Abstract
Background & aims
5-aminosalicylic acid (5-ASA) is a mainstay therapeutic agent in chronic ulcerative colitis (CUC) where it reverses crypt architectural changes and reduces colitis-associated cancer (CAC). The present study addressed the possibility that 5-ASA reduces β-catenin-associated progenitor cell activation, Akt-phosphorylated β-cateninSer552 (P-β-catenin), and colitis-induced dysplasia (CID).
Methods
Effects of 5-ASA on P-β-catenin staining and function were assessed by IHC and qRT-PCR in biopsies of CUC in mild or “refractory” severe mucosal inflammation. Effects of 5-ASA on epithelial proliferation, and activation of Akt and β-catenin were assessed in IL-10−/− colitis and CID by IHC and Western blotting. Dysplasia was assessed by counting the number and lengths of lesions per colon.
Results
Data from IL-10−/− and human colitis samples show 5-ASA reduced Akt activation and P-β-catenin levels in the mid and upper crypt. Reductions in P-β-catenin in CUC biopsies with severe inflammation suggested that 5-ASA reduced P-β-catenin levels in tissue refractory to 5-ASA’s anti-inflammatory effects. In IL-10−/− mice, 5-ASA reduced CID concordant with inhibition of crypt Akt and β-catenin signaling.
Conclusions
The results are consistent with the model that 5-ASA contributes to chemoprevention in CAC by reducing β-catenin signaling within intestinal progenitors.
Introduction
Chronic ulcerative colitis (CUC) increases the risk of colorectal cancer (CRC) 10-fold over age-matched controls1–3. 5-aminosalicylic acid (ASA) is the primary anti-inflammatory therapy for ulcerative colitis (UC). Meta-analysis of human data indicate that 5-ASA reduces the risk for colitis-associated cancer (CAC)4, possibly in a dose-dependent manner5. In vitro and in vivo data suggest 5-ASA assists CRC chemoprevention through several mechanisms6. 5-ASA has been shown to reduce epithelial proliferation and increase apoptosis7,8 through a variety of indirect and direct mechanisms. 5-ASA is a reactive oxygen scavenger9, reduces NF-κB activation10 and inhibits production of inflammatory cytokines11,12, prostaglandins and leukotrienes13. 5-ASA inhibits epithelial phosphoinositide-3 kinase (PI3K) signaling14–16 possibly by binding to peroxisome proliferator–activated receptor gamma (PPARγ) expressed by intestinal epithelial cells16. Recent data suggest that PPARγ increases expression of phosphatase and tensin homologue (PTEN)17, a tumor suppressor protein that inhibits PI3K signaling18. These findings raise the possibility that 5-ASA assists in cancer chemoprevention in part, due to PTEN-mediated inhibition of PI3K signaling.
Our understanding of the pathways that regulate intestinal epithelial proliferation derives, in part, from observations in human dysplasia and genetically altered mouse models. Studies from patients with familial adenomatous polyposis, adenomas and sporadic CRC indicate that inactivating mutations in the adenomatous polyposis coli (APC) gene predisposes individuals to polyps and CRC15,19–21. Studies in mouse and human tissue indicate that a major reason for the neoplastic effect of APC relates to its role in degradation of β-catenin. APC participates in a β-catenin degradation complex with axin, GSK3β and CK-122. Thus, inactivating mutations in members of this complex or mutations in β-catenin itself lead to β-catenin stabilization and nuclear relocalization23–27. Thus, nuclear β-catenin is the hallmark of active Wnt signaling and is frequently observed in tumor cells28,29. In the nucleus, β-catenin binds T-cell factor-4, inducing transcription of a set of target genes involved in cell proliferation, migration, sorting and Paneth cell differentiation30. Effects of increased β-catenin signaling can be studied in vivo by over expressing a “stabilized” mutant allele of the β-catenin gene (Ctnnb1lox(ex3)). Overexpression of mutant β-catenin within intestinal stem cells expands secretory and absorptive compartments with generation of adenomas23,31,32. Inappropriate β-catenin stabilization also results from targeted deletion of one allele of APC which promotes polyp formation26. Deficient APC function reduces phosphorylation of the β-terminus of β-catenin associated with degradation in the cytosol. Barker et al found that APC deficiency in upper crypts led to β-catenin stabilization and enhanced proliferation of progenitor cells localized to the transit-amplifying compartment33. Their data suggests that β-catenin signaling may regulate proliferative activities of intestinal stem cells in lower crypts and progenitor cells in transit-amplifying zones.
The involvement of PI3K signaling in β-catenin signaling has been suggested in several systems where increased PI3K was associated with intestinal dysplasia. Studies have focused on effects of disruption of PTEN. He et al found that Ser552-phosphorylation of β-catenin (P-β-catenin) occurred in the nuclei of cells at sites of crypt fissioning within polyps in a murine model of Cowden’s disease (MxCre/PTENfl/fl mice)34. Furthermore, these results combined with in vitro data from Fang et al35 suggests that Ser552-phosphorylation of the C-terminus of β-catenin by the PI3K-activated serine threonine kinase, Akt, stimulates transcriptional expression of Wnt/β-catenin target genes. In studies by Marsh et al, PTEN deletion was combined with epithelial APC deficiency36. Deficiency of both PTEN and APC led to activation of Akt in crypt enterocytes and enhanced nuclear localization of β-catenin with development of large invasive adenocarcinomas. Evidence that PTEN deficiency increased Akt activation and promoted more aggressive dysplastic transformation raises the possibility that PI3K signaling affects β-catenin activation.
Studies presented here address the possibility that 5-ASA affects β-catenin signaling in crypt epithelial cells. Cells with P-β-catenin were detected within areas of increased proliferation in human and mouse colitis. Human data suggest 5-ASA reduced numbers of P-β-catenin positive cells in severely inflamed colitis (i.e. tissue resistant to anti-inflammatory changes of 5-ASA). 5-ASA treatment of IL-10−/− mice reduced levels of P-Akt and P-β-catenin while impairing the progression from colitis to dysplasia in a dose dependent manner. Given these results, we propose 1) 5-ASA directly inhibits epithelial β-catenin signaling in colitis, and 2) 5-ASA-mediated reduction in epithelial β-catenin plays a role in reducing the risk for colitis-induced dysplasia (CID) and CAC. As increased levels of stabilized β-catenin are closely linked to neoplastic transformation in a range of tissues, these findings may explain why 5-ASA provides chemoprevention in patients with chronic colitis.
Materials and Methods
Human Colonic Specimens
See supplemental methods.
Animals
C57BL/6 IL-10−/− (IL-10−/−) mice were used for this study. Mice were purchased from Jackson Laboratories (Bar Harbor, ME). APCΔ468 were a gift from K. Khazaie37. Mice were maintained under specific pathogen-free conditions in the Northwestern University animal care facility and all experiments were approved by the Animal Care and Usage Committee of Northwestern University.
Real Time PCR analysis
See supplemental methods.
Induction of Colitis and Study Design
IL-10−/− mice (4–6 weeks old) were transferred to conventional housing and allowed one week to acclimate. Mice were subsequently fed chow containing piroxicam (Sigma, St. Louis, MO) to accelerate and synchronize the onset of colitis as previously described38. Piroxicam chow pellets were provided by Harlan Teklad (Madison, WI). After two weeks on regular chow, mice were sacrificed to establish pre-treatment baseline data (day 28). The chemopreventive effects of 5-ASA (Sigma) were evaluated by administration of different dosages [low dose (LD)=500 mg 5-ASA/kg of chow; high dose (HD)=1650 mg 5-ASA/kg of chow] of 5-ASA contained within pellet chow compared to a standard rodent diet from day 28 through day 70. For APCΔ468 mouse studies, 3 month old mice were fed HD 5-ASA for 2 weeks or maintained on regular chow, followed by 1 week of regular chow and sacrificed. Dosages of 5-ASA corresponded to approximately 600 mg and 2 grams/day human equivalent dose39.
Histologic Analysis
Human biopsies and mouse colons were fixed in 10% neutral buffered formalin, routinely processed, sectioned at 5µm, and stained with hematoxylin and eosin (H&E) for microscopic examination. Blinded evaluation of colitis score and dysplasia was performed independently by two investigators. Colitis in human biopsies was scored from 0 to 3 based on increasing severity of mononuclear infiltration, active cryptitis, and epithelial ulceration with IHC analysis performed on chronically inflamed adjacent mucosa. Mice were scored from 0 to 4, as previously described38.
