Abstract
The development and maturation of the oligodendrocyte requires a series of highly orchestrated events that coordinate the proliferation and differentiation of the oligodendrocyte precursor cell (OPC) as well as the spatiotemporal regulation of myelination. In recent years, widespread interest has been devoted to the therapeutic potential of adult OPCs scattered throughout the central nervous system (CNS). In this review, we highlight molecular mechanisms controlling OPC differentiation during development and the implication of these mechanisms on adult OPCs for remyelination. Cell-autonomous regulators of differentiation and the heterogeneous microenvironment of the developing and the adult CNS may provide coordinated inhibitory cues that ultimately maintain a reservoir of uncommitted glia.
Introduction
The process of myelination is an exquisite and dynamic example of cell–cell interaction, which consists of the concentric wrapping of multiple layers of membrane around an axon. This process requires a series of highly orchestrated events that balance both intrinsic and extrinsic mechanisms to coordinate the spatiotemporal regulation of myelination. Myelin is a product of vertebrate evolution that maximizes the efficiency and velocity of action potentials transmitted through nerve cells. Demyelination as a result of disease or injury severely disrupts the efficient transmission of the action potential, ultimately resulting in a loss of function. To effectively treat these devastating conditions, it is essential to expand our knowledge concerning the generation and maturation of the myelin-forming cells and the processes that lead to myelination.
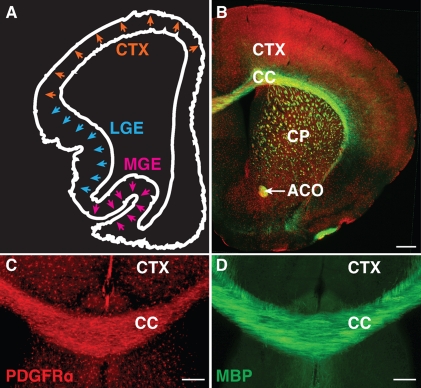
In the CNS, oligodendrocytes are responsible for the formation of myelin that surrounds axons. Although most oligodendrocyte precursor cells (OPCs) differentiate into myelinating oligodendrocytes, there remains a sizeable population of OPCs that remain undifferentiated after the completion of myelination. In recent years, adult OPCs have generated much interest as a reservoir of cells with the potential to self-renew, differentiate, and remyelinate the CNS (Gensert and Goldman, 1997; Keirstead et al., 1998; Levison et al., 1999; Nishiyama et al., 1999; Horner et al., 2000; Levine et al., 2001; Dawson et al., 2003; Windrem et al., 2004; Rivers et al., 2008). Although adult OPCs are also thought to serve other functions within the nervous system (Paukert and Bergles, 2006; Nishiyama et al., 2009), their potential for recruitment into demyelinated lesions points to an ideal therapeutic role, as adult OPCs represent an endogenous source of progenitor cells, making up 2–9% of the CNS cell population (Dawson et al., 2003). These cells seem to be distributed throughout the CNS, remaining in the undifferentiated state even after other OPCs differentiate and myelinate axons. Current evidence suggests that these adult OPCs express the same markers (PDGFR-α and NG2) as their developmental counterparts and appear morphologically similar (Nishiyama et al., 1996, 1999; Dawson et al., 2000, 2003; Wilson et al., 2006; Franklin and Ffrench-Constant, 2008). It remains unclear as to why these particular progenitors differ from their myelinating counterparts during development and remain as uncommitted cells. Understanding the developmental origins of adult OPCs would begin to address whether these cells have the intrinsic capabilities to differentiate and myelinate as opposed to having a different cellular identity. Are adult OPCs derived from the same population as OPCs during development, or are they a separate population of cells? The answer appears complex, as during development, OPCs are heterogeneous in their spatiotemporal origin (Fig. 1 A). OPCs arise from multiple regions of the ventricular zone in a sequential manner. The first wave of OPCs that populate the forebrain originates from the medial ganglionic eminence and anterior entopeduncular area. These OPCs are followed by a second wave from the lateral ganglionic eminence and caudal ganglionic eminence. Finally, a third collection of OPCs originates within the postnatal cortex. These respective populations are identified not only by their spatiotemporal differences but also by their differential transcription factor expression (Kessaris et al., 2006). Although these studies suggest the presence of heterogeneous OPC populations, the majority of the OPCs differentiate into oligodendrocytes and myelinate axons regardless of their origin. Despite originating from multiple regions at different times throughout development, OPCs are found evenly distributed throughout the adult brain in both gray and white matter alike (Fig. 1, B–D).
Figure 1.
Developmental origins of OPCs and the final distribution of adult OPCs in the telencephalon. (A) Schematic illustration outlining the origin of OPCs in the telencephalon during development. As indicated by the arrows, OPCs arise first from the medial ganglionic eminence (MGE) at embryonic day 12.5 followed by the lateral ganglionic eminence (LGE) several days later. Cortically derived OPCs appear soon after birth (adapted from Richardson et al., 2006). (B) Despite their spatial and temporal differences in origin, OPCs are evenly distributed throughout the adult brain and are found in regions such as the cortex (CTX), corpus callosum (CC), caudate putamen (CP), and anterior commissure (ACO). PDGFR-α (red) indicates the presence of OPCs. Myelin basic protein (MBP; green) identifies the heavily myelinated white matter tracts. Bar, 500 µm. (C and D) Magnified view of the adult brain, showing the presence of PDGFR-α–expressing adult OPCs dispersed throughout both gray matter, as represented by the cortex, and white matter tracts such as the corpus callosum. Myelin basic protein illustrates the myelinated fibers. Bar, 100 µm.
Additionally, compensatory mechanisms are observed when specific OPC populations are eliminated, whereby OPCs from different regions replace eradicated cells, resulting in normal oligodendrocyte and myelin phenotype (Kessaris et al., 2006; Kirby et al., 2006). Given the interchangeable nature of these OPCs despite spatiotemporal differences and the lack of evidence for adult OPCs originating from a separate lineage, it is reasonable to assume that insight into the mechanisms responsible for differentiation during development may provide valuable information concerning the presence and potential utility of the adult OPC.
