Abstract
To test the hypothesis that estrogens alter insulin action, we evaluated the effects of intravenous conjugated estrogens (CE) on insulin-stimulated steady-state glucose infusion rate (SSGIR) and suppression of plasma glycerol in postmenopausal women (mean ± SD; 56 ± 4 yr; n = 12) not using hormone replacement. SSGIR and glycerol were measured during a two-stage (8 and 40 mU · m−2· min−1) hyperinsulinemic euglycemic clamp on 2 days, with or without a 2.5-mg intravenous CE bolus. Serum estradiol concentrations were increased ~200% on the estrogen (EST) compared with the control (CON) days. Serum insulin was reduced (P < 0.01) during stage 2 of the clamp for EST (63.3 ± 12.8 μU/ml) vs. CON (78.2 ± 15.8 μU/ml). Mean SSGIR and plasma glycerol did not differ between CON and EST days. With adjustment for differences in insulin concentration between conditions, stage 2 glucose disposals were significantly higher (8.63 vs. 7.20 mg · kg−1 · min−1) and plasma glycerol concentrations were significantly lower (29.4 vs. 35.0 μmol/l) for EST vs. CON. Our findings suggest that acute CE administration increases insulin clearance and action in postmenopausal women.
Keywords: euglycemic clamp, lipolysis, conjugated estrogens
THE PHYSIOLOGICAL EFFECTS of ovarian hormones on the glucoregulatory or antilipolytic actions of insulin are relatively unknown (35). Studies that have examined insulin action across the menstrual cycle (41) and during normal pregnancy (24) have reported reductions in insulin action when both estrogen and progesterone are elevated. However, these studies cannot separate out the independent effects of estrogen and progesterone, which may have opposing effects on insulin action. Consistent with this possibility, insulin action is impaired after ovariectomy in rats and nonhuman primates and is restored after estrogen, but not progesterone, replacement (5, 6, 31, 47). Furthermore, the plasma concentration of estradiol may determine whether insulin action is improved or impaired (21, 22).
In postmenopausal women, exogenous hormone replacement has been associated with improved insulin action (10, 16, 18, 26, 38), although this is not a uniform finding (15, 29, 42). Discordant results among clinical studies may be due to variations in hormone treatment (e.g., route of administration, opposition by progestins) or to confounding lifestyle factors (e.g., physical activity, adiposity). The latter possibility is important, because accumulation of adipose tissue after menopause, particularly in the abdominal region, is accelerated and associated with increased risk for glucose intolerance, insulin resistance, and development of type 2 diabetes mellitus (DM) (2, 13, 40, 46). Clinical trials have demonstrated that hormone replacement therapy attenuates age-related increases in abdominal adiposity (i.e., reduced waist size and trunk fat) in postmenopausal women (17, 19, 27). Whether estrogens have an effect on insulin action that is independent of changes in adiposity is unclear. Recent evidence suggests that hormone replacement therapy may reduce the incidence of diabetes by as much as 35% in postmenopausal women (27). Thus elucidating the physiological interactions between sex hormones and insulin action should lead to a better understanding of the progressive risk for type 2 DM in older women.
The purpose of this study was to determine whether whole body insulin action (stimulation of glucose uptake and suppression of lipolysis) is acutely altered by estrogen administration in postmenopausal women. Accordingly, we evaluated glucose infusion rates (GIR) and plasma glycerol concentrations in postmenopausal women during a two-stage hyperinsulinemic euglycemic clamp procedure on two separate occasions, with and without a 2.5-mg intravenous bolus of conjugated estrogens (CE). We hypothesized that raising plasma estradiol to premenopausal midluteal concentrations would acutely improve insulin action in postmenopausal women.
METHODS
Subjects
Twelve healthy, postmenopausal women (56 ± 4 yr) not on hormone replacement therapy were studied. Postmenopausal status was defined as cessation of menses for ≥1 yr or hysterectomy with FSH >30 IU/ml. Before study enrollment, each woman provided informed consent to participate according to the Colorado Multiple Institutional Review Board. Women were excluded from the study if they had a history of hormone-sensitive cancer, fasting plasma glucose >125 mg/dl, uncontrolled hypertension (resting systolic blood pressure >150 mmHg or diastolic >90 mmHg), thyroid dysfunction (TSH <0.5 or >5.0 μU/ml), hypertriglyceridemia (fasting triglycerides >400 mg/dl), or abnormal liver function.
Body composition assessment
Total and regional body composition (fat mass and fat-free mass) were determined by dual-energy X-ray absorptiometry using a Lunar DPXIQ (Software v. 4.38; Lunar, Madison, WI). The recommendation of the manufacturer was used to define the trunk region. Waist circumference was measured as the minimum circumference between the top of the iliac crest and the distal end of the rib cage along the midaxillary line.
Aerobic fitness
Maximal oxygen consumption, as an index of aerobic fitness, was measured during exhaustive treadmill exercise using an on-line, open-circuit spirometry system (VMAX; SensorMedics, Yorba Linda, CA).
