Abstract
The success of tissue engineering applications can potentially be dramatically improved with the addition of adjuncts that increase the proliferation and differentiation of progenitor or stem cells. Platelet-rich plasma (PRP) has recently emerged as a potential biologic tool to treat acute and chronic tendon disorders. The regenerative potential of PRP is based on the release of growth factors that occurs with platelet rupture. Its autologous nature gives it a significant advantage in tissue engineering applications. To test whether PRP may be useful specifically for cartilage regeneration, a cell culture experiment was devised in which mesenchymal stem cells (MSCs) were grown in control media or media enhanced with inactivated, buffered PRP. Proliferation 7 days after PRP treatment was increased: 1.041 versus 0.199 for the control media cells (p < 0.001). The messenger RNA (mRNA) level of the osteogenic marker RUNX2 was 52.84 versus 26.88 for the control group (p < 0.005). Likewise the mRNA level of the chondrogenic markers Sox-9 and aggrecan was 29.74 versus 2.29 for the control group (p < 0.001) and 21.04 versus 1.93 (p < 0.001), respectively. These results confirm that PRP enhances MSC proliferation and suggest that PRP causes chondrogenic differentiation of MSC in vitro.
Introduction
Platelet-rich plasma (PRP) is an emerging biologic tool in orthopedic surgery and regenerative medicine. It has the significant advantage over other potential therapies in that it is autologous. It has many potential uses in tissue regeneration by inducing proliferation and differentiation of stem cells in vitro, being added to cell-seeded constructs at the time of implantation, and also can be introduced alone to locally induce endogenous regeneration. In general, PRP can be defined as plasma with enriched levels of platelets relative to whole blood. The white blood cell concentration may also be increased. Within platelets are alpha granules that contain powerful growth factors, including transforming growth factor beta and vascular endothelial growth factor. As the concentration of platelets rises, the amount of growth factor increases linearly.9
Initially, PRP has been utilized in fracture repair as a method of improving fusion or union rates based on the pioneering work of Marx et al.18 They used PRP for bone graft augmentation in oral and maxillofacial surgery and found significantly improved fusion rates and bone density in the mandible. However, results using PRP in inducing osteogenesis are mixed. Bone graft enhancement in nonmembranous sites has not been confirmed. In some studies, PRP has been shown to decrease spine fusion rates.7,27 Also, PRP in animal studies inhibits demineralized bone matrix–mediated bone formation.22
PRP may, however, help initiate, enhance, or accelerate soft tissue healing. In a recent pilot investigation Mishra and Pavelko found that PRP may be an alternative to surgery for patients with chronic elbow tendinosis.19 Sanchez et al. also noted that PRP improved the clinical outcome of patients undergoing Achilles tendon repair surgery.23 Several other authors have further suggested that PRP improves wound healing in total knee replacements and may improve healing of rotator cuff tears treated surgically.5,10,12 In vitro investigations have confirmed that PRP enhances proliferation of a variety of human cell types. Anitua et al. noted increase cell growth and increased synthesis of vascular endothelial growth factor in tendon cells when grown in the presence of PRP.1,2 PRP also enhances anabolic gene expression patterns in flexor digitorum superficialis tendons.24 Lucarelli et al. found that 10% PRP induced marked bone marrow cell proliferation.16 Other investigators have found that PRP enhances mesenchymal stem cell (MSC) proliferation.14,25 Finally, PRP also stimulates porcine chondrocyte proliferation and matrix biosynthesis.3
It is important to note that not all PRP is the same. Some combination of calcium and thrombin has been utilized to activate PRP in most studies. In the present investigation, however, the PRP was not activated. This formulation was chosen because it has been found to be effective in vivo in a recent human study.19 Further, activation of PRP for most orthopedic applications can be expected to occur by exposing it to the collagen that is abundant in most musculoskeletal tissue. This in vivo activation may occur over time creating a sustained release of growth factors. Finally, elimination of thrombin in PRP formulations is of value because bovine thrombin has been shown to have potentially significant immunologic and bleeding side effects.15 Recombinant thrombin may be an alternative, but would clearly add to the cost of any treatment.
In the present investigation, we studied the proliferation and differentiation of MSCs. Several messenger RNA (mRNA) differentiation markers were selected. RUNX2 is a transcriptional factor that regulates bone and cartilage development. Sox-9 is a master protein that directs progenitor cells along a chondrogenic line, and aggrecan is a significant structural component of articular cartilage. These proteins were chosen to specifically evaluate the MSC for chondrogenic differentiation in the presence or absence of PRP. Skin fibroblasts were initially studied to evaluate cell proliferation in varying concentrations of PRP.