Immunohistochemical Analysis
See supplemental methods.
Epithelial Cell Isolation with Leukocyte Depletion
Intestinal crypt epithelial isolation was done according to Traber et al40. Briefly, the colon was flushed, filleted open longitudinally and washed thoroughly (0.15M NaCl, 1mM DTT, 40 µg/mL PMSF). Colons were incubated for 45 minutes in EDTA buffer [109mM NaCl, 2.4mM KCl, 4.3mM KH2PO4,, 4.3mM Na2HPO4, 2mM EDTA, 10mM glucose, 5mM glutamine, 0.5mM DTT and 40µg/ml PMSF (pH7.4)] with agitation at room temperature. CD45-positive cells were depleted from the resulting supernatant using rat anti-mouse CD45 antibody (BD Pharmingen, San Diego, CA) mixed with sheep anti-rat IgG Dynabeads (Invitrogen, Carlsbad, CA) per manufacturers’ protocol. Flow cytometry confirmed CD45+ cells were <1% of the remaining isolated epithelial cells (data not shown).
Western Blotting
Nuclear and cytosolic extracts were purified as described by Pajari et al41, and the purity of the fractions were checked by enrichment of histone H3 or α1 tubulin (Sigma). See supplemental methods.
Statistical Analysis
All data were analyzed by Student’s t-test. P-values less than 0.05 were considered statistically significant.
Results
5-ASA reduces P-β-catenin levels in human CUC
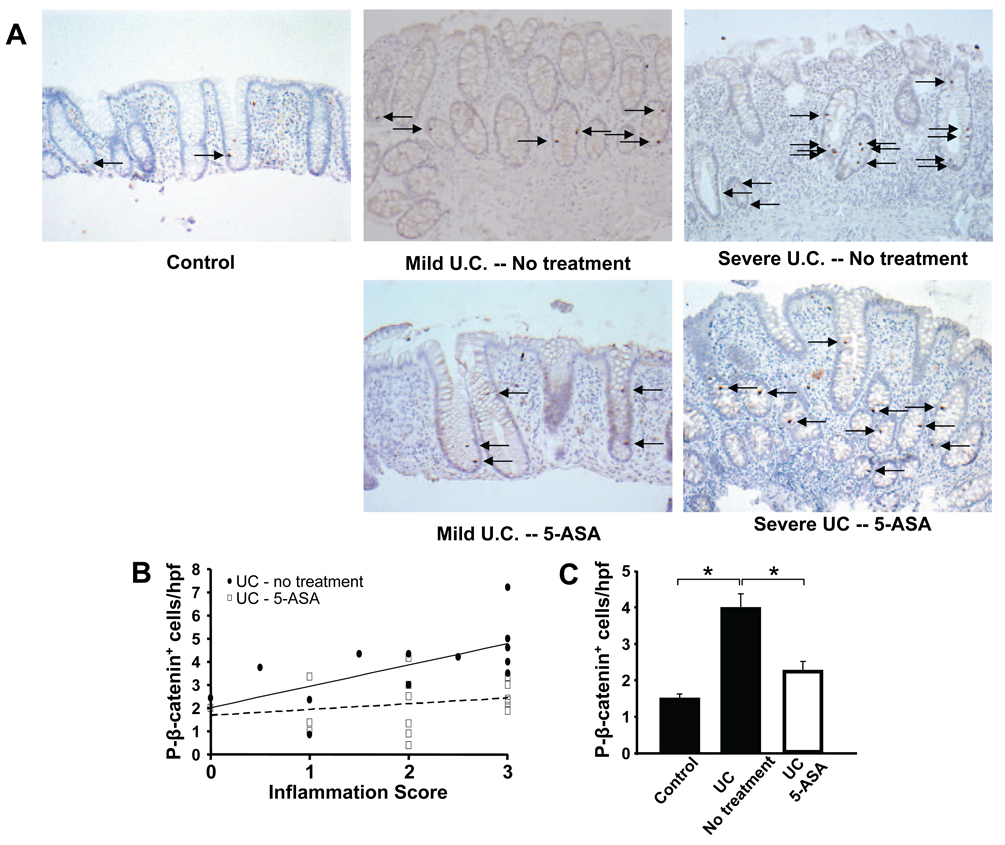
β-catenin-mediated signaling has been identified as a key regulator of epithelial proliferative responses42. We postulated that β-catenin would be increased in repair responses as seen in CUC. To detect intestinal epithelial cells with activated β-catenin signaling, we stained for P-β-catenin in sections from controls as well as mild and severe CUC. As shown in Figure 1, P-β-catenin staining increased in CUC. Specifically, increases in numbers of epithelial cells staining positive for P-β-catenin (P-β-catenin+) paralleled changes in mucosal inflammation (Fig. 1B, solid line). Close examination of tissues revealed that as inflammation increased, the number of P-β-catenin+ epithelial cells expanded from lower crypts (controls) to mid and upper crypt regions in CUC (Fig. 1A).
Figure 1. 5-ASA treatment reduces P-β-catenin signaling in human UC.
Human biopsy samples from control and UC patients obtained during colonoscopy were processed for assessment of histologic inflammation scores and immunohistochemical analysis with P-β-catenin Ab. Data are presented as means ± SEM; *P<0.01 (A) Representative sigmoid colon tissue stained for nuclear P-β-catenin (arrows) from control, chronic inflamed mucosa obtained from adjacent untreated, mild and severely inflamed UC (top panels), and 5-ASAtreated (≥2.4g/d) mild and severely inflamed UC (see methods; original magnification=20×). (B) Enumeration of P-β-catenin+ cells/40× field versus inflammatory scores, (C) mean P-β-catenin+ cells/40× field with or without 5-ASA treatment. (D) Histologic scores demonstrate comparable levels of inflammation between treatment groups segregated into mild or severe inflammation based on tissue histology (see Methods). (E) Mean number of P-β-catenin+ cells/hpf in mild and severely-inflamed UC biopsies ± 5-ASA treatment. (F) Increased β-catenin target gene mRNA expression in untreated colitis is attenuated by 5-ASA, as measured by real-time RT-PCR. Results are shown as fold induction in relation to mRNA expression of Gapdh.
To address effects of 5-ASA on epithelial P-β-catenin levels, tissues from 5-ASA-treated patients were examined. Analysis revealed that P-β-catenin+ levels increased 2.7-fold in CUC tissues compared to controls. By comparison, P-β-catenin levels increased 0.6-fold in 5-ASA-treated CUC compared to controls representing a 72% reduction of CUC-induced P-β-catenin signaling (p<0.05, Fig. 1C). These results suggested 5-ASA inhibited β-catenin signaling in epithelial cells in CUC. Next, we examined whether 5-ASA affects β-catenin activation in tissues when inflammation was unabated. To address the relative contribution of 5-ASA’s anti-inflammatory effect on the change in P-β-catenin levels, tissues from 5-ASA-treated patients were segregated into mild and severe inflammation based on histology criteria (Fig. 1D, methods). To be clear, these selected 5-ASA treated patients represent a distinct group of patients that did not clinically respond to 5-ASA and continued to have active inflammation on biopsy. When these patients were compared to untreated patients with comparable levels of severe inflammation, 5-ASA still reduced numbers of P-β-catenin+ cells by 47% (p<0.05), whereas in mild colitis 5-ASA therapy reduced numbers of P-β-catenin+ cells by 37% (p=0.09) (Fig. 1E). Whereas P-β-catenin staining increased proportionately to inflammation in untreated CUC, P-β-catenin staining in 5-ASA-treated patients remained relatively constant regardless of inflammation (Fig. 1B, dashed line). The effects of 5-ASA on epithelial P-β-catenin levels in severely inflamed CUC tissue are consistent with its ability to impart selective changes in epithelial signaling.
To assess the functional consequences of 5-ASA on epithelial β-catenin signaling, we measured mRNA of three β-catenin target genes (GPR49, MMP-7, and c-Myc) by Real Time PCR. GPR49 and MMP-7 expression increased with colitis severity whereas c-Myc levels were elevated in both mild and severe CUC. Interestingly, 5-ASA treatment significantly reduced transcription of all three target genes in severe CUC samples but only c-Myc in mild disease (Fig. 1F). Cox-2 has been linked to colon carcinogenesis in several systems43. To examine the effect of 5-ASA on this inflammatory mediator, epithelial and stromal mRNA levels were assessed using LCM. Data in Supplemental figure 1 show that 5-ASA did not affect Cox-2 expression in mildly inflamed patients, but did reduce expression in refractory patients. Taken together, these data suggest that 5-ASA inhibits β-catenin signaling and Cox-2 expression in patients refractory to its anti-inflammatory effects.