Glucose disposal
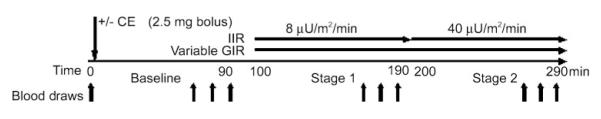
Two-stage (8 and 40 mU· m−2· min−1) hyperinsulinemic euglycemic clamps were administered according to the methods of DeFronzo et al. (12). Figure 1 illustrates the experimental protocol. For all women, clamps were performed at the General Clinical Research Center (GCRC) after a 12-h fast. An intravenous catheter was placed in an antecubital vein for the infusion of insulin and 20% dextrose. A second catheter was placed retrograde to the venous flow in the contralateral hand for blood sampling. The hand was kept in a warming box that was maintained at 60°C to produce arterialized blood samples (34). After 90 min of baseline measurement, insulin was infused at a constant rate of 8 mU· m−2· min−1 for 90 min and then increased to 40 mU· m−2· min−1 for 90 min. Plasma glucose concentrations were measured every 5 min during the insulin infusion by the glucose oxidase method on an automated glucose analyzer (YSI Instruments, Yellow Springs, OH). The dextrose infusion was variably adjusted to maintain plasma glucose at 90 mg/dl. Blood samples were collected at baseline and at 60, 75, and 90 min of each insulin stage for later determination of insulin, estradiol, estrone, catecholamines, free fatty acids (FFA), and glycerol. Whole body GIR were estimated as the steady-state glucose infusion rates (SSGIR) adjusted for fluctuations in plasma glucose concentrations during the final 30 min of each insulin infusion. The clamp procedure was performed on two occasions (in random order) in each woman, with (EST) and without (CON) a 2.5-mg intravenous bolus of CE administered at baseline. Pilot testing revealed that this dose of CE maintained estradiol levels in the premenopausal, midluteal range throughout the duration of the clamp procedure. Five of the women were randomized to receive estrogen at their first study visit, whereas the remaining seven women were randomized to estrogen at their second visit. An average of 4 ± 2 wk separated the 2 testing days.
Fig. 1.
Two-stage hyperinsulinemic euglycemic clamp procedure. CE, conjugated estrogens; IIR, insulin infusion rate; GIR, glucose infusion rate. Blood samples were obtained at time 0, and at 60, 75, and 90 min during baseline, stage 1, and stage 2.
Hormones and metabolites
Blood samples were stored at −80°C and analyzed in batches by the Core Laboratory of the GCRC. Serum insulin concentrations were determined with a double-antibody radioimmunoassay (RIA; Pharmacia Upjohn, Kalamazoo, MI). Plasma FFA (Wako Chemicals, Richmond, VA) and glycerol (R-Biopharm, Marshall, MI) were determined enzymatically. Estradiol and estrone were determined by double-antibody RIA (DPC, Los Angeles, CA and DSL, Webster, TX, respectively). Plasma epinephrine (Epi) and norepinephrine (NE) concentrations were determined by HPLC (Dionex DX-500; in-house mobile phase). Intra- and interassay coefficients of variation were as follows: 1) insulin, 5.2 and 9%; 2) FFA, 1.1 and 4.7%; 3) glycerol, 3.4 and 17.3%; 4) estradiol, 6.0 and 11.2%; 5) estrone, 8.7 and 8.6%; 6) Epi, 5.7 and 8.5%; and 7) NE, 4.1 and 7.7%.
Statistics
A two-way repeated-measures ANOVA was used to evaluate changes in primary outcome variables across the two insulin clamp stages and between the EST and the CON days. Tukey post hoc comparisons were performed when a significant day × stage interaction was observed. Main effects were considered when interaction effects were not significant. Mixed-effects models were also used to examine the effects of acute intravenous administration of CE on SSGIR and glycerol concentrations after adjustments for serum insulin concentrations observed at stages 1 and 2. Random coefficients for the intercept and slopes were included in the models to allow for subject-specific effects. When significant day × insulin interactions were observed, comparisons between the EST and CON days were performed using least square means at the average serum insulin concentrations observed across the 2 days during stage 1 (15.6 μU/ml) and stage 2 (70.8 μU/ml). The main effect of EST was considered when the interaction term was not statistically significant. Statistical significance was set at α= 0.05. Results are presented as means ± SD unless specified otherwise. All statistical analyses were performed using SAS software (SAS Institute, Cary, NC).
