Abstract
Objective
To observe the in vitro effect of Xuefu Zhuyu Decoction on endothelial progenitor cell (EPC) tube formation.
Methods
We prepared mononucleus cells from rat marrow by Ficoll density gradient centrifuge, separated EPCs by differential attachment method and observed, with inverted microscope, the effect of serum containing Xuefu Zhuyu Decoction (XFZYD) on endothelial progentor cell (EPC) tube formation.
Results
After 1 day, EPCs exposed to serum containing 5%, 10% and 15% XFZYD formed typical tube or vessel networks. Compared to the control group, the tube formation was 2 days ahead and most of the were smaller.
Conclusion
XFZYD could induce EPCs angiogenesis and hasten tube formation, especially capillary vessel.
Keywords: Xuefu Zhuyu Decoction, EPCs, tube formation
Endothelial progenitor cells (EPCs) are the precursor of angioblasts. In 1977, Asahara et al [1] reported that CD34+ hematopoietic progenitor cells from adult peripheral blood in vitro can differentiate into endothelial cells become incorporated into neovessels at sites of ischemia. Since then evidence has accumulated demonstrating that these cells [Song: which cells are these; write out name either CD34? EPCs?] not only participate in the embryonic but also postnatal vasculogenesis[2-6]. This evidence suggests that xxx [need a noun here; i'm not sure what it is] promotes the function of EPCs in angiogenesis and has challenged traditional beliefs about postnatal vasculogenesis and vascular injury repair. This study investigated the in vitro effect of EPCs on tube formation induced by the traditional Chinese herbal formulation Xuefu Zhuyu Decoction to elucidate it possible mechanism in the clinical treatment of ischemic diseases.
Methods
Preparation XFZYD–containing serum
Xuefu Zhuyu Decoction (血府逐瘀汤, Drive Out Stasis from the Mansion of Blood Decotion XFZYD) was first recorded in 1830 in Wang Qing-ren's “Yilin Gaicuo (≪医林改错》 Corrections of Errors Among Physicians)” by Wang Qing-ren. It is composed of Angelica sinensis (Oliv.) Diels 9g, Rehmannia glutinosa Libosch.9g, Prunus persica 12g, Carthamus tinctorius L.9g, Citrus aurantium L.6g, Paeonia lactiflora Pall.6g, Bupleurum chinese DC.3g, Glycyrrhiza uralensis Fisch.6g, Platycodon grandiflorum 4.5g, Ligusticum Chuanxiong Hort.4.5g, Cyathula officinalis Kuan 9g. One of the formula's main traditional indication is for the treatment of ischemic disease. Herbal material was supplied by Fujian Institute of Traditional Chinese Medicine, Fujian University of Traditional Chinese Medicine and identified by Prof. Qiu SP. Herbs were prepared by water extraction twice in a manner consistent with common usage in China and therefore involved two decoctions. The extract was filtered and and condensed to make a concentration of the preparation equivalent to 1.3g herb/ml, and then kept at 4°C.
Twelve purebred Sprague-Dawley rats (lot NO.0037614, SPF grade), 6 weeks old, were obtained from Slac Laboratory Animal Company (place in china? China?). Half were males and half were females, all in good health and weighted 150±20g. They were raised in the animal center of Fujian University of Traditional Chinese Medicine and all animal procedures were performed according to ethical principles for the care and use of laboratory animals. After 3d of suitability feed, these rats were randomly distributed into two groups. In the first group, each of the six rats was orally administrated XFZYD solution for 13ml/kg twice daily (the dosage of the medicine was equal to 10 times of the human dosage) for a total of seven days. Blood of the rats was obtained from arteria cruralis 2h within the last administration, centrifuged at 4000rpm for 30min and the serum was collected, termed as XFZYD–containing serum (XFZYD-CS). In the second group, rats were orally administrated normal saline in the same protocol; their serum was used as control serum. Both the XFZYD-CS and control serum were inactivated by heating at 56°C for 30min, filtered through a 0.22μm filter, and stored in at -20°C until use.