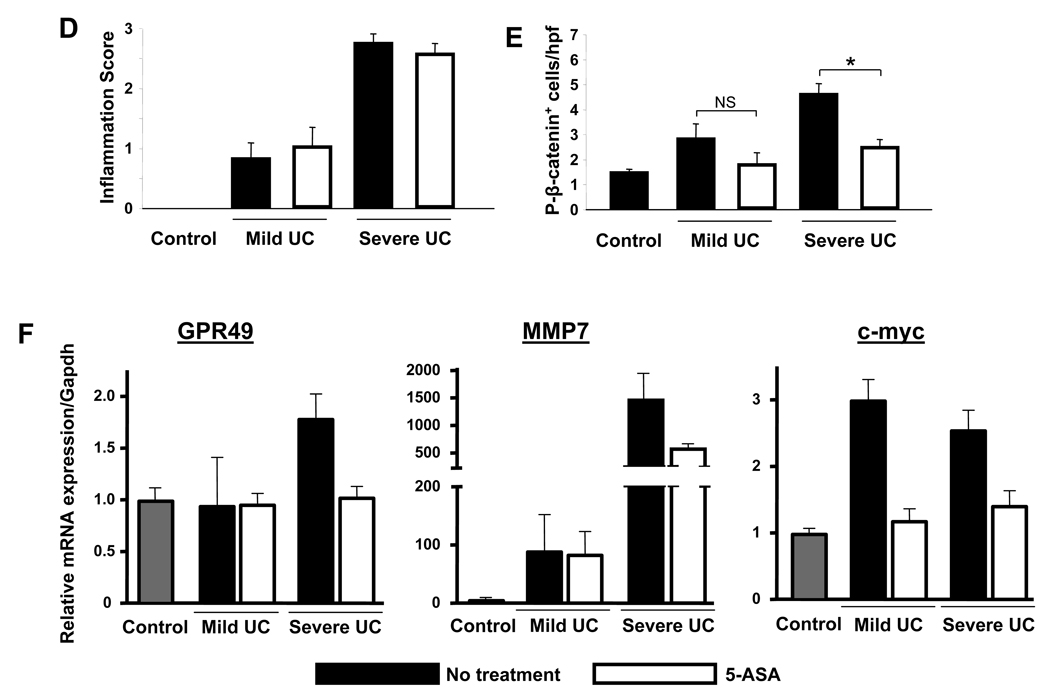
Epithelial P-Akt, P-β-catenin and BrdU incorporation are increased in colitis and dysplasia
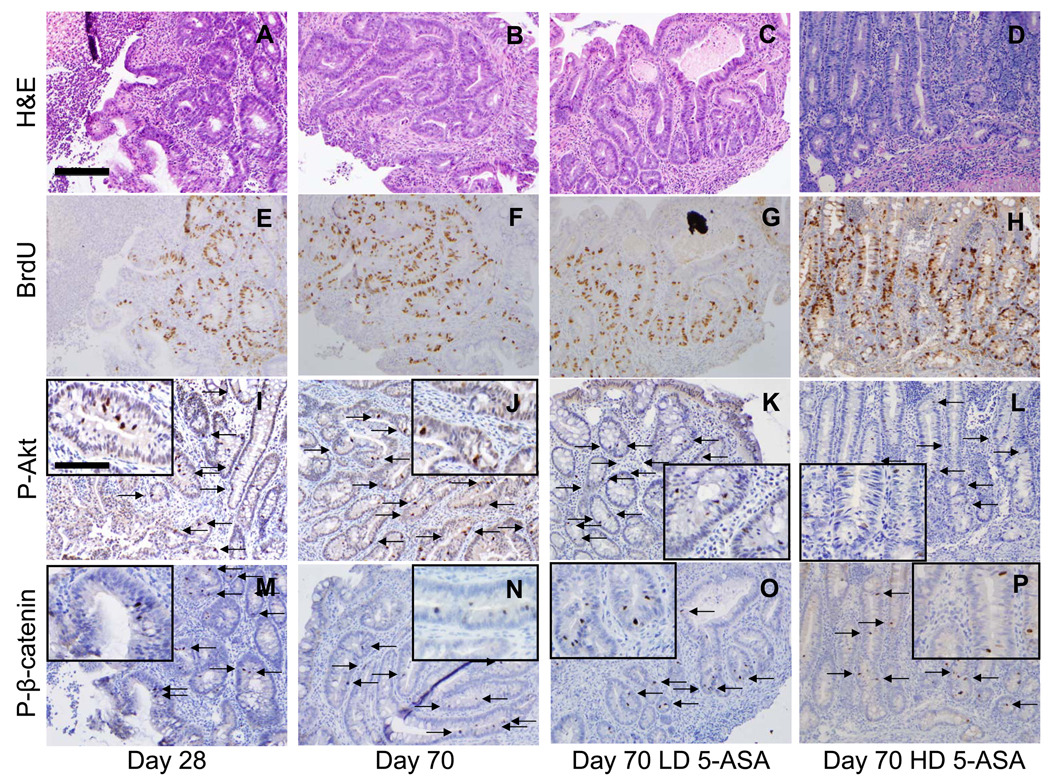
The above data suggest that 5-ASA therapy affects colitis-induced β-catenin signaling in intestinal epithelial cells. To investigate the relevance of activated β-catenin in colitis and CID, we utilized the piroxicam-induced IL-10−/− mouse model44. Analysis of tissue taken from mice from initiation to day 70 (d70) revealed inflammatory scores peaked prior to the appearance of dysplasia (Fig. 2D). Examination of colon tissue from d70 mice revealed areas of inflammation (Fig. 2B) and dysplasia (Fig. 2C) within the right colon. To assess markers of proliferation, Akt and β-catenin activation within colitis and dysplasia, tissues were stained for BrdU incorporation (Fig. 2E–G), P-Akt (Fig. 2H–J) and P-β-catenin (Fig. 2K–M). Results show that BrdU incorporation, P-Akt, and P-β-catenin-stained cells were restricted to crypt bases in control mice, whereas detection of these markers extended up to the mid and upper crypts in areas of colitis (d70). Of greater significance were observations that within dysplastic segments, BrdU+, P-Akt+ and P-β-catenin+ cells were detected within plateau areas (see insets). Counting revealed a dramatic induction of activated Akt (Fig. 2N) and P-β-catenin (Fig. 2O) in both colitis and dysplasia compared to controls (p<0.01). Taken together, assessments of BrdU, P-Akt and P-β-catenin staining in colitic mice indicated that chronic inflammation induced expansion of crypt proliferative zones populated by cells with active PI3K and β-catenin signaling. The detection of proliferating cells with P-Akt and P-β-catenin signaling within surface areas in dysplasia is consistent with the notion that ectopic P-Akt and P-β-catenin are biomarkers of colitis-induced neoplastic transformation.
Figure 2. BrdU incorporation, P-Akt and P-β-catenin levels increase as IL-10−/− colitis progresses to dysplasia.
(A–C;E–M) Sections from baseline (d0) mice and areas of colitis or dysplasia from piroxicam-treated IL-10−/− mice (d70) are shown stained for (A–C) H&E, (E–G) BrdU incorporation, (H–J) P-Akt, and (K–M) P-β-catenin. (D) Evolution of histologic inflammation (black boxes) and prevalence of dysplasia (white boxes) in IL10−/− colitis. (N) P-Akt+ and (O) P-β-catenin+ cells/100 epithelial cells for groups indicated. Representative results from 3 independent experiments are shown. Arrows indicate positive crypt based staining; arrow heads middle/upper crypt staining. 20× with 40× insets; scale bar=200µm. All values represent the mean ± SEM. *P<0.05 vs. d0.