Another fundamental question that needs to be considered is what normally happens during injury and demyelinating disease? Genetic fate-mapping studies show that adult OPCs have the capacity to myelinate continuously throughout normal adult life (Dimou et al., 2008; Rivers et al., 2008). However, in diseases such as multiple sclerosis, remyelination is limited to certain regions (forming what are known as shadow plaques, where axons are thinly remyelinated) and ultimately declining over time (Franklin, 2002). Which cells are responsible for these transient recoveries? In the adult brain, oligodendrocytes are terminally differentiated and generally do not participate in remyelination (Keirstead and Blakemore, 1997). Instead, OPCs are found in demyelinated regions, and are thus able to mobilize to damaged areas (Keirstead et al., 1998; Scolding et al., 1998; Wolswijk, 1998; Chang et al., 2000). On these accounts, remyelination by adult OPCs does not appear to be hampered by issues of access or recruitment. Additionally, although adult OPCs divide more slowly than their developmental counterparts (Shi et al., 1998; Tang et al., 2000), augmenting proliferation through increasing mitogens such as PDGF does not enhance remyelination (Woodruff et al., 2004). This further underscores OPC differentiation as the barrier impeding myelin repair rather than the recruitment and/or migration of OPCs. The fact that most of these adult OPCs remain as precursors throughout development and after injury and/or disease begs several intriguing questions that are essential to understanding cellular differentiation and the mechanisms that maintain a precursor population in the adult nervous system. Does remyelination depend on successful adult OPC differentiation? Is it possible that the inhibitory mechanisms for maintaining a precursor pool are similar to that which prevents remyelination in the adult nervous system? In this review, we will attempt to address these questions by integrating cell-autonomous differentiation events with microenvironmental cues and outline how these developmental processes may play a role after demyelination. Although it is admittedly difficult to resolve this complex issue in a single review and will require further developmental and demyelination studies, reviewing current findings should aid in formulating the most effective and efficient means to determine the relative contribution between intrinsic and extrinsic forces. Promoting remyelination in the CNS requires the establishment of a conducive setting for differentiation and myelination. This greatly depends on understanding the fine balance of developmental signals as well as cues resulting from injury or disease.
Intrinsic versus extrinsic regulation of differentiation
Early efforts addressing the mechanisms involved in OPC differentiation attempted to identify and dissect the individual components regulating this process by reducing the complexity of the environment. In vitro clonal density studies using purified OPCs provided an intriguing case for a purely cell-autonomous mechanism. In the absence of environmental cues, the presence of an intrinsic OPC differentiation program was observed where a timer mechanism was shown to regulate OPC division and maturation (Raff, 2006). In the presence of PDGF, a mitogen for OPCs, proliferation was induced with the absence of differentiation. In the presence of both PDGF and thyroid hormone, OPCs would divide six to eight times before differentiating, and progeny of a single clone grown separately would differentiate after approximately the same number of divisions as each other (Temple and Raff, 1986; Durand and Raff, 2000). This surprising finding suggested the presence of an intrinsic mechanism responsible for temporally controlling differentiation. Although OPCs were initially thought to monitor the number of divisions (Temple and Raff, 1986), it was later confirmed that the cells could somehow monitor time and not necessarily the number of divisions (Gao et al., 1997). Although this mechanism still remains unclear, thyroid hormone acts through thyroid hormone receptor-α1 to influence the activation of the timer, and various cyclin-dependent kinases, inhibitors, and other cell cycle regulatory proteins have been identified to influence both proliferation and differentiation (Durand et al., 1998; Tokumoto et al., 2001, 2002; Dugas et al., 2007). These findings suggest that differentiation is not just a default mechanism of inhibited proliferation but is dependent on other intrinsic mechanisms within the cell. Interestingly, OPCs grown in the presence of PDGF over an extended period of time in vitro appear to take on characteristics of adult OPCs, as both express similar markers and require a longer duration of time before differentiating (Tang et al., 2000). As an extension from these studies, thyroid hormone treatment to promote differentiation after demyelinating insult has shown some promise (Fernandez et al., 2004; Harsan et al., 2008).
Contrary to these findings, when OPCs are in direct contact with neurons, evidence for extrinsic regulation of differentiation prevails. These findings suggest the possibility that environmental cues may override any timer mechanism via OPC/neuronal signaling within the dynamic CNS milieu. Overexpression of PDGF by neurons in vivo leads to hyperproliferation of OPCs; however, the generation and maturation of oligodendrocytes are not inhibited or delayed, resulting in a normal myelination phenotype (Calver et al., 1998). Perhaps to perfectly myelinate the axons of the nervous system, a purely intrinsic mechanism may not be sufficient, as OPCs need to adapt to the ever-changing environment of the CNS, and contributions from the microenvironment consisting of other cell types may modulate the differentiation process. Studies coculturing purified OPCs with dorsal root ganglion neurons provide additional evidence for extrinsic regulation whereby the density of OPCs influences differentiation. Increasing the number of OPCs seeded onto neurons accelerates the process of differentiation, and this effect is induced by spatial constraints exerted by neighboring OPCs rather than a secreted or contact-mediated molecular cue (Rosenberg et al., 2008). Addition of exogenous PDGF to the cocultures enhances differentiation rather than maintaining OPCs in a proliferative state, as would be expected from the clonal density studies (Rosenberg et al., 2008). These findings imply the importance of the interaction between OPCs and their surrounding environment and that differentiation is likely dependent on extrinsic cues that activate a transcriptional program for differentiation. Understanding the balance of signals for these processes will provide valuable insight into the spatiotemporal control of differentiation during development and remyelination.