RESULTS
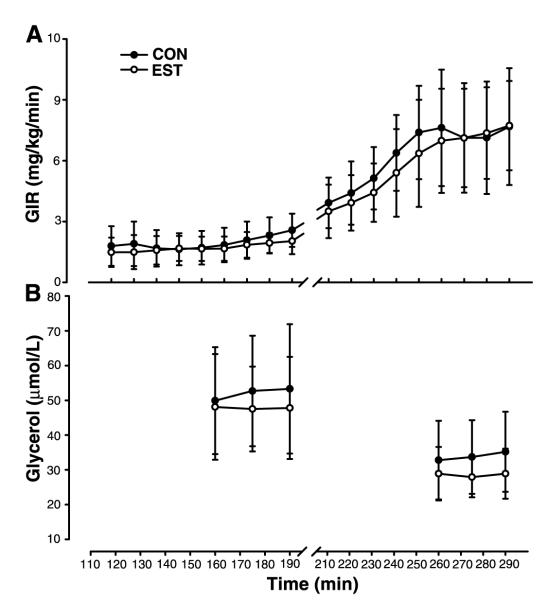
Subject characteristics and body composition are presented in Table 1. Results for the hyperinsulinemic euglycemic clamp are presented in Table 2. Mean serum estradiol and estrone concentrations were significantly (P < 0.001) increased ~200–300% on the EST day compared with the CON day. Mean plasma glucose concentrations were successfully clamped at ~90 mg/dl during the final 30 min of each insulin stage. Figure 2 illustrates the average GIR (Fig. 2A) and end-stage glycerol concentrations (Fig. 2B) during stage 1 (100–190 min; 8 mU· m−2· min−1) and stage 2 (200–290 min; 40 mU· m−2· min−1) of the hyperinsulinemic euglycemic clamp. SSGIR and glycerol concentrations, during the final 30 min of each stage, did not differ between the EST and CON days.
Table 1.
Subject characteristics (n = 12)
| Mean ± SD | Range | |
|---|---|---|
| Age, yr | 56±4 | 50–63 |
| Years postmenopausal | 9±6 | 1–16 |
| O2max, ml·kg−1 ·min−1 | 23.5±4.1 | 17.4–29.8 |
| Total cholesterol, mg/dl | 200±36 | 124–256 |
| HDL-cholesterol, mg/dl | 58±14 | 38–82 |
| LDL-cholesterol, mg/dl | 122±28 | 74–171 |
| Triglycerides, mg/dl | 108±50 | 56–201 |
| Body mass, kg | 72.1±15.1 | 54.3–100.9 |
| BMI, kg/m2 | 27.0±5.0 | 21.5–35.1 |
| Waist girth, cm | 94.9±16.3 | 75.4–118.5 |
| Fat mass, kg | 30.6±11.2 | 15.7–49.0 |
| Fat content (%body mass) | 41.2±7.1 | 28.5–51.9 |
| Fat-free mass, kg | 41.6±4.8 | 36.1–51.8 |
| Trunk fat mass, kg | 15.5±6.5 | 7.3–26.5 |
O2max, maximal oxygen consumption; HDL, high-density lipoprotein; LDL, low-density lipoprotein; BMI, body mass index.
Table 2.
Hyperinsulinemic euglycemic clamp
| Baseline |
Stage 1 |
Stage 2 |
||||
|---|---|---|---|---|---|---|
| CON | EST | CON | EST | CON | EST | |
| Estradiol, pg/ml | 11±7 | 11±7 | 10±5 | 217±79* | 10±4 | 171±59* |
| Estrone, pg/ml | 16±7 | 16±7 | 12±6 | 263±114* | 11±4 | 195±94* |
| Glucose, mg/dl | 90±9 | 91±8 | 88±3 | 90±4 | 90±6 | 90±5 |
| Insulin, μU/ml | 7±3 | 7±3 | 16±4 | 15±4 | 78±16 | 63±12* |
| Epi, pg/ml | 28±11 | 26±8 | 33±16 | 34±17 | 34±20 | 35±16 |
| NE, pg/ml | 195±83 | 217±73 | 222±70 | 252±70 | 238±90 | 232±61 |
| FFA, μeq/l | 765±214 | 717±133 | 210±109 | 192±100 | 116±28 | 116±33 |
| Glycerol, μmol/l | 81±20 | 74±15 | 41±16 | 40±16 | 34±11 | 30±7 |
Values are means ± SD. EST, estrogen day; CON, control day; Epi, epinephrine; NE, norepinephrine; FFA, free fatty acids.
P < 0.01 vs. CON day.
Fig. 2.
Mean (±SD) GIR (A) and glycerol concentrations (B) during stage 1 (100–190 min; 8 mU· m−2 · min−1) and stage 2 (200–290 min; 40 mU· m−2 · min−1) of the hyperinsulinemic euglycemic clamp. Steady-state levels for GIR (SSGIR) and glycerol concentrations at the end of each stage were not significantly different on the estrogen (EST, ○) and control (CON, •) days.