Isolation and culture of EPCs
EPCs were isolated and cultured according to previously described techniques [7]. Briefly, total mononuclear cells (MNCs) were isolated from rat bone marrow by density gradient centrifugation using Ficoll separation (Sigma, USA). The mononuclear cells were cultured at 1×106 cells/ml in the endothelial differentiation medium consisting of DMEM (Hyclone, USA) supplemented with 15% fetal bovine serum (PAA, Austria), 10ng/ml recombinant human VEGF (PeproTech,USA), 4ng/ml basic fibroblast growth factor (PeproTech, USA), 4μg/ml BPE (Gibco, USA) on gelatin–coated dishes. After 4 days in culture, non-adherent cells were washed away with PBS, new media was applied and the culture was maintained for a total 9 days, changing media every 3 days.
Tube formation assay
Attached cells were stimulated with XFZYD-CS (to make a series of final serum concentrations: 5, 10, 15% with DMEM) in the 8th day and the control group with control serum. We observed the tube formation daily and captured images using Olympus IX70 (Olympus, Japan) inverted fluorescence microscope equipped with a digital camera.
Immunohistochemistry
At the end of the culture, the cell attached to coverslips were fixed in 4% paraformaldehyde for 15min at room temperature, washed three times with PBS, DAB method protocol complied with the User Manual of MaxVision kit (Maxim, China), the diluted concentration of rabbit anti-rat von Willebrand Factor (Maxim, China) and anti-VEGFR-2 antibodies (Abcam,USA) were 400× and 200× respectively. Photomicrographs were taken with an Olympus PM-C35DX digital camera.
Results
EPC morphological change
EPCs separated from rat bone marrow are round. After 48h culture, part cells adhered to dishes. These cells gathered to form colonies in the 3th day (Fig.1A) and lined up in a typical thready appearance of EPCs (Fig.1B) in the 5th day. Then most of the cells elongated into spindle shape 2d later (Fig.1C) and formed tube in the 11th day (Fig.1D).
Fig1.
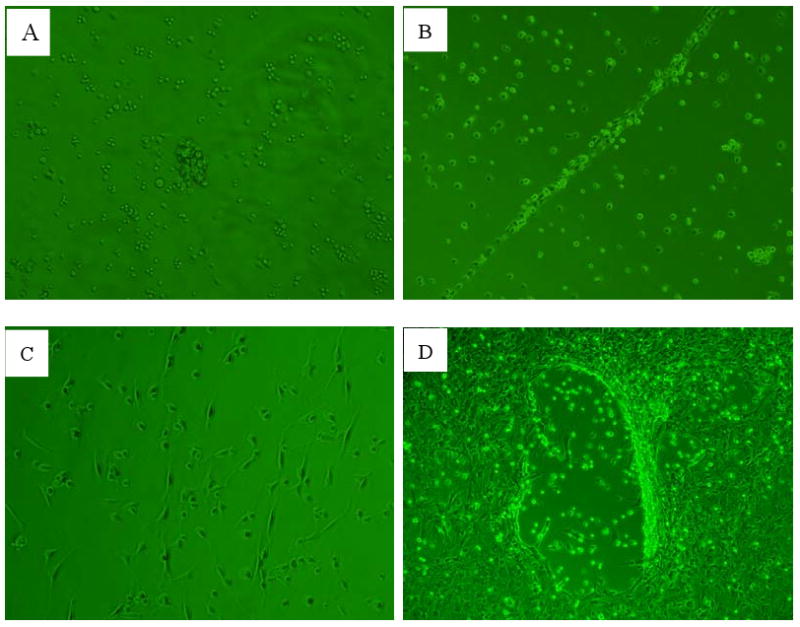
EPCs morphological change (×100).