Transcriptional regulation
In recent years, a great deal of research has been devoted to understanding the transcriptional program necessary for the generation of the oligodendroglial lineage. This investment has provided valuable insight, especially with the identification of the Olig genes (Lu et al., 2000; Tekki-Kessaris et al., 2001; Qi et al., 2002; Takebayashi et al., 2002). Olig1/2 are basic helix-loop-helix transcription factors that play multiple roles in determining the oligodendroglial lineage and are expressed in mature oligodendrocytes as well as in both developmental and adult OPCs (Zhou et al., 2000; Ligon et al., 2006). These genes are responsive to Sonic hedgehog, a ventrally expressed morphogen, which is both sufficient and necessary to induce Olig gene expression for the generation of OPCs (Lu et al., 2000). Olig1/2 knockout mice fail to develop cells of the oligodendroglial lineage (Lu et al., 2002; Zhou and Anderson, 2002), and this is attributed specifically to Olig2 function, as the Olig2-null mouse displays a similar impairment in the generation of oligodendroglia and deficits in motor neuron specification (Lu et al., 2002; Takebayashi et al., 2002). Olig1 functions later in development, as the null mouse displays a specific defect in the maturation of oligodendrocytes while maintaining a seemingly normal pool of OPCs throughout development (Xin et al., 2005). Ectopic expression of Olig2 results in an oligodendroglial fate rather than a neural fate with the induction of Sox10, a high mobility group box transcription factor expressed by oligodendrocytes (Lu et al., 2000, 2001; Zhou et al., 2000; Stolt et al., 2002; Liu et al., 2007). Interestingly, the coexpression of Olig2 with Nkx2.2 induces oligodendrocyte differentiation (Sun et al., 2001; Zhou et al., 2001; Fu et al., 2002). Olig1 participates in differentiation by up-regulating several myelin genes, including Mbp, Plp, and Mag as well as suppressing Gfap, an astrocytic gene (Xin et al., 2005; Li et al., 2007). The localization of Olig1/2 is observed in the nucleus of OPCs during development; however, although Olig2 remains in the nucleus of OPCs in the adult mouse, Olig1 is found to be cytoplasmic (Arnett et al., 2004). Could the repression of Olig1 function in a population of OPCs give rise to the adult precursor cell, and what mechanisms selectively regulate this repression? In a murine demyelination model and within tissue from multiple sclerosis patients, Olig1 is translocated into the nucleus of OPCs as in development and may be associated with oligodendrocyte differentiation in the repair process, and as anticipated, remyelination is impaired in the Olig1 knockout mouse (Arnett et al., 2004; Balabanov and Popko, 2005).
Although these studies illustrate the importance of Olig genes in OPC fate and differentiation, the extracellular pathways coupled to differentiation remains to be elucidated. By screening for molecules dynamically regulated in the Olig1 knockout mouse, GPR17, a Gi protein–coupled orphan receptor, was identified as a negative regulator of oligodendrocyte differentiation (Chen et al., 2009). GPR17 is related to P2Y (purinergic) and cysteinyl–leukotriene receptors and can be activated by nucleotides and inflammatory mediators (Ciana et al., 2006). GPR17 is expressed by OPCs and down-regulated in mature myelinating oligodendrocytes. Further studies demonstrate that GPR17 is up-regulated in mouse oligodendrocytes after demyelination, and analysis of human multiple sclerosis plaque tissue by quantitative PCR reveals an increase in GPR17 expression. To further investigate the role of this potential receptor, mice with sustained GPR17 overexpression in oligodendrocytes were generated under the control of a CNP1 promoter and exhibit severe myelin deficits. Overexpressing GPR17 in vitro results in the nuclear localization of ID2/4, which complex with Olig1/2 and inhibit OPC differentiation (Samanta and Kessler, 2004; Chen et al., 2009). Conversely, knocking out GPR17 results in precocious myelination and accelerated OPC differentiation (Chen et al., 2009). GPR17 is a novel putative extracellular receptor that inhibits OPC differentiation, and its expression in adult OPCs after demyelination deems it a valuable potential therapeutic candidate. Identifying the exogenous ligand for this receptor would provide a valuable link between the environmental and transcriptional control of oligodendrocyte differentiation.
Epigenetic regulation
To complicate things even further, although the process of OPC differentiation undoubtedly requires transcriptional changes, epigenetic mechanisms also influence OPC differentiation and can do so in a spatiotemporal-specific manner (Li et al., 2009). Additionally, several of these studies implicate conserved signaling pathways for adult OPCs after demyelinating insult. The transition from a precursor cell to a mature, myelinating oligodendrocyte requires a coordinated effort and can be achieved by modulating transcription via DNA modification. These epigenetic states can be heritable and are susceptible to environmental influences, which may allow for the dynamic interaction between intrinsic and extrinsic factors to occur. Epigenetic changes as a result of senescence may be one such example. Multiple sclerosis is a progressive disease, and the severity worsens with age (Compston and Coles, 2008). In correlation, recent findings demonstrate a decline in the efficiency of myelin repair in a cuprizone-induced model of demyelination in an age-dependent manner (Shen et al., 2008). This age-related decrease is attributed to epigenetic regulation of adult OPCs dependent on the recruitment of histone deacetylases (HDACs; Popko, 2008; Shen et al., 2008). Administering HDAC inhibitors to young and older mice result in different remyelination efficiencies (Shen et al., 2008). HDACs normally remove acetyl groups from histones to allow for the compaction of chromatin, which subsequently silences transcription. How does inhibiting transcription lead to the differentiation of OPCs? Recent evidence suggests that this is the result of the repression of pathways that normally prevent differentiation from occurring (Popko, 2008; Shen et al., 2008; Li et al., 2009). Two independent studies demonstrate that Wnt signaling is responsible for repressing OPC differentiation (Fancy et al., 2009; Li and Richardson, 2009; Rosenberg and Chan, 2009; Ye et al., 2009). The Wnt signaling pathway usually prevents the degradation of β-catenin, and knocking out HDAC1/2 in oligodendroglial cells results in the stabilization of β-catenin in the nucleus, which in turn represses Olig2 (Ye et al., 2009). These findings also demonstrate the direct association of HDAC1/2 with Tcf4/Tcf712 and β-catenin. Signaling through the Wnt pathway can interfere with this interaction and inhibit OPC differentiation (Li and Richardson, 2009; Ye et al., 2009). Both studies report that the constitutive expression of β-catenin impairs OPC differentiation and remyelination (Fancy et al., 2009; Ye et al., 2009). Knocking out adenomatous polyposis coli, a β-catenin antagonist, results in a similar phenotype (Fancy et al., 2009). Furthermore, a demyelination microarray screen identifies the increased expression of Tcf4/Tcf712 in the normal developing human CNS and tissue from multiple sclerosis lesions (Fancy et al., 2009). Tcf4/Tcf712 was originally found to be repressed by transcription factor Ying Yang 1, which also suppresses ID4, another transcriptional inhibitor of OPC differentiation (He et al., 2007). Collectively, these findings highly suggest that mechanisms which control differentiation during development likely influence differentiation after injury and/or disease. More importantly, the Wnt pathways provide a means of extracellular input, which further broadens our appreciation for the complexity of coordinating extrinsic and intrinsic mechanisms (Rosenberg and Chan, 2009).
