Abstract
The purpose of this study was to evaluate the effects of alginate composition on the neurotrophic factor release, viability, and proliferation of encapsulated neural stem cells (NSCs), as well as on the mechanical stability of the scaffold itself. Four compositions were tested: a high guluronic acid (68%) and a high mannuronic acid (54%) content alginate, with or without a poly-L-lysine (PLL) coating layer. Enzyme-linked immunosorbent assay was used to quantify the release of brain-derived neurotrophic factor, glial-derived neurotrophic factor, and nerve growth factor from the encapsulated cells. All three factors were detected from encapsulated cells only when a high L-guluronic acid alginate without PLL was used. Additionally, capsules with this composition remained intact more frequently when exposed to solutions of low osmolarity, potentially indicating superior mechanical stability. Alginate beads with a PLL-coated, high D-mannuronic acid composition were the most prone to breakage in the osmotic pressure test, and were too fragile for histology and proliferation assays after 1 week in vitro. NSCs survived and proliferated in the three remaining alginate compositions similarly over the 21-day study course irrespective of scaffold condition. NSC-seeded alginate beads with a high L-guluronic acid, non-PLL-coated composition may be useful in the repair of injured nervous tissue, where the mechanism is the secretion of neuroprotective factors. We verify the neuroprotective effects of medium conditioned by NSC-seeded alginate beads on the serum withdrawal–mediated death of PC-12 cells here.
Introduction
Alginate is a biocompatible hydrogel that has been used to encapsulate many types of cells with the purpose of immunoisolation from the host.1–4 Encapsulation protects graft cells from potential damage caused by an immune response while allowing the secretion of therapeutic agents from the cells into the surrounding host tissue. Small molecules such as glucose, oxygen, and waste products freely pass through the gel matrix. Recent studies have investigated the use of alginate as a neural stem cell (NSC) scaffold.5–7 However, the effect of alginate composition on NSC function has not been investigated.
Administration of stem cells to sites of central nervous system injury may be used as a means of replacing damaged or diseased tissue, where the focus is on directing the differentiation of implanted cells into neurons and subsequent reinnervation of host tissue. This strategy has been used to intervene in numerous sources of central nervous system injury, including Parkinson's disease, stroke, ALS, spinal cord injury, traumatic brain injury, and Huntington's disease.8–10 However, in many studies the degree of functional recovery after NSC transplantation is not explained by the quantity of differentiated graft cells alone, lending credence to a bystander or supporting role of NSCs.9,11,12 Several studies suggest that NSCs have an innate ability to promote neuroprotection and axonal regeneration of host tissue.11,13–16 Potential mechanisms include constitutive secretion of multiple neurotrophic factors,11,14–16 as well as degrading molecules that are inhibitory to axonal growth.13 NSCs derived from various sources have been found to elute nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), and matrix metalloprotease-2, which degrades chondroitin sulfate proteoglycan (a molecule inhibitory to axonal growth).6,13–15 Thus, NSCs and progenitor cells may be exploited as a sort of miniature drug factory, releasing factors that result in the desired healing response in injured nervous tissue. Encapsulation of these cells in alginate may further enhance their therapeutic utility by localizing the cells to the site of injury and isolating them from a host immune response.
Alginate is a biocompatible polysaccharide polymer composed of D-mannuronic (M) and L-guluronic acid (G) residues in varying proportions. Cross-linking and gel formation takes place when divalent cations, such as calcium, ionically bind carboxylic acid groups of blocks of guluronic residues between chains. Alginate has been used widely to encapsulate cells after ground-breaking work published by Lim and Sun in 1980, which demonstrated reversal of diabetes in rats implanted with alginate-encapsulated pancreatic islets for a period of 2–3 weeks.3 Several studies have sought to understand the relationship between alginate composition and the function of the graft.4,17–24 Two common themes emerge in the literature regarding alginate composition and graft performance: the effect of the M/G content of the alginate and the importance of a polycation coating layer. These two variables have been related to gel mechanical stability, viability of encapsulated cells, in vivo biocompatibility, and diffusion through the alginate gel. In terms of mechanical stability, alginates with a high G content are more mechanically stable than those with a high M content.25 However, high G alginate has been shown to initially inhibit the metabolic and secretory activity of cells due to growth inhibition, theoretically because a higher strength gel is more difficult for proliferating cells to displace.17,21 Beads composed of high G alginate are also known to be more porous than high M alginate, thus enhancing diffusion of molecules into and out of the matrix.26 Poly-L-lysine (PLL) coating is commonly employed as a means of strengthening the alginate bead and providing a barrier to immune system components such as IgG.27,28 However, the PLL coating layer may itself cause an unfavorable foreign body response and slight toxicity to encapsulated cells, and its use remains controversial.19,20,22,23,29–31