Materials and Methods
The PRP was prepared from whole blood using the Medtronic Magellan device (Medtronic, Minneapolis MN). It was then buffered to physiologic pH using 8.4% sodium bicarbonate. The platelet concentration in the PRP was 700% above baseline. The PRP culture media was then standardized across all experiments at 1 million platelets/mL.
Initial experiments to validate our approach and optimize the PRP concentration were conducted using human skin fibroblasts and CD34-positive cells. The fibroblasts were grown in either D-MEM/F-12 with 10% fetal bovine serum (control) or 90% control media and 10% buffered PRP (PRP media). PRP in concentrations of 0.1%, 1%, and 5% was also evaluated. The PRP was not activated in any way. Control and PRP media wells were initially seeded with 10,000 cells each and incubated at 37°C for 7 days. CD34-positive cells from G-CSF–mobilized peripheral blood from healthy volunteers were also tested in vitro. They were grown in Xvivo15 (Cambrex, East Rutherford, NJ) supplemented with 2-mercaptoethanol, penicillin, and streptomycin. PRP in 1%, 5%, and 20% was then added to this media. Five hundred cells were initially seeded per well and incubated at 37°C for 5 days.
Human MSCs (HMSCs) were then purchased from a commercial source (Cambrex) and cultured in DMEM media with 10% fetal bovine serum, 2% L-glutamine, and 1% penicillin/streptomycin. HMSCs were grown in control media or control media with 10% PRP by volume. Again, the PRP was not activated with thrombin or calcium. Cell proliferation in eight trials was then measured at 7 days by MTT assay (Molecular Probes, Eugene, OR). Briefly, cells were seeded at a density of 5000 cells/well in a 96-well plate and allowed to attach for 4 h. Then PRP was added at the concentration of 10%. Media was changed once, and then at the seventh day, the cells were labeled with MTT and absorbance was read at 570 nm.
mRNA levels for RUNX2, Sox-9, and aggrecan were quantified using real-time RT-PCR and normalized against the housekeeping gene 18s. Expression of these genes has been utilized as a marker of osteogenic and chondrogenic differentiation in the prior literature. RUNX2 is a transcription factor that is required for osteogenic differentiation,29 and is commonly used as a marker of early osteogenic differentiation.17 Sox-9 is a transcription factor required for expression of cartilage matrix proteins and indicative of early chondrogenic differentiation. Aggrecan is a cartilage matrix protein critical for maintaining proteoglycan content and, thus, associated with cartilage matrix synthesis. Both aggrecan and Sox-9 are effective markers of chondrogenic differentiation.21 For RUNX2, PRP was added for 15 min and RNA was isolated immediately. For Sox-9 and aggrecan, the MSCs were grown in chondrogenic media (Cambrex) for 21 days with PRP added for the first 7 days. Real-time quantitative RT-PCR was performed using the ABI prism 7900 detection system (Foster City, CA). The primer and probes of 18S, RUNX2, SOX-9, and aggrecan were obtained from a commercial source (Applied Biosystems, Foster City, CA). Total RNA was extracted using Trizol reagent and used for cDNA synthesis by reverse transcription. cDNA samples were then subjected to PCR analysis. The results were quantified using the relative standard curve method and normalized to the internal control. Statistical evaluation was conducted on the basis of ANOVA and Student's post hoc t-tests.
Results
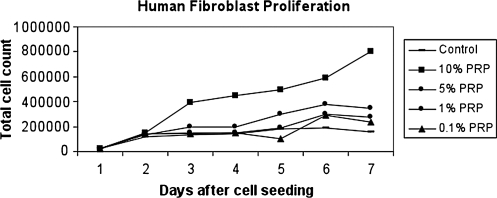
The results for the fibroblasts' dose–response experiment are presented in Figure 1. All wells showed an increase in cell number over the 7 days studied. Further, at each time-point examined, there was a general increase in cell number with increasing concentration of PRP. The 10% PRP concentration had the largest effect on cell proliferation with dramatically higher cell numbers observed than all other concentrations at days 3–7.
FIG. 1.
Daily cell numbers over 7 days as a function of PRP concentration in human fibroblasts.