Axonal inhibition of differentiation
Throughout development, OPCs are in constant contact with axons, suggesting that glial–neuronal communication is essential for regulating OPC development, including the timing of differentiation and myelination. During development, the axon expresses numerous inhibitory cues preventing OPC differentiation to ensure the proper spatial arrangement of oligodendrocytes and temporal control of myelination along axon tracts. However, many of these same extrinsic signals expressed along axons are reexpressed during demyelinating conditions, which may impair efforts to promote remyelination. Identifying these molecular cues and understanding how they influence differentiation of OPCs may provide new insight into providing a permissive environment for myelin repair.
The neural cell adhesion molecule (NCAM) is expressed ubiquitously by nearly all neurons, and axons express the polysialylated (PSA) form during development before the onset of myelination (Jakovcevski et al., 2007). This modification reduces homophilic interactions between NCAMs as a result of the negative charge and/or hydration volume of the PSA (Kleene and Schachner, 2004). In addition, PSA-NCAM can modulate heterophilic interactions with other glycans, such as heparan sulfate proteoglycans, which are also expressed by OPCs (Winkler et al., 2002). Correlative studies report a decrease in expression of PSA-NCAM before the onset of oligodendrocyte myelination, and prematurely eliminating PSA-NCAM from neurons in vitro enhances differentiation and myelination (Charles et al., 2000), suggesting an inhibitory role when present (Jakovcevski et al., 2007). As adhesion is a process that is required for numerous cellular processes, including migration and other forms of cell–cell interaction, adult OPCs may require a permissive substrate lacking PSA-NCAM to differentiate and remyelinate. Indeed, PSA-NCAM is reexpressed in multiple sclerosis lesions but not in regions where remyelination occurs (Charles et al., 2002).
Another axonal inhibitor of OPC differentiation is the Notch receptor Jagged1 (Wang et al., 1998). Notch is an evolutionarily conserved transmembrane protein, whereby activation through its putative receptors Jagged and Delta results in the cleavage of its intracellular domain. Upon cleavage, Notch is translocated to the nucleus and modulates transcription to influence cell fate and differentiation (Kopan and Ilagan, 2009). Notch1 is thought to act through Hes5, a basic helix-loop-helix transcription factor, which in turn suppresses transcription of myelin genes (Wang et al., 1998; Liu et al., 2006). Knocking out Notch1 in vivo results in premature differentiation and ectopic oligodendrocyte formation in the gray matter (Genoud et al., 2002). Inhibiting γ-secretase (the protease involved in Notch1 cleavage) in vitro also enhances differentiation (Watkins et al., 2008). Targeted deletion of Notch1 in Olig1-expressing OPCs results in precocious differentiation, and consistent with its inhibitory effect on OPC differentiation during development, remyelination is enhanced in this mouse model (Zhang et al., 2009). As adult OPCs still express Notch1 (Wang et al., 1998), further investigation is required to determine the possible role for Notch signaling in maintaining adult OPCs and the lack of differentiation and myelination after demyelinating insult.
A potent example of an axonal inhibitor of oligodendrocyte differentiation that has been examined in various demyelinating animal models is the leucine-rich repeat and Ig domain–containing, Nogo receptor–interacting protein (LINGO-1; Mi et al., 2005, 2007, 2008, 2009; Lee et al., 2007). Function blocking LINGO-1 using an anti–LINGO-1 antibody has proved to enhance functional recovery and remyelination after experimental autoimmune encephalomyelitis, lysolecithin treatment, and after cuprizone-induced demyelination (Mi et al., 2007, 2009). Although these results illustrate great therapeutic potential, the mechanisms that control this inhibition remain unclear. It seems that both oligodendrocytes and neurons express LINGO-1, and disruption of LINGO-1 functions on oligodendrocytes or neurons, respectively, is sufficient to promote differentiation and myelination (Mi et al., 2005; Lee et al., 2007). Additionally, LINGO-1 activates the Rho family GTPase RhoA and decreases Fyn expression and activation (Mi et al., 2005). These intracellular signaling molecules have both been implicated in oligodendrocyte differentiation (Liang et al., 2004; Goto et al., 2008; Rajasekharan et al., 2009). Further studies concerning the developmental regulation of the axonal expression of LINGO-1 should illuminate this paracrine relationship between axons and OPCs, which may also be relevant after injury and disease.
Connective tissue growth factor (CTGF) is another paracrine signal expressed and secreted by neurons to inhibit OPC differentiation. Ectopic expression of CTGF in vivo through adenovirus transduction reduces the number of oligodendrocytes formed during development (Stritt et al., 2009). CTGF is thought to sequester and thereby antagonize the function of insulin-like growth factor 1, which has been implicated in stimulating OPC differentiation (McMorris et al., 1986; Hsieh et al., 2004). Suppression of CTGF relies on the transcription factor serum response factor (SRF), permitting differentiation during development (Stritt et al., 2009). SRF is a transcription factor with multiple binding partners such as TCFs and myocardin-related transcription factors (MRTFs) and can be activated by growth factors, such as NGF and brain-derived neurotrophic factor, or through neuronal activity (Wickramasinghe et al., 2008; Knöll and Nordheim, 2009). Deletion of SRF specifically in neurons results in an increase in the number of OPCs and inhibits the maturation and differentiation processes (Knöll and Nordheim, 2009; Stritt et al., 2009). These results clearly illustrate the shared importance and reciprocal nature of understanding transcriptional, epigenetic, and the extrinsic signals that modulate oligodendrocyte maturation. By fully considering the mechanisms required for the activation of these processes, we hope to gain valuable insight into establishing an environment conducive for OPC differentiation during development and remyelination.
Tapping into the glial reservoir
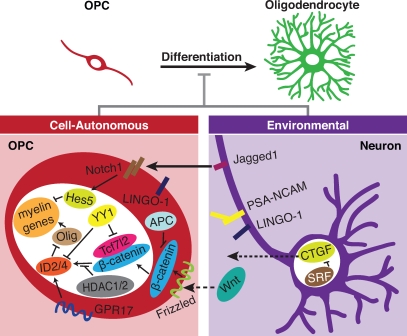
One of the most fundamental questions in biology is whether it is possible to resolve pathological states by recapitulating development. The OPC is a prototypical cell for answering this question, as an abundant number of endogenous undifferentiated precursors exist in the adult nervous system. Additionally, the simplicity in the binary decision to become a terminally differentiated oligodendrocyte or remain a precursor cell reduces the complexity when addressing cell fate decisions. Making the commitment to differentiate during development is a complex process, as it requires the coordination of transcriptional machinery in conjunction with epigenetic regulation within the cell. Ongoing research has also provided evidence for the impact of extrinsic factors on OPC differentiation. Curiously, many of these environmental influences appear to be inhibitory in nature, with the axon delaying OPC differentiation rather than promoting it. Fig. 2 summarizes the molecular components discussed in this review, with all of them playing a role during developmental OPC differentiation, and many of these inhibitory factors, originating from both the axon and within the OPC itself, appear to prevent adult OPCs from reaching their potential to participate in myelin repair. Both contact-mediated cues and secreted factors participate in inhibiting differentiation, although there is still much to learn as to how these environmental signals converge upon intracellular pathways as well as transcriptional regulators responsible for maintaining OPCs in an undifferentiated state (Fig. 2).