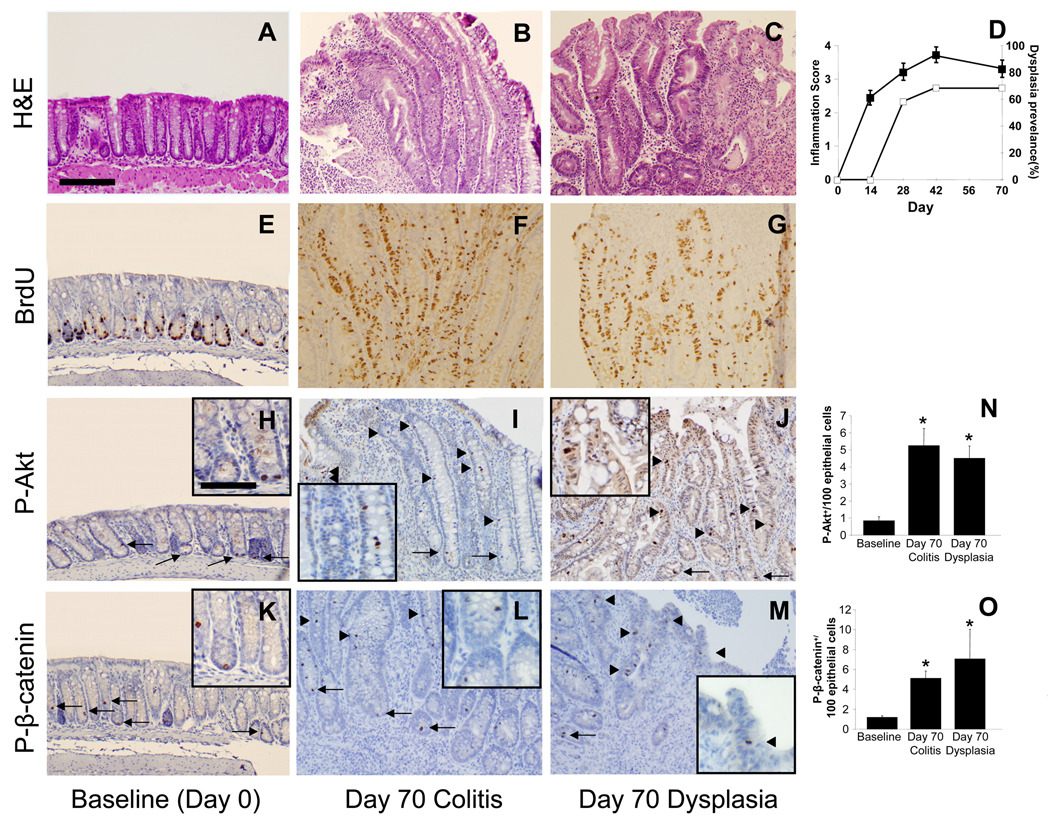
5-ASA reduces Akt-mediated β-catenin signaling in IL-10−/− CAC
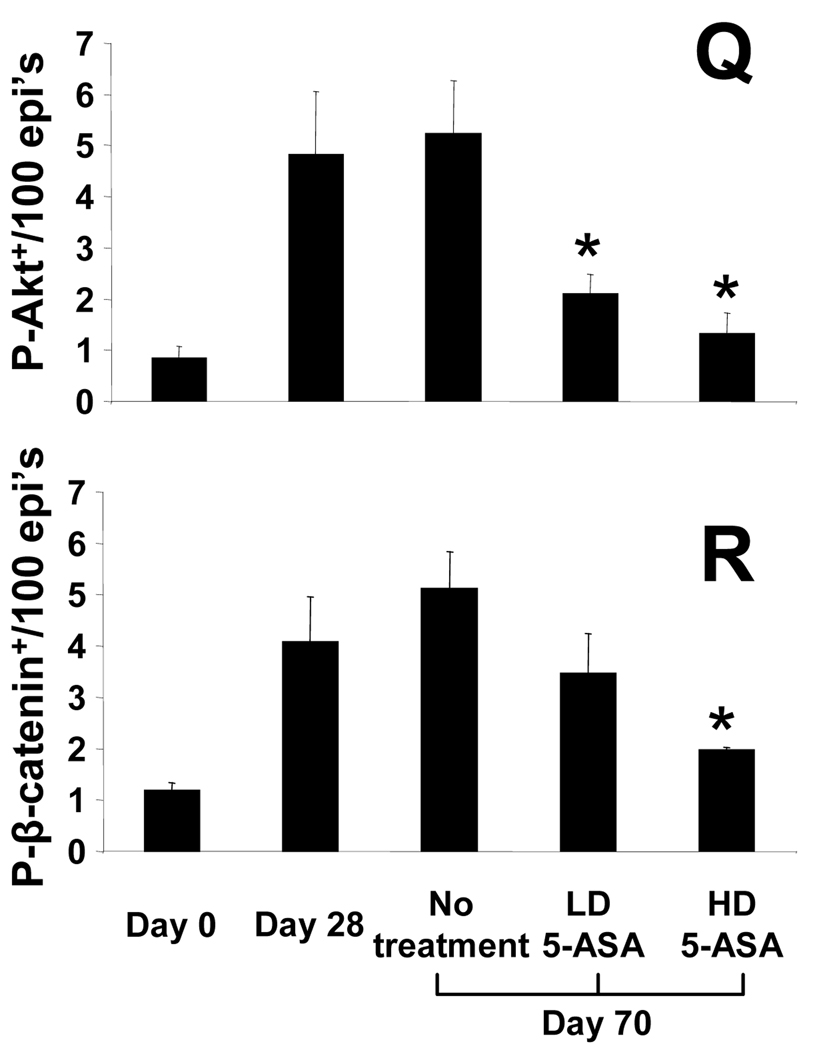
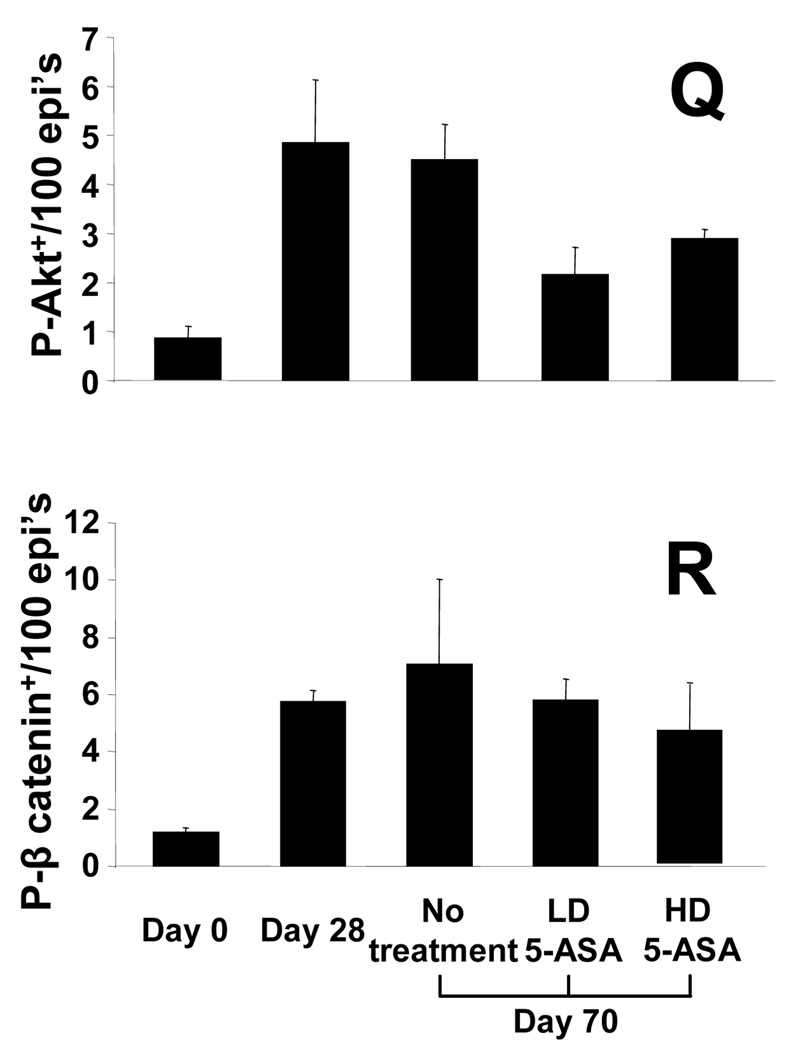
We next examined the effects of 5-ASA on proliferation and signaling for Akt and β-catenin in chronic colitis. (No effects of 5-ASA in uniflamed mice were detected, data not shown). Data in Fig. 3 show that low dose (LD) and high dose (HD) 5-ASA reduced BrdU incorporation in mid and upper crypt regions in a dose-dependent manner. By comparison, HD 5-ASA reduced BrdU labeling more in upper crypt regions than observed in LD 5-ASA-treated mice (Fig. 3G, H). To examine whether 5-ASA treatment affected Akt and β-catenin signaling in a similar dose-dependent manner, sections were stained for P-Akt and P-β-catenin. Examination of sections indicated that 5-ASA reduced levels of both markers in the upper crypts. Cell counting data indicated that LD 5-ASA reduced numbers of P-Akt+ cells by 60% (p<0.05) and P-β-catenin+ cells by 35%. Effects of HD 5-ASA treatment were more pronounced as numbers of P-Akt+ cells decreased by 75% and P-β-catenin+ cells by 61% (p<0.02 for both). Thus, 5-ASA reduces Akt and β-catenin signaling in epithelial cells within mid to upper crypts of colitis tissue.