In light of the extensive research indicating a relationship between alginate composition and encapsulated cell function, as well as the limited amount of data on NSC encapsulation in alginate, the effects of M/G content and PLL coating on entrapped cortical NSCs were investigated. Among the conditions tested, we show that neurotrophic factor release and mechanical stability in response to an osmotic challenge were the most favorable with a high G scaffold without a PLL coating layer. NSCs survived and proliferated in alginate regardless of the compositions tested. Neurotrophic factor release and bioactivity assay data substantiated the use of NSCs encapsulated in alginate to heal injured nervous tissue via a bystander mechanism. These scaffolded cells have therapeutic potential in treating nervous system injuries in future studies, and current work in our lab is investigating their ability to repair a cortical lesion in the adult rat brain.32
Materials and Methods
Materials
Cortical NSCs, nestin antibody, neuronal class III β-tubulin (TUJ-1) antibody, and all NSC cell culture reagents were purchased from StemCell Technologies (Vancouver, BC). 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay and enzyme-linked immunosorbent assay (ELISA) kits were obtained from Promega Corporation (Madison, WI). Alginate was from NovaMatrix (Drammen, Norway). Live/Dead Assay and PC-12 medium reagents were from Invitrogen Corporation (Carlsbad, CA). BD Biocoat collagen–coated plates were from BD Biosciences (San Jose, CA). Centricon filters and NG-2 antibody were from Millipore Corporation (Billerica, MA). Secondary antibodies were from Molecular Probes (Eugene, OR). PC-12 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). All other reagents were from Sigma-Aldrich (St. Louis, MO).
Cortical NSC culture and encapsulation in alginate
E14 murine cortical NSCs were cultured and expanded with 20 ng/mL epidermal growth factor according to the supplier's protocol with a penicillin–streptomycin antibiotic supplement. The culture and stem cell characteristics of these cells have been described.33,34 Cell encapsulation (on Day 0) was achieved by mixing a cell slurry with alginate 50:50 and dropping into a 0.1 M calcium chloride solution for 10 min. The encapsulation yielded beads with a final concentration of 500,000 cells/mL in 1% w/v alginate (approximately 650 cells per bead). The weight percentage was chosen based on a recommendation reported in the literature.21 Cells were approximately 90% viable as assessed by trypan blue staining before encapsulation. Bead size was approximately 1 mm in diameter (1.38 ± 0.19 mm, mean ± SD, n = 158) immediately after production, and size was controlled by parallel air flow through a glass atomizer. Four different conditions were employed to optimize the encapsulation procedure: a high G alginate (68% G content, molecular weight = 219,000g/mol) or a high M alginate (54% M content, molecular weight = 222,000 g/mol), with or without a PLL coating layer. The conditions chosen have been shown to have differing mechanical strengths based on M/G content, and molecular weights were closely matched to eliminate this factor as an experimental variable.25,35 These conditions are abbreviated as G, M, G-PLL, and M-PLL. All alginates were highly purified and sterile.
PLL coating was achieved as previously described.36 Briefly, beads were rinsed in physiological saline before a 10 min incubation in 0.1% PLL-HCl (15,000–30,000 MW) in saline. After additional saline rinsing, beads were incubated for 10 min in 0.1% alginate in saline. Beads received a final rinse before being returned to medium. Beads were cultured in static transwell dishes containing 500,000 cells (encapsulated or unencapsulated) that were allowed to proliferate in the presence of epidermal growth factor over time.
ELISA
At 1, 4, 7, 14, and 21 day time points, medium samples were collected for quantification of growth factor release with ELISA (n = 5 per condition). Untreated medium and supernatant from nonencapsulated cells were used as controls. Supernatant was concentrated with YM-3 Centricon filters at 4°C. Promega Emax ELISA kits for NGF, BDNF, and GDNF were used according to the manufacturer's protocol. Average values below the lowest dilution above zero of the standard curve for each kit were considered to be nondetectable.
Alginate mechanical stability
The mechanical stability of the alginate beads was assessed using a semi-quantitative osmotic pressure test.37 Testing was conducted at 1, 7, 14, and 21 day time points. Alginate beads were exposed to solutions of low osmolarity (0, 2.8, and 11.1 mOsm saline with medium used as a control) for a period of 3 h, as previously described.37 Thirty beads per condition were assessed visually for breakage at each time point. The number of intact capsules was compared between conditions by an observer blinded to the experimental condition.
NSC proliferation and viability in alginate
Beads were collected for quantification of proliferation with an MTS assay and viability with propidium iodide staining at the same time points as ELISA. In the MTS assay, a tetrazolium compound is bioreduced by metabolically active cells into a formazan product that is quantified by a plate reader. Twenty beads per well (n = 4 wells per condition per day) were assayed for each condition in accordance with previously published methods and sample sizes in similar studies.38
To assess the viability of the cells in the alginate, beads were exposed to propidium iodide for 1.5 h and counterstained with Hoechst for the final 10 min (n = 4 per condition). Results were assessed by obtaining the percentage of propidium iodide positive nuclei by counting under a Zeiss Axioplan microscope. Slides were evaluated by an observer blinded to the experimental condition.