A. EPCs colonies after 2d culture.
B. EPCs linear array after 4d culture.
C. EPCs spindle shape after 6d culture.
D. EPCs tube formation after 10d culture.
EPC differentiation analysis
EPCs cultured 14d for expressing more marker proteins were used to analysis their differentiation state. Immunohistochemistry results showed that EPCs spread out in flat or spindle, elliptic nucleolus of all groups stained blue and the cytoplasm of control group light blue (Fig.2A). EPCs differentiation into endothelial cell made the cytoplasm stain brown [Song: do you mean ‘stained the cells brown’?] by VEGFR-2 (Fig.2B) and vWF (Fig.1C) antibody.
Fig2.
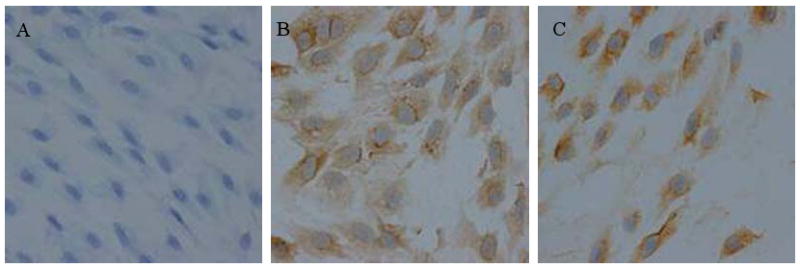
EPCs immunohistochemistry (×400).
A. Control group.
B. VEGFR-2 group.
C. vWF group.
Effect on EPC tube formation by XFZYD
One day after the induction of XFZYD-CS, all three medicine concentration groups showed typical tube or vessel network (Fig.3B-3D). Among them, the size of the tube in 5% XFZYD-CS group was so small that it had been showed in twofold microscopic fields than others. The control group showed a similar structure 2d later in [is the correct word ‘in” or “until” or “at” the 11th day (Fig.1D,4A) and this time passed the suitable opportunity for observing tube or network formation. [do you think i'm reading them correctly] Compared the remain 11th day tubes (Fig.4B) and primary 9th day tubes (Fig.3B-3D) of herbal groupto the 11th day of control group, although individual big tube could be seen (Fig.3D), most tube of herbal groups were smaller than that of the control group. Above result suggested that XFZYD not only speeded the tube formation but also helped to form capillary vessel.
Fig3.

EPCs tube formation in the 9th day of culture.
A. Control group (×100).
B. 5% XFZYD-CS group (×200).
C. 10% XFZYD-CS group (×100).
D. 15% XFZYD-CS group (×100).
Fig4.

EPCs tube formation in the 11th day of culture (×100).
A. Control group.
B. XFZYD-CS group
Discussion
EPCs separated by differential attachment method from rat bone marrow in this study showed a serial [no “a” if it is serial or do you mean ‘a series of’/Janet can you tell what they're saying?] typical morphological characteristics such as linear array, tube formation and [Song: delete “so on” and add another example if you can] during culture. Immunohistochemistry results further illustrated that these cells could express endothelial markers VEGFR-2 and vWF under endothelial media and showed the ability to differentiate into endothelial cell. All above changes met EPC characteristics.
It should be highlighted that these EPC induce tube formation occured 1d after the induction[janet wat is the word they need?] of XFZYD and 2d ahead of the control group. While there are many report about MNCs from human peripheral blood forming tube structure[8-9], there are no reports of rat bone marrow derived EPCs forming such typical vessel network in vitro without angiogenesis assay kit. A regular angiogenesis assay kit can show vessel network in a specific phase by seeding a certain amount of endothelial cells in the supplied media. EPCs culture media in this research was similar to the kit media, so tube formation could be observed during culture process. Like the kit, the observation of tube formation had time limit. Our group failed to show vessel network many times by seeding digested EPCs into kit media, but the seeding of human umbilical vein endothelial cells using the same kit worked. This result suggested that with the kit and our protocol were accurate. The reason for the EPCs failure in the assay kit may relate to the digestion injury or incompletion differentiation.