Figure 2.
Intrinsic and extrinsic mechanisms prevent the differentiation of OPCs to myelinating oligodendrocytes. These mechanisms act during development and in some cases after injury and disease. Solid arrows indicate contact-mediated interactions such as Jagged1 expressed on axons acting through Notch1 on OPCs, leading to the suppression of myelin genes via Hes5. Dashed arrows indicate secreted molecules such as CTGF from the neuron and Wnt from a yet-unidentified source. Although its ligand has not been determined, GPR17 expressed by OPCs is likely sensitive to environmental signals.
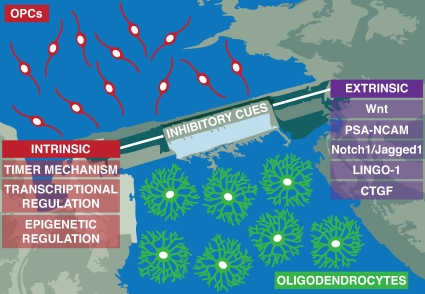
Furthermore, unlike the peripheral nervous system, the CNS has no capacity for removing inhibitors that prevent differentiation and remyelination (Vargas and Barres, 2007). It is not clear as to why there are numerous inhibitory cues and few inductive factors; perhaps this allows for the finer control of the differentiation process or even for the maintenance of adult precursors. Despite our lack of understanding, it appears that overriding these inhibitory cues expressed during injury and disease may be the first practical step to understanding restoration. Conversely, a concerted effort to identify inductive cues will also enrich our understanding of the balance of signals involved in OPC differentiation during development and remyelination. Fig. 3 illustrates a summary of this review, in which yet-unidentified inductive cues for OPC differentiation are represented by the school of oligodendrocyte precursors heading toward the dam. The barrier itself is an analogy for inhibitory cues and is composed of myriad cell-autonomous and microenvironmental signals. During injury and disease, we propose that the removal of the barrier (inhibitory cues) is necessary in order for remyelination to occur, represented by oligodendrocytes downstream of the reservoir. Likewise, if one fills the dam high enough through inductive means, can one overcome the barricade, i.e., balance of induction and inhibition? Also, what determines the fate of individual OPCs, making one more likely to remain uncommitted in adulthood, whereas others will terminally differentiate? Our illustration cannot represent the intricate geometry of the CNS where adult OPCs seem to reside throughout. However, the seemingly arbitrary distribution of adult OPCs can be deceiving; in fact, the extrinsic influences discussed in this review may be working collectively through constructing unique, dispersed microenvironments where adult OPCs inhabit. Even though Occam’s razor would suggest otherwise, the CNS has to rely on complex and robust mechanisms for maintaining precursor cells, and this may require a dynamic array of intercellular interactions. If this is the case, it will be challenging to identify the components involved in adult OPC maintenance within the complex CNS neuropil. Grasping the elusive nature of these endogenous precursors will also allow us to exploit their potential during injury and disease.
Figure 3.
A proposed model for the maintenance of adult OPCs. OPCs (red) reside in a reservoir and are upstream of the dam. They are inhibited from differentiating into oligodendrocytes (green) by several cell-autonomous (intrinsic) and microenvironmental (extrinsic) inhibitory cues. Presumably unidentified inductive cues may act to overcome the inhibitory barrier and allow differentiation to occur.
Acknowledgments
We are grateful to Dr. Q. Richard Lu and Sheila Rosenberg for insightful discussions and critically reviewing our manuscript.
We are thankful for support from the National Multiple Sclerosis Society (Career Transition Award TA 3008A2/T to J.R. Chan) and the National Institutes of Health/National Institute of Neurological Disorders and Stroke (grant NS062796-01 to J.R. Chan).
Footnotes
Abbreviations used in this paper:
- CTGF
- connective tissue growth factor
- HDAC
- histone deacetylase
- MRTF
- myocardin-related transcription factor
- NCAM
- neural cell adhesion molecule
- OPC
- oligodendrocyte precursor cell
- PSA
- polysialylated
- SRF
- serum response factor
References
- Arnett H.A., Fancy S.P.J., Alberta J.A., Zhao C., Plant S.R., Kaing S., Raine C.S., Rowitch D.H., Franklin R.J.M., Stiles C.D. 2004. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 306:2111–2115 10.1126/science.1103709 [DOI] [PubMed] [Google Scholar]
- Balabanov R., Popko B. 2005. Myelin repair: developmental myelination redux? Nat. Neurosci. 8:262–264 10.1038/nn0305-262 [DOI] [PubMed] [Google Scholar]
- Calver A.R., Hall A.C., Yu W.P., Walsh F.S., Heath J.K., Betsholtz C., Richardson W.D. 1998. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 20:869–882 10.1016/S0896-6273(00)80469-9 [DOI] [PubMed] [Google Scholar]
- Chang A., Nishiyama A., Peterson J., Prineas J., Trapp B.D. 2000. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 20:6404–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P., Hernandez M.P., Stankoff B., Aigrot M.-S., Colin C., Rougon G., Zalc B., Lubetzki C. 2000. Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc. Natl. Acad. Sci. USA. 97:7585–7590 10.1073/pnas.100076197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles P., Reynolds R., Seilhean D., Rougon G., Aigrot M.-S., Niezgoda A., Zalc B., Lubetzki C. 2002. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain. 125:1972–1979 10.1093/brain/awf216 [DOI] [PubMed] [Google Scholar]
- Chen Y., Wu H., Wang S., Koito H., Li J., Ye F., Hoang J., Escobar S.S., Gow A., Arnett H.A., et al. 2009. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 12:1398–1406 10.1038/nn.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P., Fumagalli M., Trincavelli M.L., Verderio C., Rosa P., Lecca D., Ferrario S., Parravicini C., Capra V., Gelosa P., et al. 2006. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 25:4615–4627 10.1038/sj.emboj.7601341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A., Coles A. 2008. Multiple sclerosis. Lancet. 372:1502–1517 10.1016/S0140-6736(08)61620-7 [DOI] [PubMed] [Google Scholar]