Immunocytochemistry of cells in alginate
Beads were collected at the same time points as ELISA for histology (n = 4 per condition). For all staining applications, beads were briefly fixed in phosphate buffered saline–buffered 4% paraformaldehyde for 10 min followed by dehydration in graded washes of ethanol up to 70%. The samples were embedded in paraffin and sectioned on a microtome at a 5 μm thickness. Sections were de-paraffinized, rehydrated, blocked with 10% normal goat serum, and stained with markers of differentiation. Specifically, immunocytochemistry for TUJ-I (neuronal precursors), GFAP (astrocytes), NG-2 (oligodendrocyte precursors), and nestin (undifferentiated NSCs) were performed based on a previously described method at 1:500, 1:80, 1:500, and 1:80 concentrations, respectively.39 Tris buffered saline was used in place of phosphate buffered saline to improve bead stability during staining by avoiding phosphate binding of calcium cross-links. Nuclei were counterstained with Hoechst. As a negative control, Tris buffered saline was used in place of primary antibody. Analysis of results was a qualitative assessment of histology.
Bioactivity of conditioned medium from NSC-seeded beads
To verify the potential ability of cortical NSCs to exert neuroprotective effects via a bystander mechanism when encapsulated in alginate, a serum-withdrawal model of apoptosis in PC-12 cells was used.6 PC-12 cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated horse serum and 5% fetal bovine serum on type IV collagen–coated 96-well plates, based on methods previously described.40 To obtain conditioned medium, NSCs were encapsulated in 1% high G alginate, and medium was collected 48 h later. The high G alginate scaffold was chosen for this experiment based on its improved mechanical stability and support of neurotrophic factor release as demonstrated in this study. Nonseeded alginate beads conditioned the control medium. Fifty percent of serum-containing medium was removed and replaced with either NSC-conditioned or control medium (from empty capsules). Control cells were given a medium change only. Twenty-four and 48 hours later, PC-12s were evaluated for viability with a Live/Dead kit. The center of each well was imaged with the 20 × objective of a Leica inverted microscope, and live and dead cells were counted by an observer blinded to the experimental condition with ImageJ software (U. S. National Institutes of Health, Bethesda, MD). The experiment was repeated, and a univariate ANOVA model that factored in time and trial was used to evaluate the combined data. Cell viability was evaluated as a percent of control.
Statistical analysis
Differences between controls and experimental conditions for ELISA, MTS values, NSC, and PC-12 viability were analyzed with standard analysis of variance (ANOVA) techniques and a Tukey posthoc test where significance was noted. Osmotic pressure test data were analyzed with logistic regression due to the binary response variable (1 = intact bead, 0 = broken bead). Histology was evaluated on a qualitative basis. Statistical significance was defined at the 0.05 level.
Results
ELISA
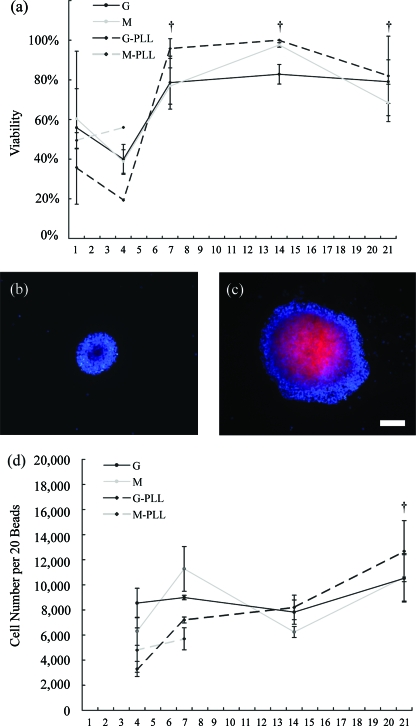
The amount of NGF secreted from the unencapsulated cells (roughly between 10 and 90 pg/million cells/day depending on time point; Table 1) was similar to that previously reported from the C17.2 NSC line derived from cerebellum (approximately 10 pg/million cells/day), and NGF secreted from cells in G capsules also fell within this range.15 GDNF secretion from unencapsulated and G encapsulated cells on day 14 was somewhat higher than the value reported for the C17.2 clone (70 pg/million cells/day) (Table 1).15 No secretion of NGF or GDNF was detected at any time point from cells encapsulated in other scaffold conditions. NGF was detected on fewer days from G encapsulated cells compared with unencapsulated cells (Table 1). BDNF was detected from all conditions, both encapsulated and unencapsulated, at the 4 day time point, and values were generally higher than that reported for C17.2 cells (10 pg/million cells/day) (Table 1).15 The 4 day time point is also when cell viability tended to be at its lowest levels for scaffold conditions; it is unknown if the two phenomena are related (Fig. 2a). NGF levels for unencapsulated cells were higher at 7 and 21 days than at initial time points; no other significant differences were found.
