Abstract
Context
It has been suggested that the propensity to store fat in the gluteal-femoral region may be cardioprotective.
Objective
The primary aim of this study was to test whether the favorable associations of leg fat with risk factors for cardiovascular disease persist after controlling for the highly unfavorable effects of abdominal (visceral or sc) adiposity in postmenopausal women.
Study Participants
The study included 95 postmenopausal women [age, 60 ± 8 yr (mean ± SD)].
Main Outcomes
Whole-body and regional fat distribution was measured using dual-energy x-ray absorptiometry and abdominal computed tomography. Markers of insulin resistance and dyslipidemia were determined from oral glucose tolerance tests and fasted lipid and lipoprotein measurements, respectively. Primary outcomes were: fasting insulin (INS0), area under the insulin curve (INSAUC), product of the oral glucose tolerance test insulin and glucose AUC (INSAUC —GLUAUC), serum triglycerides (TG), and high-density lipoprotein (HDL) cholesterol.
Results
Controlling for trunk fat revealed a favorable effect of leg fat on INS0, INSAUC, INSAUC × GLUAUC, TG, and HDL. However, after controlling for either visceral or sc abdominal adiposity, TG was the only risk factor for which the favorable effect of leg fat persisted.
Conclusions
The lack of an association between leg fat and most of the risk factors, after adjusting for abdominal visceral or sc fat, suggests an overriding deleterious influence of abdominal adiposity on cardiovascular risk. Nevertheless, our finding that regional adipose tissue depots have apparent independent and opposing effects on serum TG supports the need for further research into the physiological mechanisms governing these effects.
Obesity has long been considered a risk factor for metabolic and cardiovascular disease (CVD), but over the past 20 yr, it has become increasingly apparent that it is abdominal adiposity that is more closely associated with disease risk. The higher prevalence of CVD in men than in women is likely due, in part, to the fact that men tend to accumulate fat in the abdominal region, whereas women tend to store fat in gluteal-femoral regions, at least before the menopause (1). Perhaps not coincidentally, both abdominal fat accumulation and the incidence of CVD accelerate in women after the menopause (2, 3).
The harmful association of abdominal fat, and specifically visceral fat, with disease risk is well-established (4–6). It is less clear whether lower-body fat stores, which are predominant in most women, have independent effects on CVD risk. Because obesity, in general, is linked with increased CVD risk, it may seem likely that lower-body fat would confer either neutral or deleterious effects that are simply less potent than those of abdominal fat. However, in contrast to this, we (7) and others (8–17) have observed favorable associations of lower-extremity fat with common risk factors for CVD. In our previous study of postmenopausal women (7), leg fat was not a consistent simple correlate of insulin resistance or dyslipidemia. However, after statistically controlling for the potent deleterious associations between trunk fat and CVD risk factors, leg fat emerged as being favorably associated with triglycerides (TG), high-density lipoprotein (HDL)-cholesterol, and insulin resistance. Because leg fat is stored primarily in sc depots, we speculated that the apparent protective effect of leg fat could simply be indicative of a propensity to store fat sc in the abdominal region, away from the visceral regions that are more strongly associated with disease risk. If our hypothesis is correct, the favorable effects related to increased leg fat could have reflected a small visceral fat depot within the trunk region. However, we could not evaluate this in our previous study, because the procedure used to measure trunk fat (dual-energy x-ray absorptiometry; DXA) does not discriminate sc and visceral abdominal fat.
Therefore, the aim of the current study was to determine whether our previous finding, that leg fat had favorable associations with risk factors for CVD in postmenopausal women after controlling for the deleterious effects of trunk fat, persists when controlling instead for the effects of abdominal (either visceral or sc) adiposity. We postulated that the strength of the favorable associations of leg fat with CVD risk factors, which we found after controlling for trunk fat, would be diminished when controlling for visceral adiposity. As a secondary aim, we further determined whether the deleterious effects of trunk fat on CVD risk factors were mediated primarily by visceral or sc abdominal adiposity.
Subjects and Methods
Subjects
We measured body composition and select disease risk factors in 95 healthy postmenopausal women [age, 60 ± 8 yr (mean ± SD)]. All women were at least 1 yr past menopause (average time since last menses, 11 ± 9 yr), were not on hormone therapy or glucose- or lipid-lowering drugs, were not current smokers, and did not have diabetes mellitus as assessed by an oral glucose tolerance test (18). The nature, purpose, and risks of the study were explained verbally and in writing to each subject during the consent process. All of the participants provided written informed consent to participate in the study, which was approved by the Colorado Multiple Institutional Review Board.