- Dawson M.R.L., Levine J.M., Reynolds R. 2000. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J. Neurosci. Res. 61:471–479 [DOI] [PubMed] [Google Scholar]
- Dawson M.R.L., Polito A., Levine J.M., Reynolds R. 2003. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 24:476–488 10.1016/S1044-7431(03)00210-0 [DOI] [PubMed] [Google Scholar]
- Dimou L., Simon C., Kirchhoff F., Takebayashi H., Götz M. 2008. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 28:10434–10442 10.1523/JNEUROSCI.2831-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas J.C., Ibrahim A., Barres B.A. 2007. A crucial role for p57(Kip2) in the intracellular timer that controls oligodendrocyte differentiation. J. Neurosci. 27:6185–6196 10.1523/JNEUROSCI.0628-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand B., Raff M. 2000. A cell-intrinsic timer that operates during oligodendrocyte development. Bioessays. 22:64–71 [DOI] [PubMed] [Google Scholar]
- Durand B., Fero M.L., Roberts J.M., Raff M.C. 1998. p27Kip1 alters the response of cells to mitogen and is part of a cell-intrinsic timer that arrests the cell cycle and initiates differentiation. Curr. Biol. 8:431–440 10.1016/S0960-9822(98)70177-0 [DOI] [PubMed] [Google Scholar]
- Fancy S.P.J., Baranzini S.E., Zhao C., Yuk D.-I., Irvine K.-A., Kaing S., Sanai N., Franklin R.J.M., Rowitch D.H. 2009. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23:1571–1585 10.1101/gad.1806309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez M., Giuliani A., Pirondi S., D’Intino G., Giardino L., Aloe L., Levi-Montalcini R., Calzà L. 2004. Thyroid hormone administration enhances remyelination in chronic demyelinating inflammatory disease. Proc. Natl. Acad. Sci. USA. 101:16363–16368 10.1073/pnas.0407262101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R.J.M. 2002. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 3:705–714 10.1038/nrn917 [DOI] [PubMed] [Google Scholar]
- Franklin R.J.M., Ffrench-Constant C. 2008. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 9:839–855 10.1038/nrn2480 [DOI] [PubMed] [Google Scholar]
- Fu H., Qi Y., Tan M., Cai J., Takebayashi H., Nakafuku M., Richardson W.D., Qiu M. 2002. Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development. 129:681–693 [DOI] [PubMed] [Google Scholar]
- Gao F.B., Durand B., Raff M. 1997. Oligodendrocyte precursor cells count time but not cell divisions before differentiation. Curr. Biol. 7:152–155 10.1016/S0960-9822(06)00060-1 [DOI] [PubMed] [Google Scholar]
- Genoud S., Lappe-Siefke C., Goebbels S., Radtke F., Aguet M., Scherer S.S., Suter U., Nave K.-A., Mantei N. 2002. Notch1 control of oligodendrocyte differentiation in the spinal cord. J. Cell Biol. 158:709–718 10.1083/jcb.200202002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensert J.M., Goldman J.E. 1997. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 19:197–203 10.1016/S0896-6273(00)80359-1 [DOI] [PubMed] [Google Scholar]
- Goto J., Tezuka T., Nakazawa T., Sagara H., Yamamoto T. 2008. Loss of Fyn tyrosine kinase on the C57BL/6 genetic background causes hydrocephalus with defects in oligodendrocyte development. Mol. Cell. Neurosci. 38:203–212 10.1016/j.mcn.2008.02.009 [DOI] [PubMed] [Google Scholar]
- Harsan L.-A., Steibel J., Zaremba A., Agin A., Sapin R., Poulet P., Guignard B., Parizel N., Grucker D., Boehm N., et al. 2008. Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J. Neurosci. 28:14189–14201 10.1523/JNEUROSCI.4453-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Dupree J., Wang J., Sandoval J., Li J., Liu H., Shi Y., Nave K.-A., Casaccia-Bonnefil P. 2007. The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation. Neuron. 55:217–230 10.1016/j.neuron.2007.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner P.J., Power A.E., Kempermann G., Kuhn H.G., Palmer T.D., Winkler J., Thal L.J., Gage F.H. 2000. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 20:2218–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J., Aimone J.B., Kaspar B.K., Kuwabara T., Nakashima K., Gage F.H. 2004. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 164:111–122 10.1083/jcb.200308101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I., Mo Z., Zecevic N. 2007. Down-regulation of the axonal polysialic acid-neural cell adhesion molecule expression coincides with the onset of myelination in the human fetal forebrain. Neuroscience. 149:328–337 10.1016/j.neuroscience.2007.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead H.S., Blakemore W.F. 1997. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J. Neuropathol. Exp. Neurol. 56:1191–1201 10.1097/00005072-199711000-00003 [DOI] [PubMed] [Google Scholar]
- Keirstead H.S., Levine J.M., Blakemore W.F. 1998. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 22:161–170 [DOI] [PubMed] [Google Scholar]
- Kessaris N., Fogarty M., Iannarelli P., Grist M., Wegner M., Richardson W.D. 2006. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9:173–179 10.1038/nn1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby B.B., Takada N., Latimer A.J., Shin J., Carney T.J., Kelsh R.N., Appel B. 2006. In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development. Nat. Neurosci. 9:1506–1511 10.1038/nn1803 [DOI] [PubMed] [Google Scholar]
- Kleene R., Schachner M. 2004. Glycans and neural cell interactions. Nat. Rev. Neurosci. 5:195–208 10.1038/nrn1349 [DOI] [PubMed] [Google Scholar]
- Knöll B., Nordheim A. 2009. Functional versatility of transcription factors in the nervous system: the SRF paradigm. Trends Neurosci. 32:432–442 10.1016/j.tins.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Kopan R., Ilagan M.X. 2009. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 137:216–233 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee X., Yang Z., Shao Z., Rosenberg S.S., Levesque M., Pepinsky R.B., Qiu M., Miller R.H., Chan J.R., Mi S. 2007. NGF regulates the expression of axonal LINGO-1 to inhibit oligodendrocyte differentiation and myelination. J. Neurosci. 27:220–225 10.1523/JNEUROSCI.4175-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J.M., Reynolds R., Fawcett J.W. 2001. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 24:39–47 10.1016/S0166-2236(00)01691-X [DOI] [PubMed] [Google Scholar]