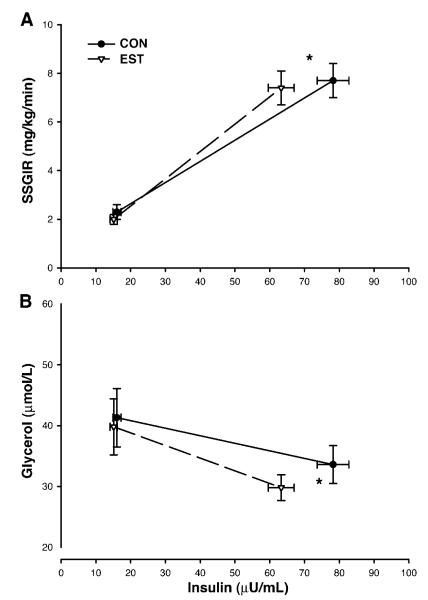
There was a significant (P < 0.01) day × stage interaction for insulin concentrations such that serum insulin concentrations during stage 1 were not significantly different between EST day (15.1 ± 3.6 μU/ml) and CON day (16.1 ± 4.1 μU/ml) but were significantly lower during stage 2 on the EST day (63.3 ± 12.8 μU/ml) compared with the CON day (78.2 ± 15.8 μU/ml). Despite lower serum insulin concentrations on the EST day, mean SSGIR during stages 1 and 2 were not significantly different between EST day (2.01 ± 0.75 and 7.43 ± 2.42 mg· kg−1 · min−1) and CON day (2.29 ± 0.96 and 7.69 ± 2.54 mg· kg−1 · min−1; Fig. 3A) in the ANOVA model. After serum insulin concentrations in the mixed-effects model were controlled for, SSGIR remained nonsignificantly different between the EST day (2.3 ± 0.9 mg· kg−1 · min−1) and CON day (2.2 ± 1.1 mg· kg−1 · min−1) during stage 1 (mean serum insulin 15.6 μU/ml). However, SSGIR was significantly higher (P = 0.01) on the EST day (8.6 ± 2.5 mg· kg−1 · min−1) than on the CON day (7.2 ± 3.5 mg· kg−1 · min−1) during stage 2 (mean serum insulin 70.8 μU/ml).
Fig. 3.
Mean (±SE) insulin-stimulated SSGIR (A) and glycerol concentrations (B) during the final 30 min of each insulin clamp stage on the estrogen (EST, Δ) and control (CON, •) days. Stage 2 serum insulin concentrations were lower on the EST day than on the CON day. After differences in stage 2 insulin concentrations were controlled for, there were significant (*P < 0.05) main effects of estrogen on SSGIR and glycerol.
The day × stage interaction for the suppression of glycerol was not statistically significant (P = 0.27) in the ANOVA model. There was a trend (P = 0.09, 40% statistical power) for a main effect of day (i.e., estrogen) on glycerol concentrations such that plasma glycerol tended to be lower during stages 1 and 2 on the EST day (40 ± 16 and 30 ± 7 μmol/l) compared with the CON day (41 ± 16 and 34 ± 11 μmol/l; Fig. 3B). After serum insulin concentrations in the mixed-effects model were controlled for, glycerol levels were not different between the EST day (40 ± 16 μmol/l) and CON day (41 ± 16 μmol/l) during stage 1 (mean serum insulin 15.6 μU/ml) but were significantly lower (P < 0.05) on the EST day (29 ± 7 μmol/l) than on the CON day (35 ± 11 μmol/l) during stage 2 (mean serum insulin 70.8 μU/ml).
DISCUSSION
The major new finding of this study was that acute intravenous administration of CE resulted in a significant reduction in serum insulin concentrations during a hyperinsulinemic euglycemic clamp procedure; this suggests that whole body insulin clearance was increased. The lower insulin levels on the EST day compared with CON day were not accompanied by proportional reductions in GIR or serum glycerol concentrations, suggesting enhanced insulin action.
Ovarian hormones and carbohydrate metabolism
The role of ovarian hormones in glucose and insulin metabolism is unclear. Reduced insulin sensitivity has been observed during the midluteal, compared with the midfollicular, phase of the menstrual cycle (41) as well as during normal pregnancy (24), suggesting that ovarian hormones have a detrimental effect on carbohydrate metabolism. However, both estrogen and progesterone concentrations are elevated during the luteal phase of the menstrual cycle and during late pregnancy, making it difficult to determine the independent effects of these hormones. In the ovariectomized rat model, glucoregulatory action worsens (i.e., insulin resistance) after ovariectomy and is restored with estrogen, but not progesterone, replacement (5, 31). Similarly, insulin action has been shown to be impaired by ovariectomy in nonhuman primates and restored by estrogen-only replacement, but not by progesterone-only or combined estrogen and progesterone replacement (6, 47). Thus the respective roles of estrogens and progestins on carbohydrate metabolism appear to be opposing. In the current study, we acutely administered estrogens to postmenopausal women to determine whether there are acute, independent effects of estrogens on insulin action.
Estrogen replacement and insulin clearance
Prospective, randomized trials have consistently observed reduced fasting insulin concentrations in women treated with hormone replacement compared with those on placebo (4, 7–9, 16, 18, 20, 45). We (18) and others (4, 10, 32, 36, 45) have observed decreased insulin responses to an oral or intravenous glucose challenge in women treated with hormone replacement. It was not possible in these studies to determine whether the lower insulin levels were due to increased clearance or decreased secretion. However, there is evidence to suggest that estrogens may increase insulin secretion, as C-peptide concentrations increase in response to both oral CE (43) and transdermal estradiol (4, 10). Thus, if the net effect is a reduction in circulating insulin, estrogens must increase insulin clearance to a greater extent than they increase insulin secretion. Cagnacci et al. (4) examined this issue by estimating changes in hepatic insulin clearance from the molar ratio of C-peptide to insulin (fasted and integrated areas during a glucose challenge) in women treated with either transdermal estradiol or oral CE. Treatment with transdermal estradiol resulted in higher C-peptide concentrations but lower fasted and integrated insulin responses, suggesting increased hepatic insulin clearance. An increase in insulin clearance was not observed in response to oral CE. The discordant responses to transdermal and oral estrogen may be related to the first-pass hepatic metabolism effects that occur in response to oral estrogens only. In the present study, we administered CE intravenously, thereby avoiding first-pass hepatic metabolism. Thus effects of intravenous estrogen administration may be more comparable to transdermal than to oral estrogen treatment. Our finding of lower circulating insulin levels in response to the same rate of exogenous insulin infusion on the EST day is consistent with the observation by Cagnacci et al. that hepatic insulin clearance was increased in response to transdermal estrogen therapy.