Table 1.
Neurotrophic Factor Release Varies with Scaffold Condition and Time
| |
|
Days |
||||
|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 14 | 21 | ||
| Cells | NGF | 30.98 ± 5.64 | 19.18 ± 8.17 | 74.19 ± 26.01a | 44.76 ± 9.25 | 89.20 ± 11.87b |
| GDNF | ND | ND | ND | 156.47 ± 39.47 | ND | |
| BDNF | ND | 141.89 ± 82.99 | ND | ND | ND | |
| G | NGF | 33.52 ± 10.34 | ND | 26.67 ± 12.23 | ND | ND |
| GDNF | ND | ND | ND | 296.19 ± 175.72 | ND | |
| BDNF | ND | 173.98 ± 36.52 | ND | ND | ND | |
| M | NGF | ND | ND | ND | ND | ND |
| GDNF | ND | ND | ND | ND | ND | |
| BDNF | ND | 50.96 ± 9.07 | ND | ND | ND | |
| G/PLL | NGF | ND | ND | ND | ND | ND |
| GDNF | ND | ND | ND | ND | ND | |
| BDNF | ND | 161.74 ± 52.07 | ND | ND | ND | |
| M/PLL | NGF | ND | ND | ND | ND/NP | ND/NP |
| GDNF | ND | ND | ND | ND/NP | ND/NP | |
| BDNF | ND | 102.30 ± 59.86 | ND | ND/NP | ND/NP | |
Values are given in pg/million cells/day. Unencapsulated cells secrete nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and glial-derived neurotrophic factor (GDNF) at various time points throughout the study, whereas all three factors were detected from encapsulated cells only when a high G alginate scaffold was used. BDNF was detected from all conditions at the 4 day time point, and GDNF was detected at the 14 day time point from G-encapsulated and unencapsulated cells. NGF secretion from unencapsulated cells at 21 days was significantly greater than 1 day and 4 day values (b), and secretion at 7 days was greater than 4 day values (a) (p < 0.05, ANOVA). Medium samples were assayed throughout the study regardless of the mechanical integrity of the alginate beads. Nondetectable values are denoted “ND.” Medium samples assayed from time points in which beads degraded and were no longer present are labeled “NP” (not present). Mean ± SEM is shown. G, L-guluronic acid; M, D-mannuronic acid; PLL, poly-L-lysine.
FIG. 2.
NSCs survive (a–c) and proliferate (d) in alginate regardless of the compositions tested. Viability of the cells is initially reduced after encapsulation, but significantly improves for all conditions after 1 week (a). Cells are labeled with Hoechst (blue), and propidium iodide (red) stains nuclei of cells with compromised membranes indicative of cell death (b–c). Images of G-encapsulated cells at 21 days illustrate the vulnerability of cells in the center of larger proliferating neurospheres to necrosis, presumably due to reduced nutrient and oxygen diffusion (b–c). NSCs proliferate irrespective of alginate composition, and cell number was significantly increased 21 days postencapsulation over initial 4-day quantities (d). Values were initially nondetectable by the MTS assay 1 day after encapsulation (d). M-PLL capsules were too mechanically unstable to assay after the first week. †Significantly increased versus 1 and 4 day values at the 0.05 level (ANOVA). Mean ± standard error of the mean (SEM) is shown. Scale = 100 μm. Color images available online at www.liebertonline.com/ten.
Alginate mechanical stability
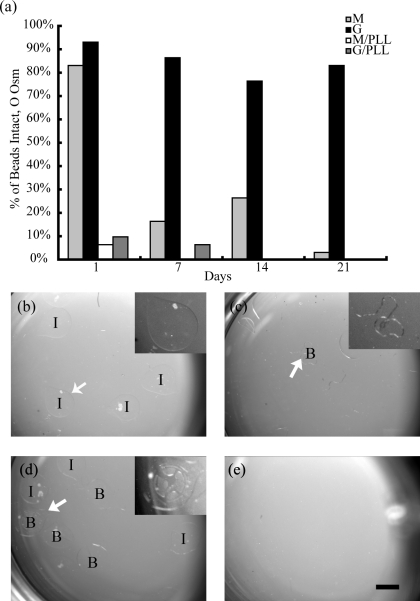
G beads were significantly more stable than all other conditions when exposed to solutions of low osmolarity (Fig. 1a, p < 0.001). The odds of beads remaining intact was increased by at least 18-fold in comparison to other conditions when a high G composition was used. Representative images of beads exposed to 0 mOsm solutions after 7 days in vitro further highlight the improved stability of high G beads compared with other alginate compositions during this test (Fig. 1b–e). High M beads were more stable than PLL-coated beads (Fig. 1a, p < 0.001). The data show that PLL coating resulted in increased incidence of bead breakage, with no significant difference between G-PLL and M-PLL beads. The presence of a PLL coating layer was visually confirmed in a separate investigation using the same coating protocol with FITC-labeled PLL and fluorescence microscopy (data not shown). There was a significant reduction of alginate stability after 24 h; the odds of beads being intact decreased ninefold after this time point (Fig. 1a, p < 0.001). No further significant changes in stability over time were observed. Similar results were found when beads were exposed to solutions of 2.8 and 11.1 mOsm saline (data not shown).