XFZYD, derived and modified from Taohong Siwu Tang(桃 红 四 物 汤, Four-Substance DecoctionwithSafflowerandPeachPit), [Song separate the words of the translation of the formula i can't seem to do this] has been widely used in the clinical practice of Chinese medicine. It is thought to, activate blood circulation without impairment of blood, removeblood stasis and promote the development of new blood, and relieve Qi stagnation and smooth Qi circulation. In a previous pilot study, our group found that XFZYD promoted tissue regeneration and new blood function [is function the right word?] by activation the hematopoietic stem cells [10]. [Song: not sure i did you sentence here accurately, please double check] Another study by our group found that XFZYD had an significant pro-angiogenesis effect on chick embryo chorioallantoic membrane (CAM)[11] and elevated the number of circulating EPCs by mobilizating [janet, help?] it from bone marrow[12]. The research reported here further confirms XFZYD's impact on inducing EPCs to angiogenesis by hastening tube formation especially capillary vessel in vitro. Our results suggest a possible mechanism for XFZYD clinical role in the treatment of ischemic diseases.
Acknowledgments
The present work is supported by the National Natural Science Foundation of China (Grant No. 30772877);Basic research fund of China Academy of Chinese medical Sciences (Grant No. ZZ2006039);Fujian Academy of Integrative medicine Foundation (Grant No.3000-905010805)
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative endothelial progenitor cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287(3):572–579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 3.Urbich C, Dimmeler S. Endothelial Progenitor Cells: Characterization and Role in Vascular Biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 4.Eguchi M, Masuda H, Asahara T. Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp Nephrol. 2007;11(1):18–25. doi: 10.1007/s10157-006-0448-1. [DOI] [PubMed] [Google Scholar]
- 5.Kawamoto A, Asahara T. Role of progenitor endothelial cells in cardiovascular disease and upcoming therapies. Catheter Cardiovasc Interv. 2007;70(4):477–484. doi: 10.1002/ccd.21292. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Zhou SH, Li XP, Shen XQ, Fang ZF. A novel hypothesis of atherosclerosis: EPCs-mediated repair-to-injure. Med Hypotheses. 2008;70(4):838–841. doi: 10.1016/j.mehy.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 7.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:E89–E93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 8.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, et al. Monocytes coexpress endothelial and macrophagocytic lineage makers and form cord-like structures in matrigel under angiogenic conditions. Cardiovasc Res. 2001;49(3):671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez Pujol B, Lucibello FC, Gehling UM, Lindemann K, Weidner N, Zuzarte ML, et al. Endothelial-like cells derived from CD14 positive monocytes. Differentiation. 2000;65(5):287–300. doi: 10.1046/j.1432-0436.2000.6550287.x. [DOI] [PubMed] [Google Scholar]
- 10.Gao D, Lin JM, Zheng LP, Lin W, Chen XZ, Song J, et al. Experimental Study on Effect of Xuefu Zhuyu Decoction on HSC from Mouse Marrow. Chinese Journal of Integrative Medicine. 2007;27(6):527–530. [PubMed] [Google Scholar]
- 11.Gao D, Song j, Hu J, Lin JM, Zheng LP, Cai J, et al. Angiogenesis promoting effects of Chinese herbal medicine for activating blood circulation to remove stasis on chick embryo chorio-allantoic membrane. Chinese Journal of Integrative Medicine. 2005;25(10):912–915. [PubMed] [Google Scholar]
- 12.Gao D, Lin W, Zheng LP, Lin JM, Chen XZ, Song j, et al. Experimental Study on Effect of Xuefu Zhuyu Decoction on EPC migrating from Rat Marrow. Chinese Journal of Integrative Medicine. 2007;5(9):829–831. [Google Scholar]