Body composition
Total and regional (trunk and leg) fat masses were determined by DXA using a Lunar DPX-IQ (Software v4.38; Lunar Corp., Madison, WI). The recommendations of the manufacturer were used to define the trunk and leg regions. Total and regional (visceral and sc) abdominal fat areas were determined by computed tomography (CT) using a General Electric (Waukesha, WI) High Speed CT. Single axial CT images (120 kV, 200–300 mA, and 10-mm slice thickness) were acquired at the level of the L2–L3 and L4–L5 intervertebral spaces. Technicians at the CT Scan Reading Center analyzed all images. Adipose tissue areas were determined using a CT intensity range (−190 to −20 Hounsfield units) from image-generated histograms of adipose and soft tissue regions. The visceral fat areas (square centimeters) were manually outlined by tracing the muscles of the abdominal wall. Fat appearing in the bowel was subtracted from the visceral fat area. The sc fat areas (square centimeters) were calculated by subtracting the visceral and bowel fat areas from the total abdominal fat area. The cylinder volume equation was used to estimate the abdominal visceral and sc fat volumes (cubic centimeters) between the L2–L3 and L4–L5 slices calculated from the measured fat areas, slice thickness, and distance between the two slices. Analysis programs were developed by the University of Colorado CT Reading Center using IDL software (RSI, Inc., Boulder, CO) on a Sparc 20 workstation (Sun Microsystems, Sunnyvale, CA).
Glucose tolerance test
A 75-g oral glucose tolerance test was administered in the morning after an overnight fast. Blood samples were obtained before and 30, 60, 90, and 120 min after glucose ingestion for glucose and insulin determinations. The total areas under the glucose curve (GLUAUC) and insulin curve (INSAUC) were calculated using the trapezoidal rule. The INSAUC and fasted insulin (INS0) were used as indices of hyperinsulinemia, and the product of the insulin and glucose areas (INSAUC × GLUAUC) was calculated as an index of peripheral insulin resistance (19–21).
Hormones and metabolites
Blood samples were stored at −80 C and analyzed, in batch, by the Core Laboratory of the General Clinical Research Center (GCRC). Serum insulin concentrations were determined with a double-antibody RIA (Diagnostic Systems Laboratories, Inc., Webster, TX). Serum glucose was measured using a hexokinase assay on a Cobra Mira Plus instrument (Roche Diagnostic Systems, Indianapolis, IN). Intra- and interassay coefficients of variation were 5.2 and 9.8% for insulin and 1.1 and 3.6% for glucose, respectively.
Blood lipids and lipoproteins
Measurements of serum lipid and lipoprotein concentrations were done by the GCRC Core Laboratory. Total cholesterol, HDL-cholesterol, and TG were measured by automated enzymatic commercial kits on a Cobra Mira Plus instrument (Roche Diagnostic Systems). Intra- and interassay coefficients of variation were as follows: 1) total cholesterol, 5.1 and 2.4%; 2) TG, 1.4 and 3.3%; 3) HDL, 4.5 and 2.9%. Low-density lipoprotein cholesterol was calculated using the Friedewald equation (22).
Statistics
The primary outcome variables (INS0, INSAUC, INSAUC × GLUAUC, TG, HDL) for analysis were chosen a priori based on our previous observations (7). Each of the primary outcome variables and each of the independent predictor variables (leg fat, trunk fat, visceral and sc abdominal fat) was log transformed before analysis to remove skewness. Quantile-quantile plots of each variable were evaluated before and after log transformation to verify that the transformed data were approximately normal. Descriptive data are presented in clinical units as median [interquartile range (IQR)] unless specified otherwise. Pearson correlation coefficients were used to assess the relationships of the primary outcome variables with each candidate predictor. Multiple linear regression models were used to test the hypothesis that lower-body adiposity had an independent and favorable relation with the metabolic risk factors of interest when controlling for trunk or abdominal adiposity. Estimated β-coefficients, their SE values (± SE), and the overall R2 values are presented for each regression model. For illustrative purposes, women were dichotomized by the median visceral fat volume and by the median leg fat mass. All statistical analyses were performed using SPSS for Windows software (v12.0; SPSS, Inc., Chicago, IL). Statistical significance was defined as an α-level of 0.05.