FIG. 1.
Neural stem cell (NSC)-seeded alginate microcapsules with a G composition have superior stability in comparison to all other conditions. G beads remain intact more frequently than M capsules after exposure to 0 mOsm 7 days after seeding (a) (p < 0.001, logistic regression). Poly-L-lysine (PLL)-coated capsules break more frequently than uncoated capsules (p < 0.001, logistic regression). Representative images of G (b), G-PLL (c), M (d), and M-PLL (e) capsules in solution illustrate the stability of G capsules (intact beads are labeled “I”) and breakage (broken beads are denoted “B”) of M and G-PLL beads. M-PLL beads are completely dissolved. Insets in (b–d) highlight representative beads (arrows) to improve observation. Error bars are not shown due to the nature of the data analysis. Scale = 1 mm.
Proliferation and viability in alginate
NSCs survive and proliferate in all alginate matrices regardless of composition (Fig. 2a–d). M-PLL capsules were too mechanically unstable to assay after the first week. After an initial drop in viability at 4 days to the 20–40% range, cell viability rebounds to over 70% thereafter regardless of encapsulation condition (Fig. 2a, p < 0.05). Cell death levels were high immediately after encapsulation, mainly due to necrosis with many cells appearing to be lysed by the procedure. Day 1 viability data likely underestimate the extent of cell death after encapsulation; cellular debris was evident at this time point, and many cells may not have been analyzed due to degradation. Representative pictures of healthy and propidium iodide–positive cells from the same sample of high G-encapsulated neurospheres are shown in Figure 2b–c. Cells at the interior of larger encapsulated neurospheres are particularly vulnerable to necrosis, presumably due to reduced nutrient and oxygen diffusion (Fig. 2c). At later time points, chromatin clumping possibly indicating apoptosis was seen in a few cells, and much lower levels of necrosis occurred. There were no significant differences in viability between alginate conditions. An MTS assay revealed cell proliferation in all alginate matrices irrespective of composition, and cell number was above the detection limit of the assay (estimated to be ∼2000 NSCs per well) beginning at 4 days postencapsulation. Cell number was significantly increased over the 4 day value by the 21 day time point (Fig. 2d, p < 0.05). Previous experiments visually validated the MTS assay of alginate beads with toluidene blue staining of paraffin sections (data not shown). The data indicate that NSCs survive and proliferate within alginate matrices after an initial drop in viability regardless of composition, likely due to the physiological challenge the encapsulation procedure presents to the cells.
Immunocytochemistry of cells in alginate
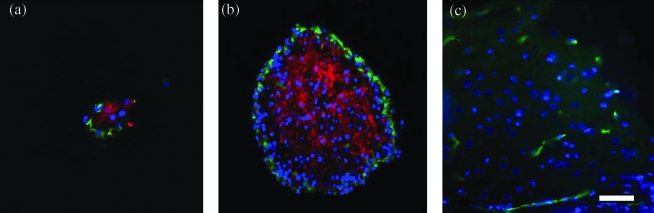
Results of immunocytochemistry were limited due to bead instability during staining, and dehydration and processing seemed to affect tissue appearance. However, preliminary results revealed that encapsulated NSCs express nestin on the periphery and GFAP in the center of larger proliferating cell masses after entrapment in alginate (Fig. 3a–b). Smaller neurospheres were often nestin positive and largely GFAP negative (data not shown). This profile of expression is typical for that observed with similar neurosphere-derived cells in culture.41 No expression of TUJ-1 was observed. Interestingly, some evidence of limited NG-2 expression was observed in one bead, indicating oligodendrocyte precursors (Fig. 3c).
FIG. 3.
Encapsulated NSCs express nestin (green) on the periphery and GFAP (red) in the center of cell masses after entrapment in alginate (a–b). Interestingly, some evidence of limited NG-2 expression (green) was observed in one bead, indicating oligodendrocyte progenitors (c). All nuclei were counterstained with Hoechst (blue). Images are from (a) G, 14 day (b) M-PLL, 14 day, and (c) G-PLL, 4 day, scaffolds and time points. Scale = 50 μm. Color images available online at www.liebertonline.com/ten.