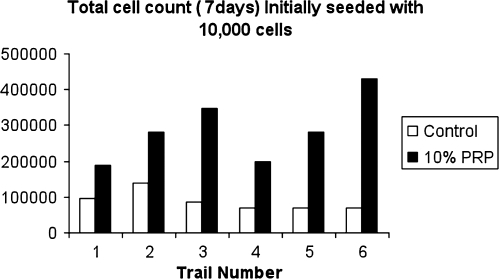
To confirm this observation, the 7-day time-point was repeated six times with the 10% concentration of PRP. The average cell count for the fibroblast experiment in the control group at 7 days was 87,700 compared to 288,000 for the PRP group (Fig. 2). For the CD34-positive cells, the counts at 5 days were 2150 for the 1% PRP (4.3 × baseline), 4329 for the 5% PRP (8.7 × baseline), and 1125 for the 20% PRP (2.3 × baseline). These data show that 20% PRP resulted in significantly less proliferation than 5% PRP. Based on the combination of these two preliminary trials, 10% PRP was chosen for the HMSC experiment.
FIG. 2.
The 7-day time-point was repeated six times with 10% PRP and compared to control. A 3.3-fold increase in cell number was observed.
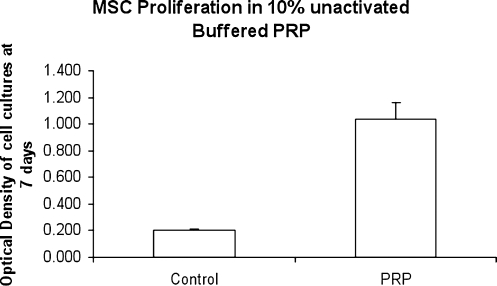
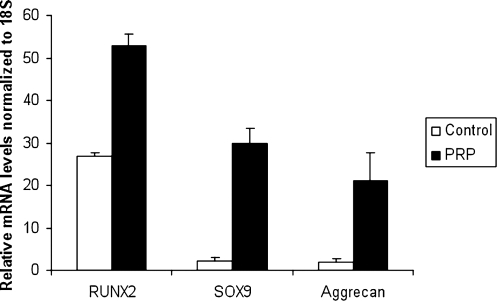
Similar to fibroblasts, PRP had a positive effect on the proliferation of HMSCs. At 7 days in culture, the MTT assay showed a fivefold increase in cellular proliferation for 10% PRP treatment relative to control (Fig. 3). In terms of gene expression, we found that markers of both osteogenic and chondrogenic differentiation were increased (Fig. 4). However, the effect on chondrogenic markers was much more profound (over 10-fold increases) than the osteogenic marker (2-fold increase). Specifically, the RUNX2 mRNA levels relative to 18S for the PRP group were 52.84 versus 26.88 for the control group (p = 0.007). In contrast, the Sox-9 mRNA levels relative to 18S were 29.74 for the PRP group versus 2.29 for the control group (p < 0.001). Likewise, aggrecan mRNA levels relative to 18S for the PRP group were 21.04 versus 1.93 for the control group (p < 0.001).
FIG. 3.
Treatment with 10% inactivated PRP results in a fivefold increase in proliferation after 7 days (p < 0.001).
FIG. 4.
PRP treatment causes an increase in expression of markers of both chondrogenic and osteogenic differentiation by human MSCs.
Discussion
PRP, a fraction of whole blood, contains powerful growth factors that affect changes in a variety of cell types, including tenocytes and MSCs.24 Woodall et al. further noted that PRP initially inhibits macrophage proliferation.28 Interestingly, PRP has also been shown to have antimicrobial effects in vitro.6 When combined with the potential for simple autologous sourcing, these properties give PRP great potential in regenerative medicine and as an adjuvant in tissue engineering.
Clinically, PRP has been shown to decrease pain and increase function in chronic elbow tendinosis patients.19 PRP has also been used in plantar fasciitis, spinal fusion, and in total knee arthroplasty with varying degrees of success.4,5,7,11,27 PRP accelerated wound healing of human skin punch wounds in a recent prospective, controlled study.13 The mechanisms by which PRP may work, however, have not been elucidated. Clearly, there are increased concentrations of growth factors in PRP, and these cytokines should influence the microenvironment where they are placed.8 Interaction between these cytokines and circulating or bone marrow–derived stem cells has been proposed, but not confirmed.