Previous hormone replacement studies that used the hyperinsulinemic euglycemic clamp technique (15, 29, 38) did not observe significant reductions in either fasted or steady-state insulin concentrations in women with normal fasted insulin levels. However, two of these studies (15, 38) reported trends toward reduced steady-state insulin concentrations after estrogen treatment of ~15 to 17%. Furthermore, the effect of estrogens on insulin clearance measured via the clamp procedure appears to be more robust in hyperinsulinemic women (3, 10). These findings suggest that the effects of estrogens on insulin clearance in normoinsu-linemic women are modest and that larger sample sizes are needed for adequate statistical power. In the present study, we observed an average estrogen-related reduction in steady-state insulin concentration of 18% during the second stage of the insulin clamp in relatively normoinsulinemic postmenopausal women. Compared with previous studies, our improved power to detect a significant effect of estrogen on insulin concentrations was likely due to our large sample size combined with a within-subject design to minimize variability. In addition, unlike previous hormone replacement interventions, we evaluated estrogen effects on insulin clearance by acutely raising estradiol to the range of normal for the midluteal phase of the menstrual cycle, thereby improving our effect size. Although it is not clear whether a reduction in plasma insulin concentration per se has clinical relevance, the induction of hyperinsulinemia in rats (30) and humans (25) has been shown to acutely alter the action of insulin, including reduced suppression of plasma FFA, increased de novo lipogenesis, and reduced glucose disposal (25, 30). Thus it is possible that an increase in insulin clearance that reduces hyperinsulinemia would itself contribute to an improved peripheral insulin action.
Estrogen replacement and peripheral insulin action
Estrogen replacement therapies have been shown to increase (3, 10, 18, 38, 43), not change (15, 29) or decrease (42), peripheral insulin action in postmenopausal women. Inconsistency among studies may be due to widely varying types of hormone treatments (e.g., varying duration of treatment, dose, route of administration, and opposition by progestins) and concurrent changes in body composition. Another source of inconsistency among studies may be variation in the methods used to assess insulin action. The prospective, randomized hormone replacement trials that have measured insulin action using the hyperinsulinemic euglycemic clamp technique have demonstrated that insulin-stimulated glucose uptake is not impaired (15, 38) and may be improved (10, 11, 29) by estrogen replacement. Consistent with these previous studies, we observed improvements in the glucoregulatory action of insulin in response to intravenous estrogen administration. Furthermore, our study extends previous observations by assessing the acute physiological effect of estrogen on insulin while eliminating confounding factors such as the first-pass hepatic effects of oral estrogens, opposition by progestins, and changes over time in body composition or physical activity.
Compared with the evidence for an effect of estrogens on glucoregulatory insulin action, the evidence for an effect on antilipolytic insulin action is more consistent. For example, sex differences in lipolysis have been observed such that basal lipolytic rates ([2H5]-glycerol rate of appearance) are higher in women compared with men matched for relative adiposity (37). Jensen et al. (26) observed higher FFA release ([14C]palmitate rate of appearance) during a pancreatic clamp in postmenopausal women who had stopped hormone replacement compared with those who had used transdermal estrogen for >2 mo. This estrogen-related attenuation of lipolysis was not explained by changes in Epi stimulation of lipolysis. Additionally, O’Sullivan and Ho (38) observed a reduction in plasma FFA during a hyperinsulinemic clamp in postmenopausal women randomized to transdermal estradiol for 12 wk. This reduction in plasma FFA was not observed in those women randomized to oral CE. Taken together, these data suggest that transdermal estrogen administration has an effect on lipid metabolism that is not catecholamine mediated and may be insulin mediated. Consistent with this possibility, we observed an improvement in the antilipolytic action of insulin (i.e., greater suppression of plasma glycerol) in response to intravenous estrogen administration.
Potential mechanisms
Estrogen receptor-deficient mice and humans are glucose intolerant and hyperinsulinemic (23, 44), suggesting an estrogen receptor-mediated effect on insulin action. Additionally, estrogens may improve insulin action through an increase in specific insulin receptor binding or insulin receptor number (39). However, upregulation of insulin receptors in response to estrogen may be tissue and dose dependent (21, 22). There is further evidence to suggest that estradiol may mimic the action of insulin in adipocytes by activating transcription factors (activator protein-1 and cAMP response element protein) that are also targeted by insulin (14). If estrogens increase adipose tissue insulin sensitivity, particularly in visceral adipose depots drained by the portal circulation, this would be predicted to decrease hepatic FFA flux and increase insulin clearance (1). In the present study, there was no effect of CE on insulin concentrations during the first, low-dose insulin stage as was observed during the second, higher-dose insulin stage. Although the stage-related differences may have been due to a threshold effect (i.e., less insulin to clear), it is also possible that the effects of estrogens on insulin concentrations are indirect and/or time dependent (e.g., through an effect on FFA or upregulation of estrogen receptors). Furthermore, if estrogens increase insulin secretion as well as clearance, the net effect on serum insulin concentration may depend on both the level and the duration of hyperinsulinemia.