Results
Body composition and metabolic characteristics of the study cohort are presented in Table 1. On average, the women were overweight (body mass index, 28 ± 6 kg/m2) and had a large waist girth (88 ± 11 cm). Pearson correlation analyses indicated that most measures of total and regional adiposity except leg fat were significantly correlated with INS0, INSAUC, INSAUC × GLUAUC, and HDL (Table 2). In contrast, only trunk fat mass and abdominal visceral fat measures were significantly correlated with TG. Abdominal visceral fat volume was the strongest correlate of each risk factor.
TABLE 1.
Body composition and metabolic characteristics of the study cohort (n = 95)
| Variable | Median (IQR) | Variable | Median (IQR) |
|---|---|---|---|
| Body mass (kg) | 75.7 (67.6, 84.7) | Total fat mass (kg) | 32.6 (27.3, 38.3) |
| Trunk fat mass (kg) | 15.8 (13.2, 18.8) | Fasted glucose (mg·dl−1) | 89 (83, 96) |
| Leg fat mass (kg) | 12.5 (10.2, 15.5) | GLUAUC (mg·dl−1·min−1·103) | 16.6 (14.3, 18.7) |
| Arm fat mass (kg) | 3.4 (2.5, 4.4) | Fasted insulin (μU·ml−1) | 7 (5, 10) |
| L2–L3 SF area (cm2) | 255 (191, 311) | INSAUC (μU·ml−1·min−1·103) | 5.3 (3.7, 7.7) |
| L4–L5 SF area (cm2) | 410 (349, 496) | INSAUC × GLUAUC (units·108) | 0.9 (0.5, 1.3) |
| SF volume (cm3) | 2998 (2389, 3469) | Total cholesterol (mg·dl−1) | 205 (186, 229) |
| L2–L3 VF area (cm2) | 108 (71, 158) | HDL-cholesterol (mg·dl−1) | 49 (42, 61) |
| L4–L5 VF area (cm2) | 103 (74, 139) | LDL-cholesterol (mg·dl−1) | 129 (109, 152) |
| VF volume (cm3) | 1013 (753, 1412) | Triglycerides (mg·dl−1) | 120 (79, 150) |
IQR, Interquartile range (25th, 75th percentile); SF, sc fat; VF, visceral fat; LDL, low-density lipoprotein. SI unit conversion factors: glucose (0.0555; mmol/liter), insulin (7.175; pmol/liter), cholesterols (0.0258; mmol/liter), triglycerides (0.0113; mmol/liter).
TABLE 2.
Pearson correlations of measures of body composition and fat distribution with dependent variables
| Primary dependent variables |
|||||
|---|---|---|---|---|---|
| INS0 | INSAUC | INSAUC × GLUAUC | TG | HDL | |
| Total fat mass (kg) | 0.429a | 0.300b | 0.311a | 0.186 | −0.292a |
| Trunk fat mass (kg) | 0.549a | 0.411a | 0.437a | 0.314a | −0.367a |
| Leg fat mass (kg) | 0.201 | 0.108 | 0.099 | −0.014 | −0.118 |
| SF volume (cm3) | 0.360a | 0.229b | 0.225b | 0.127 | −0.261b |
| VF volume (cm3) | 0.582a | 0.422a | 0.461a | 0.445a | −0.432a |
SF, sc fat; VF, visceral fat; GLU, glucose; INS, insulin; AUC, total area under the curve; TG, triglycerides; HDL, high-density lipoprotein cholesterol.
P < 0.01.
P < 0.05.