Figure 3. 5-ASA attenuates epithelial proliferation and PI3K signaling in chronic IL-10−/− colitis.
Sections from d28 and d70 colitic mice compared to LD and HD 5-ASA-treated IL-10−/− mice showing staining for (A–D) H&E, (E–H) BrdU, (I–L) P-Akt, and (M–P) P-β-catenin. Arrows indicate positive crypt based staining; arrow heads middle/upper crypt staining. LD and HD 5-ASA were given from d28 to d70 to IL-10−/− mice as 500mg and 1650mg 5-ASA/kg chow (equivalent to 600 mg and 2 grams/day human dose respectively 39). Stains showed LD and HD 5-ASA reduced BrdU+, P-Akt+ and P-β-catenin+ cells in upper crypt regions of d70 IL-10−/− mice. Scale bar=200µm. (Q,R) Cell counting show mean ± SEM for P-Akt and P-β-catenin+/100 epithelial cells for groups indicated. *P<0.05, versus d70 no treatment.
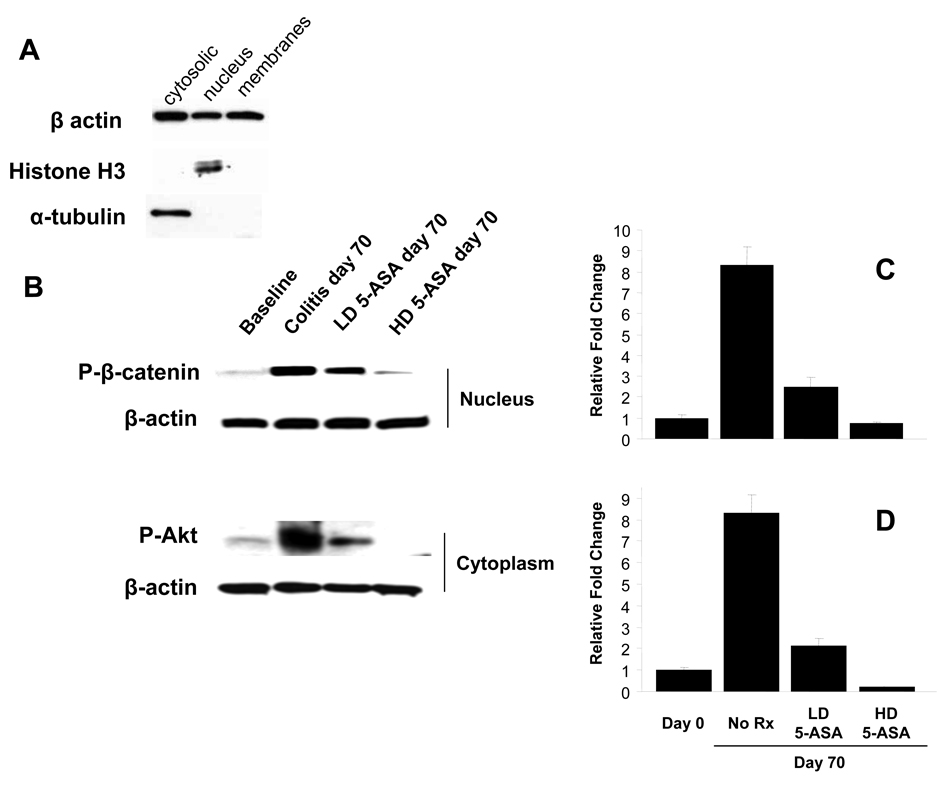
To examine effects of colitis and 5-ASA treatment on subcellular levels of P-Akt and P-β-catenin, protein extracts from leukocyte-depleted epithelial cell preparations were examined by immunoblotting. Subcellular fraction purities were confirmed (Fig. 4A) and cytoplasmic P-Akt and nuclear P-β-catenin levels quantified. Analysis of nuclear fractions revealed that colitis increased P-β-catenin levels 8-fold whereas cytoplasmic P-Akt levels increased 8.3-fold (p<0.01). Data show that LD 5-ASA reduced P-β-catenin and P-Akt levels by >70%, whereas HD 5-ASA reduced these phosphoproteins below baseline levels (Fig. 4C, D). Taken together, data support the notion that 5-ASA reduces activation of Akt and subsequent β-catenin signaling in a dose dependent fashion.
Figure 4. 5-ASA impairs epithelial β-catenin and Akt activation in colitis.
Epithelial cells were isolated and purified from IL-10−/− mice at baseline (d0) and at d70 as described in the methods. (A) Confirmation of purity of subcellular fractions (nuclear=histone H3 positive/α-tubulin negative; cytosolic=histone H3 negative/α-tubulin positive) by Western blotting. (B) Assessment of nuclear P-β-catenin and cytosolic P-Akt in colonic epithelial cells from baseline and colitic d70 IL-10−/− colitis mice with or without LD and HD 5-ASA treatment, as in Fig. 3. β-actin was used to control for protein loading. (C and D) Densitometric comparisons of data in (B) normalized to β-actin relative to d0. Values represent mean ± SEM. *P<0.01.
Effects of 5-ASA on colitis-induced dysplasia
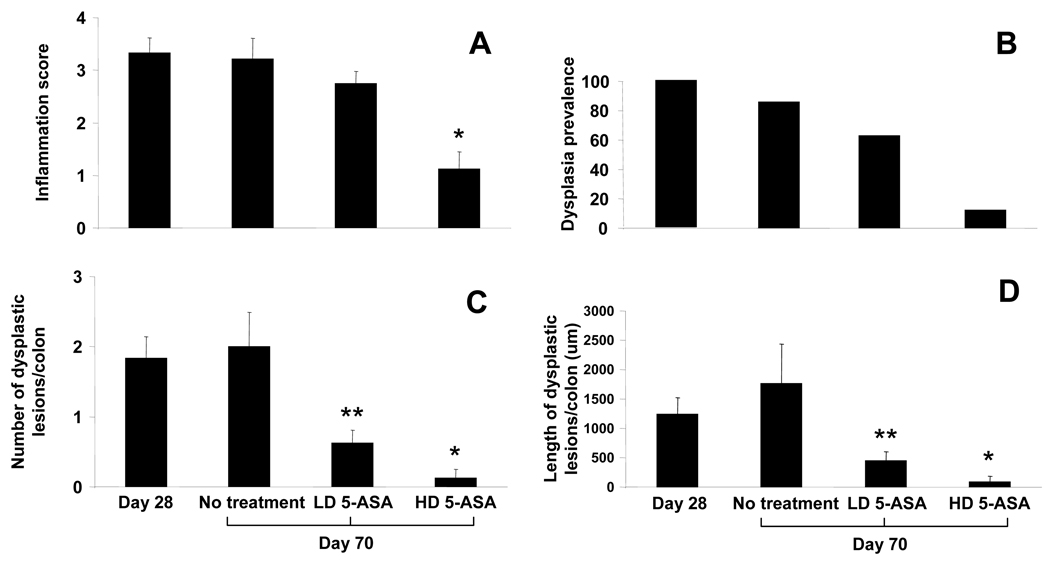
Next, we examined the possibility that 5-ASA inhibits progression of colitis to dysplasia in a dose dependent manner. Dysplasia was quantified in IL-10−/− mice by measuring the overall prevalence as well as the number and length of dysplastic lesions per colon and tissue inflammation quantified using a four point scoring system (methods). Data show that LD 5-ASA does not significantly alter levels of histologic inflammation (Fig. 5A). However, LD 5-ASA reduced the prevalence of dysplasia by 27% and significantly reduced both the number and length of dysplastic lesions/colon by 69% and 75% respectively (p<0.05). By comparison, HD 5-ASA had greater impact on tissue inflammation (Fig. 5A). HD 5-ASA reduced dysplasia prevalence by 85%, numbers of lesions by 94% and mean length of lesions by 95% (Figure 5B–D). These data indicate that in IL-10−/− colitis, 5-ASA reduced dysplasia. Notably, LD 5-ASA therapy improved dysplasia without significantly affecting mucosal inflammation while HD 5-ASA reduced dysplasia in conjunction with reducing tissue inflammation. These data are consistent with the notion that 5-ASA may be effective at reducing dysplasia risk even when it does not appreciably affect tissue inflammation.