- Levison S.W., Young G.M., Goldman J.E. 1999. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J. Neurosci. Res. 57:435–446 [DOI] [PubMed] [Google Scholar]
- Li H., Richardson W.D. 2009. Genetics meets epigenetics: HDACs and Wnt signaling in myelin development and regeneration. Nat. Neurosci. 12:815–817 10.1038/nn0709-815 [DOI] [PubMed] [Google Scholar]
- Li H., Lu Y., Smith H.K., Richardson W.D. 2007. Olig1 and Sox10 interact synergistically to drive myelin basic protein transcription in oligodendrocytes. J. Neurosci. 27:14375–14382 10.1523/JNEUROSCI.4456-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., He Y., Richardson W.D., Casaccia P. 2009. Two-tier transcriptional control of oligodendrocyte differentiation. Curr. Opin. Neurobiol. 19:479–485 10.1016/j.conb.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Draghi N.A., Resh M.D. 2004. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J. Neurosci. 24:7140–7149 10.1523/JNEUROSCI.5319-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligon K.L., Fancy S.P.J., Franklin R.J.M., Rowitch D.H. 2006. Olig gene function in CNS development and disease. Glia. 54:1–10 10.1002/glia.20273 [DOI] [PubMed] [Google Scholar]
- Liu A., Li J., Marin-Husstege M., Kageyama R., Fan Y., Gelinas C., Casaccia-Bonnefil P. 2006. A molecular insight of Hes5-dependent inhibition of myelin gene expression: old partners and new players. EMBO J. 25:4833–4842 10.1038/sj.emboj.7601352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Hu X., Cai J., Liu B., Peng X., Wegner M., Qiu M. 2007. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev. Biol. 302:683–693 10.1016/j.ydbio.2006.10.007 [DOI] [PubMed] [Google Scholar]
- Lu Q.R., Yuk D., Alberta J.A., Zhu Z., Pawlitzky I., Chan J., McMahon A.P., Stiles C.D., Rowitch D.H. 2000. Sonic hedgehog—regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 25:317–329 10.1016/S0896-6273(00)80897-1 [DOI] [PubMed] [Google Scholar]
- Lu Q.R., Cai L., Rowitch D., Cepko C.L., Stiles C.D. 2001. Ectopic expression of Olig1 promotes oligodendrocyte formation and reduces neuronal survival in developing mouse cortex. Nat. Neurosci. 4:973–974 10.1038/nn718 [DOI] [PubMed] [Google Scholar]
- Lu Q.R., Sun T., Zhu Z., Ma N., Garcia M., Stiles C.D., Rowitch D.H. 2002. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 109:75–86 10.1016/S0092-8674(02)00678-5 [DOI] [PubMed] [Google Scholar]
- McMorris F.A., Smith T.M., DeSalvo S., Furlanetto R.W. 1986. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc. Natl. Acad. Sci. USA. 83:822–826 10.1073/pnas.83.3.822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Miller R.H., Lee X., Scott M.L., Shulag-Morskaya S., Shao Z., Chang J., Thill G., Levesque M., Zhang M., et al. 2005. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 8:745–751 10.1038/nn1460 [DOI] [PubMed] [Google Scholar]
- Mi S., Hu B., Hahm K., Luo Y., Kam Hui E.S., Yuan Q., Wong W.M., Wang L., Su H., Chu T.-H., et al. 2007. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 13:1228–1233 10.1038/nm1664 [DOI] [PubMed] [Google Scholar]
- Mi S., Sandrock A., Miller R.H. 2008. LINGO-1 and its role in CNS repair. Int. J. Biochem. Cell Biol. 40:1971–1978 10.1016/j.biocel.2008.03.018 [DOI] [PubMed] [Google Scholar]
- Mi S., Miller R.H., Tang W., Lee X., Hu B., Wu W., Zhang Y., Shields C.B., Zhang Y., Miklasz S., et al. 2009. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann. Neurol. 65:304–315 10.1002/ana.21581 [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Lin X.H., Giese N., Heldin C.H., Stallcup W.B. 1996. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J. Neurosci. Res. 43:315–330 [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Chang A., Trapp B.D. 1999. NG2+ glial cells: a novel glial cell population in the adult brain. J. Neuropathol. Exp. Neurol. 58:1113–1124 10.1097/00005072-199911000-00001 [DOI] [PubMed] [Google Scholar]
- Nishiyama A., Komitova M., Suzuki R., Zhu X. 2009. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 10:9–22 10.1038/nrn2495 [DOI] [PubMed] [Google Scholar]
- Paukert M., Bergles D.E. 2006. Synaptic communication between neurons and NG2+ cells. Curr. Opin. Neurobiol. 16:515–521 10.1016/j.conb.2006.08.009 [DOI] [PubMed] [Google Scholar]
- Popko B. 2008. Epigenetic control of myelin repair. Nat. Neurosci. 11:987–988 10.1038/nn0908-987 [DOI] [PubMed] [Google Scholar]
- Qi Y., Stapp D., Qiu M. 2002. Origin and molecular specification of oligodendrocytes in the telencephalon. Trends Neurosci. 25:223–225 10.1016/S0166-2236(02)02145-8 [DOI] [PubMed] [Google Scholar]
- Raff M. 2006. The mystery of intracellular developmental programmes and timers. Biochem. Soc. Trans. 34:663–670 10.1042/BST0340663 [DOI] [PubMed] [Google Scholar]
- Rajasekharan S., Baker K.A., Horn K.E., Jarjour A.A., Antel J.P., Kennedy T.E. 2009. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 136:415–426 10.1242/dev.018234 [DOI] [PubMed] [Google Scholar]
- Richardson W.D., Kessaris N., Pringle N. 2006. Oligodendrocyte wars. Nat. Rev. Neurosci. 7:11–18 10.1038/nrn1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers L.E., Young K.M., Rizzi M., Jamen F., Psachoulia K., Wade A., Kessaris N., Richardson W.D. 2008. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11:1392–1401 10.1038/nn.2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.S., Chan J.R. 2009. Modulating myelination: knowing when to say Wnt. Genes Dev. 23:1487–1493 10.1101/gad.1824009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S.S., Kelland E.E., Tokar E., De la Torre A.R., Chan J.R. 2008. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA. 105:14662–14667 10.1073/pnas.0805640105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J., Kessler J.A. 2004. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 131:4131–4142 10.1242/dev.01273 [DOI] [PubMed] [Google Scholar]