Linear regression models (Table 3) evaluated the associations of leg fat mass with each of the primary outcomes before (model 1) and after adjusting for trunk fat mass (model 2), abdominal visceral fat volume (model 3), or abdominal sc fat volume (model 4). We first confirmed our previous observation (7) that leg fat had a favorable, independent association with CVD risk factors after adjusting for the unfavorable effects of trunk fat (Table 3, model 2). After controlling for trunk fat, leg fat became significantly and favorably related to each of the risk factors measured, with the exception of HDL (P = 0.06). For example, when regressing INS0 on leg fat without adjustment (model 1), the coefficient was positive (0.34 ± 0.17) and significant (P = 0.05) but with a low R2 (0.04). However, after adjusting for trunk fat, leg fat became significantly (P < 0.01) inversely related (−0.56 ± 0.19), and the R2 increased to 0.36 (model 2). We next determined whether the favorable independent association of leg fat mass with CVD risk factors persisted when controlling for either visceral (model 3) or sc (model 4) abdominal fat volumes, rather than trunk fat mass. After adjusting for the effects of visceral fat, leg fat was favorably associated with TG only (P < 0.05). After adjusting for sc abdominal fat, leg fat was no longer significantly related with any of the risk factors. These findings indicate an overriding effect of fat stored specifically in the abdominal (visceral or sc) regions on INS0, INSAUC, INSAUC × GLUAUC, and HDL that was not apparent when controlling for total trunk fat mass. The one exception was that adjustment for visceral fat did not override the significant (P < 0.05) independent contribution of leg fat (−0.33 ± 0.14) to TG concentrations. The independent and opposite contributions of leg fat mass and visceral fat volume to TG concentrations are illustrated in Fig. 1.
TABLE 3.
Linear regression models for each dependent variable entering DXA leg fat mass (FM) alone (model 1) and after adjustment for trunk FM (model 2), CT abdominal visceral fat volume (VFV, model 3), or sc fat volume (SFV, model 4)
| Dependent variable | Independent variables | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|
| INS0 | Leg FM | 0.34 ± 0.17 (0.05) | −0.56 ± 0.19 (<0.01) | −0.07 ± 0.16 (0.68) | −0.22 ± 0.24 (0.37) |
| Trunk FM | 1.42 ± 0.21 (<0.001) | ||||
| VFV | 0.73 ± 0.11 (<0.001) | ||||
| SFV | 0.77 ± 0.24 (<0.01) | ||||
| R2 | 0.04 | 0.36 | 0.34 | 0.14 | |
| INSAUC | Leg FM | 0.16 ± 0.15 (0.30) | −0.49 ± 0.19 (<0.05) | −0.11 ± 0.15 (0.48) | −0.19 ± 0.22 (0.40) |
| Trunk FM | 1.02 ± 0.20 (<0.001) | ||||
| VFV | 0.48 ± 0.11 (<0.001) | ||||
| SFV | 0.48 ± 0.22 (<0.05) | ||||
| R2 | 0.01 | 0.23 | 0.18 | 0.06 | |
| INSAUC × GLUAUC | Leg FM | −0.02 ± 0.15 (0.89) | −0.68 ± 0.22 (<0.01) | −0.19 ± 0.18 (0.31) | −0.25 ± 0.27 (0.36) |
| Trunk FM | 1.37 ± 0.24 (<0.001) | ||||
| VFV | 0.66 ± 0.13 (<0.001) | ||||
| SFV | 0.59 ± 0.27 (<0.05) | ||||
| R2 | 0.01 | 0.27 | 0.22 | 0.06 | |
| TG | Leg FM | −0.052 ± 0.15 (0.89) | −0.39 ± 0.15 (<0.05) | −0.33 ± 0.14 (<0.05) | −0.32 ± 0.21 (0.13) |
| Trunk FM | 0.51 ± 0.10 (<0.001) | ||||
| VFV | 0.55 ± 0.10 (<0.001) | ||||
| SFV | 0.41 ± 0.21 (0.05) | ||||
| R2 | 0.00 | 0.21 | 0.24 | 0.04 | |
| HDL | Leg FM | −0.10 ± 0.09 (0.26) | 0.21 ± 0.11 (0.06) | 0.06 ± 0.09 (0.52) | 0.13 ± 0.12 (0.30) |
| Trunk FM | −0.48 ± 0.12 (<0.001) | ||||
| VFV | −0.27 ± 0.06 (<0.001) | ||||
| SFV | −0.31 ± 0.12 (0.12) | ||||
| R2 | 0.14 | 0.17 | 0.19 | 0.08 |
Data represent β̂ ± SE β̂ (P value). HDL, High-density lipoprotein cholesterol. Contributions of the dependent variables that remained significant after adjustment are in bold type.
Fig. 1.