Figure 5. 5-ASA attenuates progression of dysplasia in IL-10−/− colitis.
Colons were removed from piroxicam-treated IL-10−/− mice on d28 (pre-treatment baseline) and d70, then processed for H&E staining. Mice received standard, LD 5-ASA, or HD 5-ASA chow from d28 through d70. (A) Histologic inflammation scores and (B) prevalence (percent mice with any detectable dysplasia) of dysplasia were determined. (C) Dysplastic burden was measured by enumerating the average number of dysplastic lesions per colon and average length of dysplastic lesions per colon (D) for groups indicated. Values represent mean ± SEM. *P<0.01, **P<0.05, versus no treatment.
Effect of 5-ASA on epithelial proliferation, P-Akt and P-β-catenin within dysplasia
Prior data showed 5-ASA treatment reduces markers of epithelial proliferation and β-catenin signaling within colitis (Fig. 5). To examine effects of 5-ASA on β-catenin signaling in dysplasia, patches of dysplastic mucosa in untreated, LD and HD-5ASA-treated mice were stained for BrdU, P-Akt, and P-β-catenin (Fig. 6). Examination of sections of reactive epithelial changes progressing to dysplasia from d28 to d70 untreated mice compared to 5-ASAtreated d70 mice showed that areas of intense crypt cell proliferation persisted regardless of 5-ASA treatment (Fig. 6E–H). Similarly, 5-ASA failed to reduce P-Akt+ (Fig. 6K, L) or P-β-catenin+ (Fig. 6O, P) cells in upper crypt regions and plateau areas. Cell counting confirmed these findings as 5-ASA failed to significantly reduce P-β-catenin-stained cells in dysplasia.
Figure 6. 5-ASA reduces epithelial proliferation and PI3K signaling in colitis associated dysplasia.
From left to right: early reactive epithelial changes adjacent to ulceration (d28) compared to dysplasia at d70 in 5-ASA-treated IL-10−/− mice. Panels show staining for (A–D) H&E, (E–H) BrdU incorporation, (I–L) P-Akt (arrows) and (M–P) P-β-catenin (arrows) within areas of dysplasia demonstrate little difference at d28 versus d70 with or without 5-ASA. (Q and R) Mean ± SEM of positively-stained cells/100 epithelial cells. 20× with 40× insets; scale bar=200µm. *P<0.05, versus no treatment, d70. Data suggest that regions of dysplasia that persist after 5-ASA treatment maintained active Akt and β-catenin signaling.
Overall, data in Fig. 5 and 6 are consistent with the notion that although 5-ASA may prevent the appearance of dysplasia by reducing Akt and β-catenin signaling, it has little if any effect on β-catenin signaling within areas of persistent dysplasia. To test whether 5-ASA affects β-catenin and the appearance of polyps in an alternative mouse model of dysplasia (mutated APC), we fed HD 5-ASA to APCΔ468 mice (Supp. fig. 2). Results show that 5-ASA reduced total numbers of polyps by 40% (95.2 ±7.6 to 57.3 ±8.6, p<0.01). We found that 5-ASA reduced the total number of P-β-catenin+ cells in intestinal polyps by 52%. Close analysis revealed that effects were largely due to reduction in the number and size of polyps as the local density of P-β-catenin+ were unaffected. Thus, data in Fig. 6 and Supp. fig. 2 indicate 5-ASA reduces the generation of dysplasia but had little effect on P-β-catenin signaling in residual dysplasia.
Discussion
Chronic inflammation serves as a stimulus for epithelial responses in colitis. In untreated CUC patients, inflammation induces epithelial proliferation45 and causes architectural distortion defined as crypt branching, loss of parallel crypt structures and variable crypt size46. In CUC patients, 5-ASA treatment reduced epithelial proliferation and reversed epithelial architectural changes, but also reduced tissue inflammation47. Thus, it remains unclear which elements of the epithelial response result from direct effects on epithelial signaling or anti-inflammatory effects on mucosal mononuclear cells. Murine studies have replicated the anti-neoplastic effect of 5-ASA in the AOM/DSS model of CAC, however no specific mechanism was described48–50. There was no significant reduction in inflammatory scores or COX-2 expression within colonic dysplasias despite reducing the tumor burden48. Furthermore, 5-ASA-enhanced PPARγ expression had anti-inflammatory and chemopreventive effects during active colitis; however, the PPARγ effect was abrogated during quiescent disease49. These data suggest that in chronic inflammation, 5-ASA exerts an inflammation-independent chemopreventive effect.
In studies presented here, epithelial P-β-catenin levels were reduced in severely inflamed tissue (Fig. 1). The effects of 5-ASA on this marker of active β-catenin signaling suggests that 5-ASA has direct effects on epithelial signaling even when examined in patients with histologically active (5-ASA refractory) disease. The findings are supported by studies in IL-10−/− mice where 5-ASA reduced epithelial PI3K/Akt and Wnt/β-catenin signaling associated with decreased epithelial proliferation. These data support the notion that 5-ASA inhibits β-catenin activation in epithelial cells independent of anti-inflammatory effects on the submucosa. These findings suggest a mechanism for 5-ASAmediated reduction in epithelial β-catenin signaling and progenitor cell activation.
The current data are consistent with the notion that 5-ASA inhibition of epithelial proliferation and progenitor cell activation result from reduced PI3K/Akt-mediated β-catenin activation. The model builds upon prior reports of 5-ASA-induced inhibition of epithelial PI3K14–16 and β-catenin signaling12 and provides a mechanism for how these pathways may interact. To illustrate sites of PI3K and β-catenin signaling, studies utilized a newly generated anti-P-β-catenin Ab generated to detect Akt-phosphorylation of the Ser552 residue in the C-terminus of β-catenin. Western blot and IHC studies detected increased nuclear P-β-catenin in areas of inflammation. Nuclear β-catenin localization is universally recognized to be associated with Wnt/β-catenin signaling28. Previous studies in small bowel polyps of MxCre/PTENfl/fl mice detected P-β-catenin staining in hyperproliferative segments of polyps, localizing to points of crypt branching34. Studies here apply these observations to 5-ASA treatment in colitis. Current data show increased P-β-catenin within crypt epithelia in colitis and demonstrate 5-ASA treatment reduces P-Akt and P-β-catenin levels (IHC and Western blot techniques) (Fig. 3, 4). Thus, current studies are consistent with the notion that 5-ASA attenuates epithelial β-catenin signaling to inhibit progenitor cell activation and crypt architectural distortion.
Although several studies suggest that 5-ASA reduces cancer risk in CUC4,5,51, not all data agrees with this association3. The current study makes several observations relevant to this debate. First, it shows that 5-ASA reduces β-catenin signaling in patients with mild and severe levels of tissue inflammation (Fig. 1) suggesting it has selective effects on epithelial β-catenin signaling. The relevance of β-catenin to CAC was supported in mouse studies shown here as well as in human studies (unpublished, Lee) where P-β-catenin levels progressively increased in CID and CAC as well as in sporadic CRC. Thus, the reduction of a relevant biomarker of neoplastic transformation by 5-ASA supports its role in chemoprevention even in refractory inflammation. Second, the reduction in nuclear P-β-catenin, P-Akt and epithelial proliferation suggest 5-ASA decreases generation of activated epithelial progenitor cells. As progenitors may acquire properties of cancer stem cells, such as self-renewal potential, a reduction in the numbers of progenitor cells may assist in chemoprevention52,53.