- Scolding N., Franklin R., Stevens S., Heldin C.H., Compston A., Newcombe J. 1998. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 121:2221–2228 10.1093/brain/121.12.2221 [DOI] [PubMed] [Google Scholar]
- Shen S., Sandoval J., Swiss V.A., Li J., Dupree J., Franklin R.J., Casaccia-Bonnefil P. 2008. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 11:1024–1034 10.1038/nn.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Marinovich A., Barres B.A. 1998. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J. Neurosci. 18:4627–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt C.C., Rehberg S., Ader M., Lommes P., Riethmacher D., Schachner M., Bartsch U., Wegner M. 2002. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 16:165–170 10.1101/gad.215802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritt C., Stern S., Harting K., Manke T., Sinske D., Schwarz H., Vingron M., Nordheim A., Knöll B. 2009. Paracrine control of oligodendrocyte differentiation by SRF-directed neuronal gene expression. Nat. Neurosci. 12:418–427 10.1038/nn.2280 [DOI] [PubMed] [Google Scholar]
- Sun T., Echelard Y., Lu R., Yuk D.I., Kaing S., Stiles C.D., Rowitch D.H. 2001. Olig bHLH proteins interact with homeodomain proteins to regulate cell fate acquisition in progenitors of the ventral neural tube. Curr. Biol. 11:1413–1420 10.1016/S0960-9822(01)00441-9 [DOI] [PubMed] [Google Scholar]
- Takebayashi H., Nabeshima Y., Yoshida S., Chisaka O., Ikenaka K., Nabeshima Y.-i. 2002. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 12:1157–1163 10.1016/S0960-9822(02)00926-0 [DOI] [PubMed] [Google Scholar]
- Tang D.G., Tokumoto Y.M., Raff M.C. 2000. Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months. J. Cell Biol. 148:971–984 10.1083/jcb.148.5.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekki-Kessaris N., Woodruff R., Hall A.C., Gaffield W., Kimura S., Stiles C.D., Rowitch D.H., Richardson W.D. 2001. Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development. 128:2545–2554 [DOI] [PubMed] [Google Scholar]
- Temple S., Raff M.C. 1986. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell divisions. Cell. 44:773–779 10.1016/0092-8674(86)90843-3 [DOI] [PubMed] [Google Scholar]
- Tokumoto Y.M., Tang D.G., Raff M.C. 2001. Two molecularly distinct intracellular pathways to oligodendrocyte differentiation: role of a p53 family protein. EMBO J. 20:5261–5268 10.1093/emboj/20.18.5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumoto Y.M., Apperly J.A., Gao F.-B., Raff M.C. 2002. Posttranscriptional regulation of p18 and p27 Cdk inhibitor proteins and the timing of oligodendrocyte differentiation. Dev. Biol. 245:224–234 10.1006/dbio.2002.0626 [DOI] [PubMed] [Google Scholar]
- Vargas M.E., Barres B.A. 2007. Why is Wallerian degeneration in the CNS so slow? Annu. Rev. Neurosci. 30:153–179 10.1146/annurev.neuro.30.051606.094354 [DOI] [PubMed] [Google Scholar]
- Wang S., Sdrulla A.D., diSibio G., Bush G., Nofziger D., Hicks C., Weinmaster G., Barres B.A. 1998. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 21:63–75 10.1016/S0896-6273(00)80515-2 [DOI] [PubMed] [Google Scholar]
- Watkins T.A., Emery B., Mulinyawe S., Barres B.A. 2008. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 60:555–569 10.1016/j.neuron.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe S.R., Alvania R.S., Ramanan N., Wood J.N., Mandai K., Ginty D.D. 2008. Serum response factor mediates NGF-dependent target innervation by embryonic DRG sensory neurons. Neuron. 58:532–545 10.1016/j.neuron.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.C., Scolding N.J., Raine C.S. 2006. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J. Neuroimmunol. 176:162–173 10.1016/j.jneuroim.2006.04.014 [DOI] [PubMed] [Google Scholar]
- Windrem M.S., Nunes M.C., Rashbaum W.K., Schwartz T.H., Goodman R.A., McKhann G., II, Roy N.S., Goldman S.A. 2004. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat. Med. 10:93–97 10.1038/nm974 [DOI] [PubMed] [Google Scholar]
- Winkler S., Stahl R.C., Carey D.J., Bansal R. 2002. Syndecan-3 and perlecan are differentially expressed by progenitors and mature oligodendrocytes and accumulate in the extracellular matrix. J. Neurosci. Res. 69:477–487 10.1002/jnr.10311 [DOI] [PubMed] [Google Scholar]
- Wolswijk G. 1998. Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J. Neurosci. 18:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff R.H., Fruttiger M., Richardson W.D., Franklin R.J.M. 2004. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 25:252–262 10.1016/j.mcn.2003.10.014 [DOI] [PubMed] [Google Scholar]
- Xin M., Yue T., Ma Z., Wu F.F., Gow A., Lu Q.R. 2005. Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice. J. Neurosci. 25:1354–1365 10.1523/JNEUROSCI.3034-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F., Chen Y., Hoang T., Montgomery R.L., Zhao X.H., Bu H., Hu T., Taketo M.M., van Es J.H., Clevers H., et al. 2009. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat. Neurosci. 12:829–838 10.1038/nn.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Argaw A.T., Gurfein B.T., Zameer A., Snyder B.J., Ge C., Lu Q.R., Rowitch D.H., Raine C.S., Brosnan C.F., John G.R. 2009. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc. Natl. Acad. Sci. USA. 106:19162–19167 10.1073/pnas.0902834106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Anderson D.J. 2002. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 109:61–73 10.1016/S0092-8674(02)00677-3 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Wang S., Anderson D.J. 2000. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 25:331–343 10.1016/S0896-6273(00)80898-3 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Choi G., Anderson D.J. 2001. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 31:791–807 10.1016/S0896-6273(01)00414-7 [DOI] [PubMed] [Google Scholar]