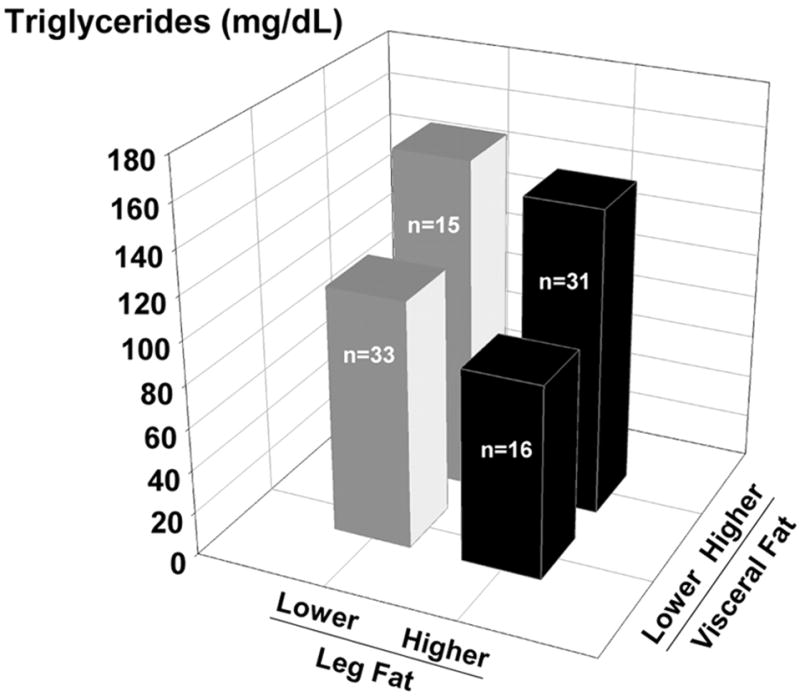
The independent and opposite contributions of lower-body and abdominal visceral adiposity to plasma TG concentrations (Systeme International conversion factor, 0.113) in postmenopausal women dichotomized based on the median values for leg fat mass and visceral fat volume.
We next confirmed that it is specifically fat stored in abdominal regions (i.e. visceral and sc), rather than the entire trunk region, that is most closely linked with metabolic risk factors. Linear regression models (Table 4) evaluated the associations of trunk fat with each of the primary outcomes before (model 1) and after adjustment for abdominal visceral (model 2) or sc (model 3) fat volumes. After adjusting for the effects of abdominal visceral fat, trunk fat was significantly and unfavorably related to INS0 only. However, after adjusting for abdominal sc fat, trunk fat remained significantly and unfavorably associated with all of the risk factors. These results suggest that the relations of trunk fat mass with the outcome variables were independent of abdominal sc adiposity but were not independent of visceral adiposity.
TABLE 4.
Linear regression models for each dependent variable entering DXA trunk fat mass (FM) alone (model 1) and either CT abdominal visceral fat volume (VFV, model 2) or sc fat volume (SFV, model 3) as independent variables
| Dependent variable | Independent variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| INS0 | Trunk FM | 1.01 ± 0.16 (<0.001) | 0.48 ± 0.23 (<0.05) | 1.40 ± 0.27 (<0.001) |
| VFV | 0.47 ± 0.15 (<0.01) | |||
| SFV | −0.45 ± 0.25 (0.08) | |||
| R2 | 0.30 | 0.37 | 0.33 | |
| INSAUC | Trunk FM | 0.66 ± 0.15 (<0.001) | 0.35 ± 0.23 (0.13) | 1.08 ± 0.26 (<0.001) |
| VFV | 0.28 ± 0.15 (0.07) | |||
| SFV | −0.47 ± 0.24 (0.05) | |||
| R2 | 0.17 | 0.20 | 0.20 | |
| INSAUC × GLUAUC | Trunk FM | 0.86 ± 0.18 (<0.001) | 0.41 ± 0.27 (0.13) | 1.49 ± 0.31 (<0.001) |
| VFV | 0.40 ± 0.18 (<0.05) | |||
| SFV | −0.72 ± 0.29 (0.01) | |||
| R2 | 0.19 | 0.23 | 0.24 | |
| TG | Trunk FM | 0.48 ± 0.15 (<0.01) | −0.07 ± 0.22 (0.76) | 0.96 ± 0.26 (<0.001) |
| VFV | 0.49 ± 0.14 (<0.001) | |||
| SFV | −0.55 ± 0.24 (<0.05) | |||
| R2 | 0.10 | 0.20 | 0.15 | |
| HDL | Trunk FM | −0.33 ± 0.09 (<0.001) | −0.09 ± 0.13 (0.48) | −0.41 ± 0.15 (<0.01) |
| VFV | −0.21 ± 0.08 (<0.05) | |||
| SFV | 0.10 ± 0.14 (0.49) | |||
| R2 | 0.14 | 0.19 | 0.14 |
Data represent β̂ ± SEβ̂ (P value). HDL, High-density lipoprotein cholesterol. Contributions of the dependent variables that remained significant after adjustment are in bold type.