Limitations
We cannot rule out the possibility that estrogens had an effect on hepatic glucose production. A study of postmenopausal women with type 2 DM suggested that estrogens might preferentially improve hepatic insulin sensitivity (i.e., more effective suppression of hepatic glucose production) (3). Because we did not assess hepatic glucose production, we do not know whether the SSGIR rates in this study accurately reflected total glucose disposal rates. However, previous research has shown complete suppression of hepatic glucose output between 60 and 120 min, depending on the rate of insulin infusion (28). On the basis of these data, hepatic glucose production should have been nearly completely suppressed during the final 30 min of our first stage, when insulin infusion was low. During the final 30 min of the second stage, when insulin infusion was higher and cumulative insulin exposure had exceeded 150 min, hepatic glucose output should have been completely suppressed, thereby increasing our confidence that SSGIR accurately reflected peripheral glucose disposal.
There is evidence that dose, route, and duration of estrogen administration are important determinants of the effects of hormone replacement therapy on insulin action (10, 32, 33). Our findings may therefore be specific to the intravenous administration of a single 2.5-mg dose of CE that raised plasma estradiol into the premenopausal, midluteal range. Furthermore, we cannot rule out the possibility that estrone or other estrogen metabolites contributed independently or synergistically to the estradiol-related effects on insulin. To our knowledge, this is the first study to measure changes in insulin action in response to acute intravenous estrogen administration.
In summary, acute intravenous administration of CE reduced serum insulin concentrations during a hyperinsulinemic euglycemic clamp, suggesting an increased insulin clearance. After adjustment for the reduced serum insulin concentrations, CE were associated with increased glucose disposal and decreased glycerol concentrations, suggesting enhanced peripheral insulin action. Recent evidence that hormone replacement therapy may reduce the incidence of diabetes by as much as 35% in postmenopausal women (27) highlights the need for future studies to elucidate the mechanisms by which estrogens influence insulin secretion, clearance, and sensitivity.
Acknowledgments
We thank Lorri Ogden (GCRC) for statistical support.
DISCLOSURE This research was supported by the following awards from the National Institutes of Health: R01 AG-18198 (W. M. Kohrt), K01 AG-19630 (R. E. Van Pelt), F32 AG-05899 (W. S. Gozansky), GCRC M01 RR-00051, and DK-48520.
REFERENCES
- 1.Bergman RN, Van Citters GW, Mittelman SD, Dea MK, Hamilton-Wessler M, Kim SP, Ellmerer M. Central role of the adipocyte in the metabolic syndrome. J Investig Med. 2001;49:119–126. doi: 10.2310/6650.2001.34108. [DOI] [PubMed] [Google Scholar]
- 2.Björntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition. 1997;13:795–803. doi: 10.1016/s0899-9007(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 3.Brussaard HE, Leuven JA Gevers, Frolich M, Kluft C, Krans HM. Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia. 1997;40:843–849. doi: 10.1007/s001250050758. [DOI] [PubMed] [Google Scholar]
- 4.Cagnacci A, Soldani R, Carriero PL, Paoletti AM, Fioretti P, Melis GB. Effects of low doses of transdermal 17-β estradiol on carbohydrate metabolism in postmenopausal women. J Clin Endocrinol Metab. 1992;74:1396–1400. doi: 10.1210/jcem.74.6.1317387. [DOI] [PubMed] [Google Scholar]
- 5.Campbell SE, Febbraio MA. Effect of the ovarian hormones on GLUT4 expression and contraction-stimulated glucose uptake. Am J Physiol Endocrinol Metab. 2002;282:E1139–E1146. doi: 10.1152/ajpendo.00184.2001. [DOI] [PubMed] [Google Scholar]
- 6.Cefalu WT, Wagner JD, Bell-Farrow AD, Wang ZQ, Adams MR, Toffolo G, Cobelli C. The effects of hormonal replacement therapy on insulin sensitivity in surgically postmenopausal cynomolgus monkeys (Macaca fascicularis) Am J Obstet Gynecol. 1994;171:440–445. doi: 10.1016/0002-9378(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 7.Cheung AP. Acute effects of estradiol and progesterone on insulin, lipids and lipoproteins in postmenopausal women: a pilot study. Maturitas. 2000;35:45–50. doi: 10.1016/s0378-5122(00)00091-8. [DOI] [PubMed] [Google Scholar]
- 8.Colacurci N, Zarcone R, Mollo A, Russo G, Passaro M, de Seta L, de Franciscis P. Effects of hormone replacement therapy on glucose metabolism. Panminerva Med. 1998;40:18–21. [PubMed] [Google Scholar]