As we found that 5-ASA inhibited β-catenin signaling in epithelia, we considered the possibility that it may impede progression of polyp formation in mice with an APC mutation. Effects of 5-ASA on polyp formation in mice with mutant APC have been previously addressed with mixed results54–56. Recent studies by Phelps et al suggest that a second mutation (e.g. KRAS) is required for progression of dysplasia in cells with deficient APC57. These studies suggest that other genetic defects are required for APC mutant cells to progress to dysplasia. We found that 5-ASA reduced polyp formation and decreased the total number of P-β-catenin+ cells within polyps in APCΔ468 mice (Supp. fig. 2). We suspect that 5-ASA may impair β-catenin signaling in populations of activated stem cells. If a second mutation is required to occur in populations of activated (APC mutant) epithelial cells, then reducing numbers of P-β-catenin+ cells may reduce the overall risk for dysplastic transformation. Data from other studies complement data here as they suggest reductions in β-catenin signaling may be due to indirect and direct effects of 5-ASA. Results from Rousseaux et al suggest 5-ASA increases PPARγ activity16 which may increase PTEN function17 and reduce PI3K signaling. Alternatively, recent studies have found that PPARγ directly reduces β-catenin signaling by cytosolic retention58 and proteasomal targeting59. Taken together, 5-ASA may inhibit PI3K-mediated β-catenin at several steps.
The insights presented apply to a number of clinical questions: (1)In patients with untreated, asymptomatic CUC, does the detection of mucosal inflammation support reinstating 5-ASA therapy?; (2)In CUC patients maintained in remission on immunomodulator and/or biologic therapy, does 5-ASA need to be continued? The current data provides a mechanistic explanation for use of 5-ASA as chemoprevention in UC. With regards to the first scenario, we would argue to reinstate 5-ASA. Based on data in Fig. 1, we predict that CUC patients with elevated mucosal inflammation will have increased numbers of cells with nuclear P-β-catenin. Furthermore, 5-ASA would be expected to lower the number of P-β-catenin+ cells even if it does not lower mucosal inflammation. Thus, 5-ASA would be expected to reduce the number of activated progenitor cells that carry an increased risk for dysplastic transformation. In the second scenario, we would propose to examine the mucosal levels of inflammation and number of P-β-catenin-stained cells. If both levels were held low by maintaining immunomodulator therapies, we would defer to results of clinical studies that support the chemopreventive benefits of 5-ASA. A third scenario to consider is whether there is a benefit to HD vs. LD 5-ASA treatment of quiescent UC? Based on mouse data, HD 5-ASA was more efficacious at preventing dysplasia. However, this also correlated with reductions in inflammatory scores. Therefore, our data does not provide evidence for using HD 5-ASA in a patient with quiescent disease who does not require an anti-inflammatory benefit. In these patients, relatively LD 5-ASA (1.2–2.4gm/d) appears adequate for chemoprevention. It remains possible that HD 5-ASA may benefit “high-risk” patients with additive risks factors (e.g. family history of spontaneous CRC or concomitant primary sclerosing cholangitis). Thus, the data presented help guide decisions regarding the use of 5-ASA in CUC patients with elevated risks for developing CAC.
Supplementary Material
Human biopsy samples from control and UC patients obtained during colonoscopy were immediately placed into RNeasy (whole tissue analysis) or fixed and processed for isolation of epithelial and stromal fractions by laser capture microdissection (LCM). Data are presented as mean ± SEM. (A) Whole tissue analysis demonstrates increased Cox-2 mRNA expression is unaffected in mild colitis, but reduced in severe 5-ASA treated UC as measured by real-time RT-PCR. (B) LCM isolated fractions showed proportionate reductions in stromal and epithelial Cox-2 gene expression. Results are shown as fold induction in relation to mRNA expression of Gapdh.
Intestinal sections from 4 month old control (untreated) APCΔ468 mice and 5-ASA treated APCΔ468 mice isolated after 2 weeks of HD 5-ASA therapy are shown stained for (A) P-β-catenin. Left panels represent normal intestinal tissue with similar P-β-catenin staining isolated to the crypts. Right panels represent areas of polyposis. (B) From left to right: prevalence of intestinal polyps, size of polyps, total P-β-catenin+ epithelial cells in all polyps/intestine, and density of P-β-catenin+ epithelial cells within individual polyps. The latter figure was determined by dividing the total number of polyp-associated P-β-catenin+ in each intestine by the size of the polyps to generate the density of P-β-catenin+ IEC’s/unit area. 20×; scale bar=200 µm. All values represent the mean ± SEM. *P<0.01 vs. untreated.
Acknowledgments
Grant Support:
NIH grants DK54778, DKAI061701 (to T.A.B.) and DK066161 (to J.B.B.)
Nonstandard abbreviations used in this paper
- ASA
amino salicylic acid
- UC
ulcerative colitis
- CAC
colitis-associated cancer
- PI3K
phosphoinositide-3 kinase
- P-β-catenin
Akt-phosphorylated β-catenin
- CRC
colorectal cancer
- PPAR-γ
peroxisome proliferator–activated receptor-gamma
- PTEN
phosphatase and tensin homologue
- APC
adenomatous polyposis coli gene
- TCF4
T-cell factor-4
- LD
low dose
- HD
high dose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
Jeffrey B. Brown: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding.
Goo Lee: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript.
Elizabeth Managlia: acquisition of data; analysis and interpretation of data
Gery R. Grimm: acquisition of data; analysis and interpretation of data.
Ramanarao Dirisina: acquisition of data.
Tatiana Goretsky: acquisition of data.
Paul Cheresh: acquisition of data; analysis and interpretation of data, statistical analysis.
Nichole R. Blatner: acquisition of data.
Khashayarsha Khazaie: critical revision of the manuscript for important intellectual content; material support.
Guang-Yu Yang: analysis and interpretation of data;
Linheng Li: analysis and interpretation of data; drafting of the manuscript: material support.
Terrence A. Barrett: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; obtained funding; study supervision
Financial Disclosure:
T.A.B. received grant support from Proctor and Gamble for this project. There are no other conflicts of interest to disclose for all authors.