Discussion
The results of this study confirmed our previous observation in postmenopausal women (7) that leg fat mass was associated with reduced CVD risk, independent of the increased risk attributable to trunk fat mass. The primary new finding of this study was that the favorable associations of leg fat with CVD risk factors did not persist, with the exception of TG, after adjusting for abdominal visceral adiposity. The relation between trunk fat mass and the risk factors was independent of abdominal sc, but not visceral, fat volume, suggesting that the associations of trunk fat with risk factors were mediated by abdominal visceral adiposity.
There has been debate over which adipose tissue regions confer the greatest increase in morbidity and mortality. Early evidence that upper-body adiposity conferred more risk than overall adiposity or lower-body adiposity came from epidemiological studies that compared waist-to-hip circumference ratio, or simply waist girth, and body mass index (6, 23). In such studies, waist-to-hip circumference ratio and waist size emerged as superior indices of disease risk compared with body mass index. The development of DXA to measure body composition allowed for a regional separation of body fat into trunk and appendicular (arm and leg) fat, and trunk fat emerged as a better correlate of disease risk compared with appendicular or whole-body adiposity (7). Furthermore, delineation of central abdominal adiposity by DXA (i.e. the L2–L4 region within the trunk) (24) and delineation of visceral and sc adiposity by CT or magnetic resonance imaging revealed central abdominal or visceral adiposity as the best correlate of disease risk (13, 24, 25). Evidence from body composition studies suggests a hierarchy among regional fat depots and their relation to disease risk such that lower-body or appendicular adiposity appears less harmful, and upper-body or abdominal (particularly visceral) adiposity appears more harmful.
There is some evidence that lower-body adiposity is actually protective against disease risk, rather than simply less harmful. Previous studies demonstrated inverse correlations of thigh or hip girth with select CVD risk factors (8, 9, 12), and reduced risk for ischemic heart disease (10) and type 2 diabetes mellitus (26). Appendicular skinfold thicknesses were also found to have an inverse relation with CVD risk (11). We (7) and others (13–17) previously observed an inverse relation between DXA-measured leg fat mass and select risk factors in women. Because the majority of fat in the legs is stored sc (27), we postulated that increased leg fat mass was simply indicative of a propensity to store fat sc and away from the abdominal visceral compartment. Further, we thought it was unlikely that sc fat would confer metabolic protection after controlling for the highly detrimental effects of abdominal visceral adiposity. However, we were unable to evaluate this in our previous study because we did not have measurements of abdominal visceral and sc fat (7).
Therefore, the primary aim of the current study was to determine whether the favorable independent associations of leg fat with CVD risk factors that we observed after controlling for trunk fat (7) persisted after adjusting for abdominal visceral or sc adiposity. We confirmed our previous finding of a favorable association of leg fat mass with risk factors, independent of trunk fat mass. However, leg fat mass was not an independent determinant of any of the risk factors after adjustment for abdominal sc adiposity, and was an independent determinant only of TG after adjustment for abdominal visceral adiposity. This suggests that abdominal fat accumulation, in either sc or visceral regions, is a potent determinant of CVD risk and that the storage of fat in non-abdominal regions does not counter these effects, with the possible exception of serum TG.