Bioactivity of conditioned medium from NSC-seeded beads
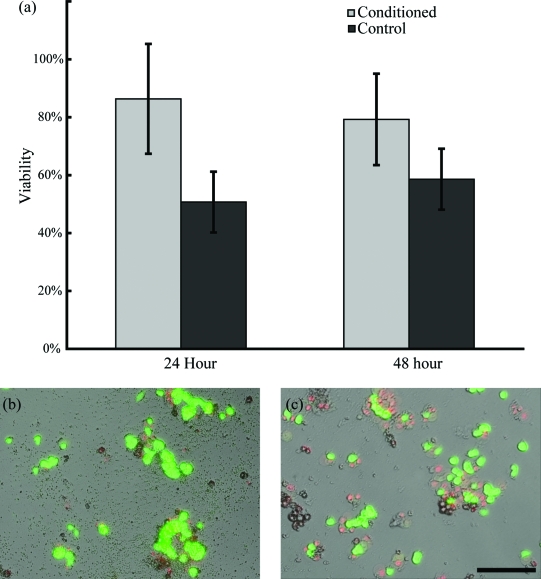
Results of a viability assay revealed a significant protective effect of conditioned medium collected from NSCs encapsulated in G beads against serum withdrawal–mediated PC-12 cell death over 48 h (Fig. 4a, p < 0.05). These results are novel for cortical NSCs, and corroborate a similar study done with hippocampal NSCs.6 Representative images of live and dead PC-12 cells are shown for NSC-conditioned and control medium treatments in Figure 4b and c, respectively.
FIG. 4.
NSC-conditioned medium from G-encapsulated cells protects PC-12 cells from serum withdrawal–mediated cell death (a) (p < 0.05, ANOVA). Representative images of live (green) and dead (red) PC-12 cells after a 50% serum withdrawal and treatment with medium conditioned by NSC-seeded (b, 75.6% viable) or unseeded (c, 49.5% viable) alginate microspheres. Results are reported as a percentage of control PC-12 cell viability. Mean ± SEM is shown. Scale = 100 μm. Color images available online at www.liebertonline.com/ten.
Discussion
Alginate composition has been shown to affect encapsulated cell proliferation and secretion of therapeutic proteins, as well as the mechanical stability of the scaffold.17,21,25 We sought to characterize the effects composition may have on NSCs, as alginate is largely unstudied as a carrier for these cells. NSCs survive and proliferate in alginate irrespective of the scaffold compositions tested in this study. Among the conditions tested, a high G content alginate without a PLL coating layer was the optimal composition based on its mechanical stability during the osmotic pressure test and support of neurotrophic factor release.
Our data reveal that cortical NSCs, even when encapsulated in alginate, secrete NGF, BDNF and GDNF into the surrounding medium. Importantly, these neurotrophic factors are known to support neuronal survival and plasticity in various models of axonopathy and neuropathy.42–46 Quantitative analysis of constitutive neurotrophic factor release from NSCs is relatively rarely reported in the literature, despite the popular theory that release of these proteins and their effects on compromised host tissue is a potential mechanism behind the cells' restorative capacity.8,9,11,14,16 Time courses of release are generally lacking, as are release profiles from scaffolded cells. Lu et al. reported detection of NGF, BDNF, and GDNF in medium collected from C17.2 NSCs after a 24 h time point, with levels in the 10–70 pg/million cells/day range.15 GDNF and NGF, but not BDNF, secretion was detected from these cells in a separate report.14 GDNF release from alginate encapsulated and unencapsulated hippocampal NSCs tended to be approximately 500–1000 pg/million cells after 72 h, as reported by Li et al.6 Therefore, the reported values for neurotrophic factor release from encapsulated and unencapsulated cells differ from 10 to 1000 pg/million cells, and our results are consistent with this range.
This study revealed that the detection of released neurotrophic factors is influenced by alginate composition and time. BDNF and GDNF are detected at single time points (4 and 14 days, respectively), while NGF release is observed throughout the study by unencapsulated and encapsulated cells. NGF is detected on fewer days (occurring only in the first week postencapsulation) from encapsulated cells in comparison to unencapsulated cells, possibly due to the initial reduction in viability of the cells by encapsulation, degradation of the NGF protein, or interactions of NGF with the alginate matrix. Alternatively, this result may be related to the apparently lower proliferation rate of NSCs in alginate matrix; the proliferation data shown in Figure 2d do not reflect the 2.5 day doubling rate unencapsulated cells typically experience in culture. GDNF and NGF are only detected from encapsulated cells when a high G, non-PLL-coated capsule is used. Meanwhile, BDNF is released from cells in all conditions tested. Why the high G alginate capsules seem more permissive to neurotrophic factor release is unclear, given that previous literature for insulin-secreting cells has suggested reduced proliferation and less secretion at early time points for cells encapsulated in high G alginate.21 No significant effect of alginate composition on NSC proliferation was demonstrated here, which may be due to the relatively low alginate weight percentage (1%) used, as well as differences in the cell types studied. Additionally, the increased gel porosity of high G alginate (which could be further enhanced by swelling) and the potential for stabilization of trophic factors by interaction with the alginate matrix are possible explanations.26,47 Since neurotrophic factors are larger than insulin (approximately 20–30 kDa vs. 5 kDa), the increased porosity of high G alginate may be necessary to allow for adequate release. The release timing of individual neurotrophic factors may be explained by cellular events and their relationship to evolving environmental cues, and the quantities and timing of neurotrophic factor release is likely to vary with culture conditions. BDNF release coincided with low cell viability, while GDNF detection occurred at a time of increased viability and cell number. It is important to note that the initial drop in viability after encapsulation could affect the types and quantities of trophic factors released from the cells. Further, the traits of the individual neurotrophic factors may have been a factor in their detection. Specifically, the relatively larger size of GDNF may have resulted in its detection at fewer time points in comparison to NGF. While electrostatic interactions with the alginate matrix require consideration, the neurotrophic factors studied here all have similar basic isoelectric points (ranging from 9 to 10), indicating that they each carry a net positive charge at physiological pH. Cellular events, protein characteristics, and their relationships to interactions with the alginate matrices may all play a role in the detection of the neurotrophic factors. However, elucidating the mechanisms behind the release profile of neurotrophic factors by the NSCs is beyond the scope of the current work.