- 9.Crook D, Godsland IF, Hull J, Stevenson JC. Hormone replacement therapy with dydrogesterone and 17 beta-oestradiol: effects on serum lipoproteins and glucose tolerance during 24 month follow-up. Br J Obstet Gynaecol. 1997;104:298–304. doi: 10.1111/j.1471-0528.1997.tb11457.x. [DOI] [PubMed] [Google Scholar]
- 10.Cucinelli F, Paparella P, Soranna L, Barini A, Cinque B, Mancuso S, Lanzone A. Differential effect of transdermal estrogen plus progestagen replacement therapy on insulin metabolism in postmenopausal women: relation to their insulinemic secretion. Eur J Endocrinol. 1999;140:215–223. doi: 10.1530/eje.0.1400215. [DOI] [PubMed] [Google Scholar]
- 11.Cucinelli F, Soranna L, Romualdi D, Musj G, Mancuso S, Lanzone A. The effect of raloxifene on glyco-insulinemic homeostasis in healthy postmenopausal women: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2002;87:4186–4192. doi: 10.1210/jc.2001-011302. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 13.Després JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–459. [PubMed] [Google Scholar]
- 14.Dos Santos EG, Dieudonne MN, Pecquery R, Le M, Giudicelli VY, Lacasa D. Rapid nongenomic E2 effects on p42/p44 MAPK, activator protein-1, and cAMP response element binding protein in rat white adipocytes. Endocrinology. 2002;143:930–940. doi: 10.1210/endo.143.3.8678. [DOI] [PubMed] [Google Scholar]
- 15.Duncan AC, Lyall H, Roberts RN, Petrie JR, Perera MJ, Monaghan S, Hart DM, Connell JM, Lumsden MA. The effect of estradiol and a combined estradiol/progestagen preparation on insulin sensitivity in healthy postmenopausal women. J Clin Endocrinol Metab. 1999;84:2402–2407. doi: 10.1210/jcem.84.7.5836. [DOI] [PubMed] [Google Scholar]
- 16.Espeland MA, Hogan PE, Fineberg SE, Howard G, Schrott H, Waclawiw MA, Bush TL. Effect of postmenopausal hormone therapy on glucose and insulin concentrations. PEPI investigators Postmenopausal Estrogen/Progestin Interventions. Diabetes Care. 1998;21:1589–1595. doi: 10.2337/diacare.21.10.1589. [DOI] [PubMed] [Google Scholar]
- 17.Espeland MA, Stefanick ML, Kritz-Silverstein D, Fineberg SE, Waclawiw MA, James MK, Greenfield M. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. J Clin Endocrinol Metab. 1997;82:1549–1556. doi: 10.1210/jcem.82.5.3925. [DOI] [PubMed] [Google Scholar]
- 18.Evans EM, Van Pelt RE, Binder EF, Williams DB, Ehsani AA, Kohrt WM. Effects of HRT and exercise training on insulin action, glucose tolerance, and body composition in older women. J Appl Physiol. 2001;90:2033–2040. doi: 10.1152/jappl.2001.90.6.2033. [DOI] [PubMed] [Google Scholar]
- 19.Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani AR. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 1997;82:414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- 20.Godsland IF, Gangar K, Walton C, Cust MP, Whitehead MI, Wynn V, Stevenson JC. Insulin resistance, secretion, and elimination in postmenopausal women receiving oral or transdermal hormone replacement therapy. Metabolism. 1993;42:846–853. doi: 10.1016/0026-0495(93)90058-v. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez C, Alonso A, Grueso NA, Diaz F, Esteban MM, Fernandez S, Patterson AM. Effect of treatment with different doses of 17-beta-estradiol on insulin receptor sub-strate-1. J Physiol. 2001;2:140–149. [PubMed] [Google Scholar]
- 22.Gonzalez C, Alonso A, Grueso NA, Esteban MM, Fernandez S, Patterson AM. Effect of treatment with different doses of 17-beta-estradiol on the insulin receptor. Life Sci. 2002;70:1621–1630. doi: 10.1016/s0024-3205(02)01489-3. [DOI] [PubMed] [Google Scholar]
- 23.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollingsworth DR. Alterations of maternal metabolism in normal and diabetic pregnancies: differences in insulin-dependent, non-insulin-dependent, and gestational diabetes. Am J Obstet Gynecol. 1983;146:417–429. doi: 10.1016/0002-9378(83)90822-0. [DOI] [PubMed] [Google Scholar]
- 25.Iozzo P, Pratipanawatr T, Pijl H, Vogt C, Kumar V, Pipek R, Matsuda M, Mandarino LJ, Cusi KJ, DeFronzo RA. Physiological hyperinsulinemia impairs insulin-stimulated glycogen synthase activity and glycogen synthesis. Am J Physiol Endocrinol Metab. 2001;280:E712–E719. doi: 10.1152/ajpendo.2001.280.5.E712. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MD, Martin ML, Cryer PE, Roust LR. Effects of estrogen on free fatty acid metabolism in humans. Am J Physiol Endocrinol Metab. 1994;266:E914–E920. doi: 10.1152/ajpendo.1994.266.6.E914. [DOI] [PubMed] [Google Scholar]