The inverse association of leg fat with TG that we observed, independent of trunk fat or visceral adiposity, remains intriguing. Although speculative, there is evidence to suggest that gluteal-femoral adipose tissue may be a fat sequestering storage depot. That is, femoral adipocytes, compared with abdominal adipocytes, have increased insulin sensitivity and increased expression of α-2 adrenergic receptors (28–30), which would act to promote storage of TG and inhibit lipolysis (i.e. reduced turnover favoring fat storage). Furthermore, in vivo measures of lipolysis (basal free fatty acid release) indicated a lower lipolytic rate in lower-body, compared with upper-body, adipose tissue (31). If gluteal-femoral adipocytes are less lipolytic and act to sequester TG, this might theoretically contribute to a reduced circulating TG. In contrast, abdominal visceral adipocytes appear to have reduced insulin sensitivity (28) and increased β-adrenergic sensitivity (32), which would potentially attenuate the suppression of lipolysis by insulin and increase the stimulation of lipolysis by catecholamines (i.e. increased turnover favoring fat mobilization). Moreover, free fatty acid release has been shown to be reduced in lower-body obese women, despite greater upper-body adiposity, when compared with nonobese women (31), supporting the possibility that gluteal-femoral fat protects against or counters free fatty acid release from upper-body fat regions.
The current study had limitations that should be noted. First, because we did not measure thigh fat by CT, we do not know whether the favorable association of lower-body adiposity with TG is specifically related to sc or im fat depots. Second, correlations do not imply causality. Thus, it is not known whether an independent increase in leg fat would promote a decrease in TG. However, there is evidence in rodents that removal (lipectomy) of sc fat promotes an increase in TG (33). Third, generalizability of the findings is limited due to the homogeneity of the study cohort. The participants were all healthy postmenopausal women; non-smokers; and not using hormones or lipid-lowering or glucose-lowering medications. It is not known whether the results are applicable to men, to younger adults, or to a less healthy population.
In summary, leg fat mass was favorably associated with serum TG, HDL-cholesterol, and markers of insulin resistance independent of trunk fat mass in healthy postmenopausal women. The lack of an association between leg fat and most of the CVD risk factors, after adjusting for abdominal visceral or sc fat, suggests an overriding deleterious influence of abdominal adiposity on CVD risk. Nevertheless, our finding that regional adipose tissue depots have apparent independent and opposing effects on serum TG supports the need for further research into the physiological mechanisms governing these effects. If leg fat does have a cardioprotective role, selective reduction of fat from this region (i.e. lipectomy) could adversely affect CVD risk.
Acknowledgments
The authors thank Pam Wolfe, M.S., for her statistical support, as well as the staffs of the University of Colorado at Denver and Health Sciences Center GCRC and Energy Balance Core of the Clinical Nutrition Research Unit for their assistance in conducting this study. We also thank the members of their research group for carrying out the day-to-day activities of the project, and the study volunteers for their time and efforts.
This work was supported by the following awards from the National Institutes of Health: R01 AG18198, AG18857, and K01 AG19630 (to R.E.V.P.), T32 AG00279 (to C.M.J.), F32 AG05899 (to W.S.G.), M01 RR00051, and P30 DK48520.
Abbreviations
- AUC
Area under the curve, for glucose (GLUAUC) or insulin (INSAUC)
- CT
computed tomography
- CVD
cardiovascular disease
- DXA
dual-energy x-ray absorptiometry
- GLU
glucose
- HDL
high-density lipoprotein
- INS
insulin
- INS0
fasting insulin
- IQR
interquartile range
- TG
triglyceride(s)
References
- 1.Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, Shimomura I, Tarui S, Matsuzawa Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207–212. [PubMed] [Google Scholar]
- 2.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 3.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 4.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjöström L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J. 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris A, Sothmann M, Hoffman R, Hennes M, Wilson C, Gustafson A, Kissebah A. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110:867–872. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 6.Pouliot MC, Després J-P, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 7.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab. 2002;282:E1023–E1028. doi: 10.1152/ajpendo.00467.2001. [DOI] [PubMed] [Google Scholar]