The mechanical stability of alginate in vivo over time remains an important challenge.29,48 In a cell encapsulation application, alginate localizes graft cells to the transplant site and isolates them from the immune response, and loss of mechanical strength may compromise these functions. Alginates with a high G content are known to be more mechanically stable than those with higher M contents, due to stabilization by additional cross-links.25 That result was verified here, where the stability of alginate capsules of varying compositions was semi-quantitatively compared by an osmotic pressure test. High G capsules remained intact far more frequently when exposed to solutions of low osmolarities compared with M beads and PLL-coated conditions, and no evidence of decreased proliferation and therapeutic factor secretion was observed. A tradeoff between cellular function and scaffold mechanical strength has been reported previously with insulin-secreting cells.21 However, this tradeoff was more pronounced for higher weight percentage (2%) alginates than the weight percentage (1%) used here. Additionally, this phenomenon was reported for a markedly different cell type than the one studied here, with presumably substantial impacts on proliferation and secretion characteristics.
It was somewhat surprising that the PLL-coated capsules were more prone to breakage than uncoated beads during the osmotic pressure test, given that PLL has been credited with improving mechanical stability.20,49 This evidence requires careful interpretation, with consideration for the consequences of saline washing, the potential effects of polycation coating on swelling behavior, the impact of alginate concentration, and the definition of mechanical stability. The results may be affected by the multiple saline washes used in the coating protocol. Monovalent sodium ions may compete with divalent calcium ions for binding sites between G residues, and ultimately break the cross-links and weaken the gel.50 We have previously reported a study in which uncoated and PLL-coated alginate disks were washed identically in saline, implanted subcutaneously in rats, and monitored over 3 months for mechanical stability, weight changes related to swelling behavior, and biocompatibility.48 PLL-coating resulted in a slight but significant increase in complex modulus 7 days after implantation in comparison to noncoated gels; no other significant differences in stability between the two conditions were noted. Therefore, PLL coating may confer a slight advantage, or at least have a nondetrimental role, on gel stability when results are not affected by saline washing. However, saline washing is a prototypic part of PLL coating protocols; thus, if coating is pursued, the current results indicate that the potentially destabilizing effects of saline washing should be considered.
In the same study, both PLL and noncoated gels initially dropped in weight after implantation, followed by similar trends in increasing weight (interpreted as swelling) for a period of 2 weeks. The weight gains were greater for noncoated than PLL-coated samples, potentially indicating greater swelling. Previous research has indicated that osmotic swelling of the bead core against a less elastic polycation membrane may eventually cause capsules to burst.51 Therefore, although the G capsules may not burst, they may swell during exposure to an osmotic challenge, likely changing diffusion properties (which may, in turn, be related to their permissiveness to neurotrophic factor release).27 The capsules in this study may be particularly vulnerable to swelling and saline effects due to the relatively low weight percentage used (1%); increased alginate concentration improves resistance to swelling.50 Finally, the way in which mechanical stability is measured (i.e., rheology in the in vivo study and osmotic pressure in the in vitro study) is likely to affect results. De Castro et al. reported PLL coating to confer superior resistance to compression, but the lowest resistance to osmotic pressure, in a mechanical stability test of three different polycation coatings.52
The authors in the current study hypothesize that alginate beads were initially weakened by saline washes before PLL coating, and swelled in response to solutions of low osmolarity against the PLL coating, causing them to burst. Thus, the mechanical stability of uncoated beads was improved compared with coated beads in the current experiment, but consideration for the effects of saline washing, swelling behavior, and changes in diffusion characteristics is required. Importantly, PLL coating has been credited with isolating alginate from IgG and complement diffusion into the gel. The coating protocol used in the experiments reported here was recommended to achieve this property.28 However, polycation coatings are still controversial after over 25 years of research due to the fact that they may directly elicit a tissue response.19,22,23,29–31 In addition to the questionable effect on in vivo biocompatibility, our data demonstrate bead breakage during an osmotic challenge and reduced neurotrophic factor detection associated with PLL coating.