The issue of activation of PRP needs to be carefully considered when evaluating the potential of PRP as a regenerative medicine adjunct. The conventional and most often taught method of using PRP involves addition of calcium and or thrombin to promote the release of the alpha granules from platelets. This process creates a gel or clot that does contain high levels of powerful growth factors. This gel would be ideal for cell-seeded scaffolds; however, it is impossible to deliver this gel without a scaffold via a small gauge needle because of its high viscosity. Moreover, the activation of PRP before application in a musculoskeletal tissue may not be needed or even desired because the abundant collagen within connective tissues serves as a potent activator of PRP.
The PRP in this investigation was also buffered to physiologic pH (7.4). This was done because in prior studies PRP was noted to be quite acidic, 6.9–7.0.19 This is important not only because the acidic version of PRP causes more initial discomfort, but also because the cytokines have different actions at different levels of pH. A recently published study speculated that PRP prepared at an alkaline pH may release additional components with stimulating effects other than or in addition to cell proliferation.26 In future investigations, the pH of the PRP and its activation status need to be reported so that comparison across studies may be possible.
Several studies now support the use of PRP to expand MSCs in vitro. Adding PRP to MSCs and then using the combination to treat bone, ligament, tendon, or cartilage may be valuable. Microfracture surgery for isolated articular cartilage lesions is one specific procedure to consider. Filling of the defect after microfracture has been correlated with clinical outcome.20 If PRP improves filling via increased stem cell proliferation or differentiation, better clinical outcomes would be anticipated. A clinical study using buffered PRP to augment microfracture surgery is presently being designed to test this hypothesis.
The potential for PRP to initiate or enhance soft tissue healing beyond orthopedic applications is also being investigated. Our institution is presently evaluating the use of PRP to enhance cardiac function after myocardial infarction in a murine model. With a better understanding of precisely how PRP works, this work may be expanded to other tissues, including spinal cord, liver, and pancreas.
In this study, we found that 10% buffered inactivated PRP significantly enhances human skin fibroblast and MSC proliferation in vitro. Although they are in vitro experiments, our findings suggest that 10% PRP may be the optimal concentration. mRNA levels of RUNX2, sox-9, and aggrecan were also significantly higher in the PRP-treated cells versus control. This suggests that PRP may selectively encourage MSC differentiation along a chondrogenic line. These in vitro data support the use of PRP in regenerative medicine, tissue engineering, and potentially to augment microfracture surgery.
Acknowledgment
This work was funded in part by NIH Grant AR45989.
Disclosure Statement
No competing financial interests exist.
References
- 1.Anitua E. Andia I. Sanchez M. Azofra J. del Mar Zalduendo M. de la Fuente M. Nurden P. Nurden A.T. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res. 2005;23:281–286. doi: 10.1016/j.orthres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 2.Anitua E. Sanchez M. Nurden A.T. Zalduendo M. de la Fuente M. Azofra J. Andia I. Reciprocal actions of platelet-secreted TGF-beta1 on the production of VEGF and HGF by human tendon cells. Plastic Reconstr Surg. 2007;119:950–959. doi: 10.1097/01.prs.0000255543.43695.1d. [DOI] [PubMed] [Google Scholar]
- 3.Akeda K. An H.S. Okuma M. Attawia M. Miyamota K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14:1272–1280. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Barrett S. Erredge S. Growth factors for chronic plantar fasciitis. Podiatry Today. 2004;17:37–42. [Google Scholar]
- 5.Berghoff W.J. Pietrzak W.S. Rhodes R.D. Platelet-rich plasma application during closure following total knee arthroplasty. Orthopedics. 2006;29:590–598. doi: 10.3928/01477447-20060701-11. [DOI] [PubMed] [Google Scholar]
- 6.Bielecki T.M. Gazdzik T.S. Arendt J. Szczepanski T. Krol W. Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in-vitro study. J Bone Joint Surg Br. 2007;89:417–420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 7.Carreon L. Glassman S.D. Anekstein Y. Puno R.M. Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine. 2005;30:243–246. doi: 10.1097/01.brs.0000160846.85397.44. [DOI] [PubMed] [Google Scholar]