- 8.Terry RB, Stefanick ML, Haskell WL, Wood PD. Contribution of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism. 1991;40:733–740. doi: 10.1016/0026-0495(91)90093-c. [DOI] [PubMed] [Google Scholar]
- 9.Sjostrom CD, Hakangard AC, Lissner L, Sjostrom L. Body compartment and subcutaneous adipose tissue distribution—risk factor patterns in obese subjects. Obes Res. 1995;3:9–22. doi: 10.1002/j.1550-8528.1995.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 10.Kahn HS, Austin H, Williamson DF, Arensberg D. Simple anthropometric indices associated with ischemic heart disease. J Clin Epidemiol. 1996;49:1017–1024. doi: 10.1016/0895-4356(96)00113-8. [DOI] [PubMed] [Google Scholar]
- 11.Hunter GR, Kekes-Szabo T, Snyder S, Nicholson C, Nyikos I, Berland L. Fat distribution, physical activity, and cardiovascular risk factors. Med Sci Sports Exerc. 1997;29:362–369. doi: 10.1097/00005768-199703000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Seidell JC, Perusse L, Despres J-P, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74:315–321. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 13.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr. 1997;65:855–860. doi: 10.1093/ajcn/65.3.855. [DOI] [PubMed] [Google Scholar]
- 14.Tankó LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 15.Tankó LB, Bagger YZ, Alexandersen P, Christiansen C. Peripheral adiposity and cardiovascular disease. Circulation. 2003;108:e164. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 16.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CDA, Yudkin JS, Heine RJ, Nujpels G, Seidell JC. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: The Hoorn Study. Diabetes Care. 2004;27:372–377. doi: 10.2337/diacare.27.2.372. [DOI] [PubMed] [Google Scholar]
- 17.Okura T, Nakata Y, Yamabuki K, Tanaka K. Regional body composition changes exhibit opposing effects of coronary heart disease risk factors. Arterioscler Thromb Vasc Biol. 2004;24:923–929. doi: 10.1161/01.ATV.0000125702.26272.f6. [DOI] [PubMed] [Google Scholar]
- 18.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 19.Levine R, Haft DE. Carbohydrate homeostasis. I. N Engl J Med. 1970;283:175–183. doi: 10.1056/NEJM197007232830405. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Evans EM, Van Pelt RE, Binder EF, Williams DB, Ehsani AA, Kohrt WM. Effects of HRT and exercise training on insulin action, glucose tolerance, and body composition in older women. J Appl Physiol. 2001;90:2033–2040. doi: 10.1152/jappl.2001.90.6.2033. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Björntorp P. The associations between obesity, adipose tissue distribution and disease. Acta Med Scand. 1988;723(Suppl):121–134. doi: 10.1111/j.0954-6820.1987.tb05935.x. [DOI] [PubMed] [Google Scholar]
- 24.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women. Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 25.Despres J-P. The insulin resistance-dyslipidemic syndrome of visceral obesity: effect on patient’s risk. Obes Res. 1998;6:S8–S17. doi: 10.1002/j.1550-8528.1998.tb00683.x. [DOI] [PubMed] [Google Scholar]
- 26.Freedman DS, Rimm AA. The relation of body fat distribution, as assessed by six girth measurements, to diabetes mellitus in women. Am J Public Health. 1989;79:715–720. doi: 10.2105/ajph.79.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 28.Richelsen B, Pedersen SB, Moller-Pedersen T, Bak JF. Regional differences in triglyceride breakdown in human adipose tissue: effects of catecholamines, insulin, and prostaglandin E2. Metabolism. 1991;40:990–996. doi: 10.1016/0026-0495(91)90078-b. [DOI] [PubMed] [Google Scholar]
- 29.Richelsen B. Increased α2- but similar β-adrenergic receptor activities in subcutaneous gluteal adipocytes from females compared with males. Eur J Clin Invest. 1986;16:302–309. doi: 10.1111/j.1365-2362.1986.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 30.Mauriège P, Galitzky J, Berlan M, Lafontan M. Heterogeneous distribution of β and α-2 adrenoceptor binding sites in human fat cells from various fat deposits: functional consequences. Eur J Clin Invest. 1987;17:156–165. doi: 10.1111/j.1365-2362.1987.tb02395.x. [DOI] [PubMed] [Google Scholar]
- 31.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest. 1991;88:609–613. doi: 10.1172/JCI115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wahrenberg H, Lönnqvist F, Arner P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J Clin Invest. 1989;84:458–467. doi: 10.1172/JCI114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber RV, Buckley MC, Fried SK, Kral JG. Subcutaneous lipectomy causes a metabolic syndrome in hamsters. Am J Physiol Regul Integr Comp Physiol. 2000;279:R936–R943. doi: 10.1152/ajpregu.2000.279.3.R936. [DOI] [PubMed] [Google Scholar]