- 27.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E. Glycemic effects of postmenopausal hormone therapy: the Heart and Estrogen/progestin Replacement Study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 28.Katz H, Butler P, Homan M, Zerman A, Caumo A, Cobelli C, Rizza RA. Hepatic and extrahepatic insulin action in humans: measurement in the absence of non-steady-state error. Am J Physiol Endocrinol Metab. 1993;264:E561–E566. doi: 10.1152/ajpendo.1993.264.4.E561. [DOI] [PubMed] [Google Scholar]
- 29.Kimmerle R, Heinemann L, Heise T, Bender R, Weyer C, Hirschberger S, Berger M. Influence of continuous combined estradiol-norethisterone acetate preparations on insulin sensitivity in postmenopausal nondiabetic women. Menopause. 1999;6:36–42. [PubMed] [Google Scholar]
- 30.Koopmans SJ, Kushwaha RS, DeFronzo RA. Chronic physiologic hyperinsulinemia impairs suppression of plasma free fatty acids and increases do novo lipogenesis but does not cause dyslipidemia in conscious normal rats. Metabolism. 1999;48:330–337. doi: 10.1016/s0026-0495(99)90081-1. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai S, Holmang A, Bjorntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand. 1993;149:91–97. doi: 10.1111/j.1748-1716.1993.tb09596.x. [DOI] [PubMed] [Google Scholar]
- 32.Lindheim SR, Duffy DM, Kojima T, Vijod MA, Stanczyk FZ, Lobo RA. The route of administration influences the effect of estrogen on insulin sensitivity in postmenopausal women. Fertil Steril. 1994;62:1176–1180. doi: 10.1016/s0015-0282(16)57181-7. [DOI] [PubMed] [Google Scholar]
- 33.Lindheim SR, Presser SC, Ditkoff EC, Vijod MA, Stanczyk FZ, Lobo RA. A possible bimodal effect of estrogen on insulin sensitivity in postmenopausal women and the attenuating effect of added progestin. Fertil Steril. 1993;60:664–667. doi: 10.1016/s0015-0282(16)56218-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu D, Moberg E, Kollind M, Lins PE, Adamson U, MacDonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35:287–290. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- 35.Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci (Colch) 2002;102:151–166. doi: 10.1042/cs1020151. [DOI] [PubMed] [Google Scholar]
- 36.Lobo RA, Pickar JH, Wild RA, Walsh BW, Hirvonen E. Metabolic impact of adding medroxyprogesterone acetate to conjugated estrogen therapy in postmenopausal women. Obstet Gynecol. 1994;84:987–995. [PubMed] [Google Scholar]
- 37.Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab. 2001;281:E1333–E1339. doi: 10.1152/ajpendo.2001.281.6.E1333. [DOI] [PubMed] [Google Scholar]
- 38.O’Sullivan AJ, Ho KKY. A comparison of the effects of oral and transdermal estrogen replacement on insulin sensitivity in postmenopausal women. J Clin Endocrinol Metab. 1995;80:1783–1788. doi: 10.1210/jcem.80.6.7775623. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen SB, Borglum JD, Moller-Pedersen T, Richelsen B. Effects of in vivo estrogen treatment on adipose tissue metabolism and nuclear estrogen receptor binding in isolated rat adipocytes. Mol Cell Endocrinol. 1992;85:13–19. doi: 10.1016/0303-7207(92)90120-u. [DOI] [PubMed] [Google Scholar]
- 40.Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: a controlled longitudinal study. Ann Intern Med. 1995;123:673–675. doi: 10.7326/0003-4819-123-9-199511010-00005. [DOI] [PubMed] [Google Scholar]
- 41.Pulido JME, Salazar MA. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30:19–22. doi: 10.1016/s0188-0128(98)00008-6. [DOI] [PubMed] [Google Scholar]
- 42.Ryan AS, Nicklas BJ, Berman DM. Hormone replacement therapy, insulin sensitivity, and abdominal obesity in postmenopausal women. Diabetes Care. 2002;25:127–133. doi: 10.2337/diacare.25.1.127. [DOI] [PubMed] [Google Scholar]
- 43.Saglam K, Polat Z, Yilmaz MI, Gulec M, Akinci SB. Effects of postmenopausal hormone replacement therapy on insulin resistance. Endocrine. 2002;18:211–214. doi: 10.1385/ENDO:18:3:211. [DOI] [PubMed] [Google Scholar]
- 44.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 45.Spencer CP, Godsland IF, Cooper AJ, Ross D, Whitehead M, Stevenson JC. Effects of oral and transdermal 17betaestradiol with cyclical oral norethindrone acetate on insulin sensitivity, secretion, and elimination in postmenopausal women. Metabolism. 2000;49:742–747. doi: 10.1053/meta.2000.6238. [DOI] [PubMed] [Google Scholar]
- 46.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Waist circumference vs. body mass index for prediction of disease risk in postmenopausal women. Int J Obes Relat Metab Disord. 2001;25:1183–1188. doi: 10.1038/sj.ijo.0801640. [DOI] [PubMed] [Google Scholar]
- 47.Wagner JD, Thomas MJ, Williams JK, Zhang L, Greaves KA, Cefalu WT. Insulin sensitivity and cardiovascular risk factors in ovariectomized monkeys with estradiol alone or combined with nomegestrol acetate. J Clin Endocrinol Metab. 1998;83:896–901. doi: 10.1210/jcem.83.3.4628. [DOI] [PubMed] [Google Scholar]