- 8.El-Sharkawy H. Kantarci A. Deady J. Hasturk H. Liu H. Alshahat M. van Dyke T.E. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 9.Eppley B.L. Woodell J.E. Higgins J. Platelet quantification and growth factor analysis from platelet rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 10.Gamradt S.C. Rodeo S.A. Warren R.F. Platelet rich plasma in rotator cuff repair. Tech Orthop Surg. 2007;22:26–33. [Google Scholar]
- 11.Gardner M.J. Demetrakopoulos D. Klepchick P.R. Mooar P.A. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty: an analysis of the hemoglobin, narcotic requirement and range of motion. Int Orthop. 2006;3:304–313. doi: 10.1007/s00264-006-0174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molloy T. Wang Y. Murrell G.A.C. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hom D.B. Linzie B.M. Huang T.C. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174–183. doi: 10.1001/archfaci.9.3.174. [DOI] [PubMed] [Google Scholar]
- 14.Kocaoemer A. Kern S. Klueter H. Bieback K. Human AB-serum as well as thrombin-activated platelet-rich-plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells. Stem Cells. 2007;25:1270–1278. doi: 10.1634/stemcells.2006-0627. [DOI] [PubMed] [Google Scholar]
- 15.Lawson J.H. The clinical use and immunologic impact of thrombin in surgery. Semin Thromb Hemost. 2006;32 Suppl 1:98–110. doi: 10.1055/s-2006-939559. [DOI] [PubMed] [Google Scholar]
- 16.Lucarelli E. Beccheroni A. Donati D. Sangiorgi L. Cenacchi A. del Vento A.M. Meotti C. Bertoja A.Z. Giardino R. Fornasari P.M. Mercuri M. Picci P. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24:3095–3100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- 17.Li Y.J. Batra N.N. You L. Meier S.C. Coe I.A. Yellowley C.E. Jacobs C.R. Oscillatory fluid flow affects human marrow stromal cell proliferation and differentiation. J Orthop Res. 2004;22:1283–1289. doi: 10.1016/j.orthres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Marx R. Carlson E. Eichstaedt R. Schimmele S. Strauss J. Georgeff K. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 19.Mishra A. Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34:1774–1778. doi: 10.1177/0363546506288850. [DOI] [PubMed] [Google Scholar]
- 20.Mithoefer K. Williams R.J. Warren R.F. Potter H.G. Chondral resurfacing of articular cartilage defects in the knee with micro-fracture technique. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 (Pt 2):294–304. doi: 10.2106/JBJS.F.00292. [DOI] [PubMed] [Google Scholar]
- 21.Miyanishi K. Trindade M.C. Lindsey D.P. Beaupre G.S. Carter D.R. Goodman S.B. Shurman D.J. Smith R.L. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vivo. Tissue Eng. 2006;12:1419–1428. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- 22.Ranly D.M. Lohmann C.H. Andreacchio D. Boyan B.D. Schwartz Z. Platelet- rich plasma inhibits demineralized bone matrix-induced bone formation in nude mice. JBJS. 2007;1:139–147. doi: 10.2106/JBJS.F.00388. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez M. Anitua E. Axofra J. Andia I. Padilla S. Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35:245–251. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 24.Schnabel L.V. Mohammed H.O. Miller B.J. McDermott W.G. Jacobson M.S. Santangelo K.S. Fortier L.A. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Ortho Res. 2007;25:230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 25.Vogel J.P. Szalay K. Geiger F. Kramer M. Richter W. Kasten P. Platelet-rich plasma improves expansion of human mesenchymal stem cells and retains differentiation capacity and in vivo bone formation in calcium phosphate. Platelets. 2006;17:462–469. doi: 10.1080/09537100600758867. [DOI] [PubMed] [Google Scholar]
- 26.Wahlstrom O. Linder C. Kalen A. Magnusson P. Variation of pH in lysed platelet concentrates influences proliferation and alkaline phosphatase activity in human osteoblast-like cells. Platelets. 2007;18:113–118. doi: 10.1080/09537100600800537. [DOI] [PubMed] [Google Scholar]
- 27.Weiner B.K. Walker M. Efficacy of autologous growth factors in lumbar intertransverse fusions. Spine. 2003;28:1968–1970. doi: 10.1097/01.BRS.0000083141.02027.48. [DOI] [PubMed] [Google Scholar]
- 28.Woodall J.R. Tucci M. Mishra A. Benghuzzi H. Cellular effects of platelet rich plasma: a study on HL-60 macrophage-like cells. Biomed Sci Instrum. 2007;43:266–271. [PubMed] [Google Scholar]
- 29.Yang X. Karsenty G. Transcription factors in bone: developmental and pathological aspects. Trends Mol Med. 2002;8:340–345. doi: 10.1016/s1471-4914(02)02340-7. [DOI] [PubMed] [Google Scholar]