1016/s0306-4522(02)00488-8. [DOI] [PubMed] [Google Scholar]
- Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002b;950:261–267. doi: 10.1016/s0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Stone EA. Effect of stress on norepinephrine-stimulated cyclic AMP formation in brain slices. Pharmacol. Biochem. Behav. 1978;8:583–591. doi: 10.1016/0091-3057(78)90392-1. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat. Rev. Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei MA, Bloom SR. Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett. 1999;457:157–161. doi: 10.1016/s0014-5793(99)01030-3. [DOI] [PubMed] [Google Scholar]
- Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology. 2008;87:71–90. doi: 10.1159/000110802. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Ramesh V, Strecker RE, McCarley RW. Microdialysis perfusion of orexin-A in the basal forebrain increases wakefulness in freely behaving rats. Arch. Ital. Biol. 2001;139:313–328. [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2003;26:25–28. [PubMed] [Google Scholar]
- Tsuda A, Tanaka M, Ida Y, Shirao I, Gondoh Y, Oguchi M, Yoshida M. Expression of aggression attenuates stress-induced increases in rat brain noradrenaline turnover. Brain Res. 1988;474:174–180. doi: 10.1016/0006-8993(88)90680-4. [DOI] [PubMed] [Google Scholar]
- van den Pol TM, Nolan MF, Lee K, Richardson PJ, Buijs RM, Davies CH, Spanswick D. Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol. 2003;549:809–821. doi: 10.1113/jphysiol.2002.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur. J Neurosci. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908;18:459–482. [Google Scholar]
- Yoshida Y, Fujiki N, Nakajima T, Ripley B, Matsumura H, Yoneda H, Mignot E, Nishino S. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur. J. Neurosci. 2001;14:1075–1081. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- Yoshimichi G, Yoshimatsu H, Masaki T, Sakata T. Orexin-A regulates body temperature in coordination with arousal status. Exp. Biol. Med. 2001;226:468–476. doi: 10.1177/153537020122600513. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J. Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Onaka T, Sakurai T, Yada T. Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport. 2002;13:1351–1353. doi: 10.1097/00001756-200207190-00027. [DOI] [PubMed] [Google Scholar]