References
- 1.Karlen P, Lofberg R, Brostrom O, et al. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 1999;94:1047–1052. doi: 10.1111/j.1572-0241.1999.01012.x. [DOI] [PubMed] [Google Scholar]
- 2.Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol. 2005;100:1345–1353. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 5.Rubin DT, LoSavio A, Yadron N, et al. Aminosalicylate therapy in the prevention of dysplasia and colorectal cancer in ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:1346–1350. doi: 10.1016/j.cgh.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:160–170. doi: 10.1038/ncpgasthep0696. [DOI] [PubMed] [Google Scholar]
- 7.Orchard T, Probert CS, Keshav S. Review article: maintenance therapy in patients with ulcerative colitis. Aliment Pharmacol Ther. 2006;24 Suppl 1:17–22. doi: 10.1111/j.1365-2036.2006.03071.x. [DOI] [PubMed] [Google Scholar]
- 8.Reinacher-Schick A, Seidensticker F, Petrasch S, et al. Mesalazine changes apoptosis and proliferation in normal mucosa of patients with sporadic polyps of the large bowel. Endoscopy. 2000;32:245–254. doi: 10.1055/s-2000-135. [DOI] [PubMed] [Google Scholar]
- 9.Ahnfelt-Ronne I, Nielsen OH, Christensen A, et al. Clinical evidence supporting the radical scavenger mechanism of 5-aminosalicylic acid. Gastroenterology. 1990;98:1162–1169. doi: 10.1016/0016-5085(90)90329-y. [DOI] [PubMed] [Google Scholar]
- 10.Egan LJ, Mays DC, Huntoon CJ, et al. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem. 1999;274:26448–26453. doi: 10.1074/jbc.274.37.26448. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser GC, Yan F, Polk DB. Mesalamine blocks tumor necrosis factor growth inhibition and nuclear factor kappaB activation in mouse colonocytes. Gastroenterology. 1999;116:602–609. doi: 10.1016/s0016-5085(99)70182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenson WF, Lobos E. Sulfasalazine inhibits the synthesis of chemotactic lipids by neutrophils. J Clin Invest. 1982;69:494–497. doi: 10.1172/JCI110474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharon P, Ligumsky M, Rachmilewitz D, et al. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978;75:638–640. [PubMed] [Google Scholar]
- 14.Monteleone G, Franchi L, Fina D, et al. Silencing of SH-PTP2 defines a crucial role in the inactivation of epidermal growth factor receptor by 5-aminosalicylic acid in colon cancer cells. Cell Death Differ. 2006;13:202–211. doi: 10.1038/sj.cdd.4401733. [DOI] [PubMed] [Google Scholar]
- 15.Kinzler KW, Nilbert MC, Su LK, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 16.Rousseaux C, Lefebvre B, Dubuquoy L, et al. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrow B, Evers BM. Activation of PPARgamma increases PTEN expression in pancreatic cancer cells. Biochem Biophys Res Commun. 2003;301:50–53. doi: 10.1016/s0006-291x(02)02983-2. [DOI] [PubMed] [Google Scholar]
- 18.Leslie NR, Downes CP. PTEN: The down side of PI 3-kinase signalling. Cell Signal. 2002;14:285–295. doi: 10.1016/s0898-6568(01)00234-0. [DOI] [PubMed] [Google Scholar]
- 19.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 20.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 21.Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 22.Shitashige M, Hirohashi S, Yamada T. Wnt signaling inside the nucleus. Cancer Sci. 2008;99:631–637. doi: 10.1111/j.1349-7006.2007.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harada N, Tamai Y, Ishikawa T, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wielenga VJ, Smits R, Korinek V, et al. Expression of CD44 in Apc and Tcf mutant mice implies regulation by the WNT pathway. Am J Pathol. 1999;154:515–523. doi: 10.1016/S0002-9440(10)65297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romagnolo B, Berrebi D, Saadi-Keddoucci S, et al. Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated beta-catenin. Cancer Res. 1999;59:3875–3879. [PubMed] [Google Scholar]
- 26.Sansom OJ, Reed KR, Hayes AJ, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreu P, Colnot S, Godard C, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. doi: 10.1242/dev.01700. [DOI] [PubMed] [Google Scholar]
- 28.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 29.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 30.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, Gibson P, Currle DS, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 34.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang D, Hawke D, Zheng Y, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282:11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh V, Winton DJ, Williams GT, et al. Epithelial Pten is dispensable for intestinal homeostasis but suppresses adenoma development and progression after Apc mutation. Nat Genet. 2008;40:1436–1444. doi: 10.1038/ng.256. [DOI] [PubMed] [Google Scholar]
- 37.Gounaris E, Tung CH, Restaino C, et al. Live imaging of cysteine-cathepsin activity reveals dynamics of focal inflammation, angiogenesis, and polyp growth. PLoS One. 2008;3:e2916. doi: 10.1371/journal.pone.0002916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berg DJ, Zhang J, Weinstock JV, et al. Rapid development of colitis in NSAID-treated IL-10-deficient mice. Gastroenterology. 2002;123:1527–1542. doi: 10.1053/gast.2002.1231527. [DOI] [PubMed] [Google Scholar]
- 39.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 40.Traber PG, Gumucio DL, Wang W. Isolation of intestinal epithelial cells for the study of differential gene expression along the crypt-villus axis. Am J Physiol. 1991;260:G895–G903. doi: 10.1152/ajpgi.1991.260.6.G895. [DOI] [PubMed] [Google Scholar]
- 41.Pajari AM, Rajakangas J, Paivarinta E, et al. Promotion of intestinal tumor formation by inulin is associated with an accumulation of cytosolic beta-catenin in Min mice. Int J Cancer. 2003;106:653–660. doi: 10.1002/ijc.11270. [DOI] [PubMed] [Google Scholar]
- 42.Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- 43.Backlund MG, Mann JR, Dubois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;69 Suppl 1:28–32. doi: 10.1159/000086629. [DOI] [PubMed] [Google Scholar]
- 44.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franklin WA, McDonald GB, Stein HO, et al. Immunohistologic demonstration of abnormal colonic crypt cell kinetics in ulcerative colitis. Hum Pathol. 1985;16:1129–1132. doi: 10.1016/s0046-8177(85)80181-7. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins D, Balsitis M, Gallivan S, et al. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol. 1997;50:93–105. doi: 10.1136/jcp.50.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Odze R, Antonioli D, Peppercorn M, et al. Effect of topical 5-aminosalicylic acid (5-ASA) therapy on rectal mucosal biopsy morphology in chronic ulcerative colitis. Am J Surg Pathol. 1993;17:869–875. doi: 10.1097/00000478-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Clapper ML, Gary MA, Coudry RA, et al. 5-aminosalicylic acid inhibits colitis-associated colorectal dysplasias in the mouse model of azoxymethane/dextran sulfate sodium-induced colitis. Inflamm Bowel Dis. 2008;14:1341–1347. doi: 10.1002/ibd.20489. [DOI] [PubMed] [Google Scholar]
- 49.Ikeda I, Tomimoto A, Wada K, et al. 5-aminosalicylic acid given in the remission stage of colitis suppresses colitis-associated cancer in a mouse colitis model. Clin Cancer Res. 2007;13:6527–6531. doi: 10.1158/1078-0432.CCR-07-1208. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki S, Sakamoto S, Mitamura T, et al. Preventive effects of sulphasalazine on colorectal carcinogenesis in mice with ulcerative colitis. In Vivo. 2000;14:463–466. [PubMed] [Google Scholar]
- 51.van Staa TP, Card T, Logan RF, et al. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–1578. doi: 10.1136/gut.2005.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 53.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 54.Ritland SR, Leighton JA, Hirsch RE, et al. Evaluation of 5- aminosalicylic acid (5-ASA) for cancer chemoprevention: lack of efficacy against nascent adenomatous polyps in the Apc(Min) mouse. Clin Cancer Res. 1999;5:855–863. [PubMed] [Google Scholar]
- 55.MacGregor DJ, Kim YS, Sleisenger MH, et al. Chemoprevention of colon cancer carcinogenesis by balsalazide: inhibition of azoxymethane-induced aberrant crypt formation in the rat colon and intestinal tumor formation in the B6-Min/+ mouse. Int J Oncol. 2000;17:173–179. doi: 10.3892/ijo.17.1.173. [DOI] [PubMed] [Google Scholar]
- 56.Koelink PJ, Robanus-Maandag EC, Devilee P, et al. 5-Aminosalicylic acid inhibits colitis-associated but not sporadic colorectal neoplasia in a novel conditional Apc mouse model. Carcinogenesis. 2009;30:1217–1224. doi: 10.1093/carcin/bgp113. [DOI] [PubMed] [Google Scholar]
- 57.Phelps RA, Chidester S, Dehghanizadeh S, et al. A two-step model for colon adenoma initiation and progression caused by APC loss. Cell. 2009;137:623–634. doi: 10.1016/j.cell.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujisawa T, Nakajima A, Fujisawa N, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) suppresses colonic epithelial cell turnover and colon carcinogenesis through inhibition of the beta-catenin/T cell factor (TCF) pathway. J Pharmacol Sci. 2008;106:627–638. doi: 10.1254/jphs.fp0071766. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, Wang H, Zuo Y, et al. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Human biopsy samples from control and UC patients obtained during colonoscopy were immediately placed into RNeasy (whole tissue analysis) or fixed and processed for isolation of epithelial and stromal fractions by laser capture microdissection (LCM). Data are presented as mean ± SEM. (A) Whole tissue analysis demonstrates increased Cox-2 mRNA expression is unaffected in mild colitis, but reduced in severe 5-ASA treated UC as measured by real-time RT-PCR. (B) LCM isolated fractions showed proportionate reductions in stromal and epithelial Cox-2 gene expression. Results are shown as fold induction in relation to mRNA expression of Gapdh.
Intestinal sections from 4 month old control (untreated) APCΔ468 mice and 5-ASA treated APCΔ468 mice isolated after 2 weeks of HD 5-ASA therapy are shown stained for (A) P-β-catenin. Left panels represent normal intestinal tissue with similar P-β-catenin staining isolated to the crypts. Right panels represent areas of polyposis. (B) From left to right: prevalence of intestinal polyps, size of polyps, total P-β-catenin+ epithelial cells in all polyps/intestine, and density of P-β-catenin+ epithelial cells within individual polyps. The latter figure was determined by dividing the total number of polyp-associated P-β-catenin+ in each intestine by the size of the polyps to generate the density of P-β-catenin+ IEC’s/unit area. 20×; scale bar=200 µm. All values represent the mean ± SEM. *P<0.01 vs. untreated.