Our results indicate that a high G alginate without PLL coating was the most favorable composition for NSC encapsulation among the conditions tested based on superior mechanical stability during the osmotic pressure test and support of neurotrophic factor release. However, several opportunities for further development remain. Alternative polycations or a different polycation coating procedure may allow for tailoring diffusivity properties without a loss in mechanical stability or neurotrophic factor detection.29 Alginate weight percentage variation, bead size reduction, and incorporation of interpenetrating networks are additional examples of methods that may improve results.21,53–55 We are currently evaluating the ability of a high G, non-PLL-coated alginate scaffold seeded with NSCs to repair a cortical lesion in vivo, and early results are promising.32
Acknowledgments
Special thanks to Soumya Yandamuri for performing blinded cell counts. The authors gratefully thank Dr. David Mooney and Dr. David Martin and their students for assistance in cell encapsulation training. Thanks to Joe Kazemi and the Center for Statistical Consultation and Research (CSCAR) at the University of Michigan for their assistance with statistical methods. This project was supported by the Center for Neural Communication Technology (NIBIB, P41 EB002030), an NSF Graduate Research Fellowship, and a Rackham Engineering Award from the University of Michigan.
Disclosure Statement
No competing financial interests exist.
References
- 1.Borlongan C.V. Skinner S.J. Geaney M. Vasconcellos A.V. Elliott R.B. Emerich D.F. Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke. 2004;35:2206. doi: 10.1161/01.STR.0000138954.25825.0b. [DOI] [PubMed] [Google Scholar]
- 2.Joki T. Machluf M. Atala A. Zhu J. Seyfried N.T. Dunn I.F. Abe T. Carroll R.S. Black P.M. Continuous release of endostatin from microencapsulated engineered cells for tumor therapy. Nat Biotechnol. 2001;19:35. doi: 10.1038/83481. [DOI] [PubMed] [Google Scholar]
- 3.Lim F. Sun A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 4.Wikstrom J. Elomaa M. Syvajarvi H. Kuokkanen J. Yliperttula M. Honkakoski P. Urtti A. Alginate-based microencapsulation of retinal pigment epithelial cell line for cell therapy. Biomaterials. 2008;29:869. doi: 10.1016/j.biomaterials.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 5.Ashton R.S. Banerjee A. Punyani S. Schaffer D.V. Kane R.S. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials. 2007;28:5518. doi: 10.1016/j.biomaterials.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Li X. Liu T. Song K. Guan S. Zhu L. Ge D. Cui Z. Effect of neural stem cells on apoptosis of PC12 cells induced by serum deprivation. Biotechnol prog. 2007;23:952. doi: 10.1021/bp070060+. [DOI] [PubMed] [Google Scholar]
- 7.Li X. Liu T. Song K. Yao L. Ge D. Bao C. Ma X. Cui Z. Culture of neural stem cells in calcium alginate beads. Biotechnol prog. 2006;22:1683. doi: 10.1021/bp060185z. [DOI] [PubMed] [Google Scholar]
- 8.Lindvall O. Kokaia Z. Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10 Suppl:S42. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 9.Martino G. Pluchino S. The therapeutic potential of neural stem cells. Nat Rev. 2006;7:395. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 10.Gaillard A. Prestoz L. Dumartin B. Cantereau A. Morel F. Roger M. Jaber M. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci. 2007;10:1294. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- 11.Ourednik J. Ourednik V. Lynch W.P. Schachner M. Snyder E.Y. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20:1103. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 12.Pluchino S. Zanotti L. Rossi B. Brambilla E. Ottoboni L. Salani G. Martinello M. Cattalini A. Bergami A. Furlan R. Comi G. Constin G. Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 13.Heine W. Conant K. Griffin J.W. Hoke A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004;189:231. doi: 10.1016/j.expneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Llado J. Haenggeli C. Maragakis N.J. Snyder E.Y. Rothstein J.D. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004;27:322. doi: 10.1016/j.mcn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Lu P. Jones L.L. Snyder E.Y. Tuszynski M.H. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 16.Teng Y.D. Lavik E.B. Qu X. Park K.I. Ourednik J. Zurakowski D. Langer R. Snyder E.Y. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99:3024. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constantinidis I. Rask I. Long R.C., Jr. Sambanis A. Effects of alginate composition on the metabolic, secretory, and growth characteristics of entrapped beta TC3 mouse insulinoma cells. Biomaterials. 1999;20:2019. doi: 10.1016/s0142-9612(99)00104-0. [DOI] [PubMed] [Google Scholar]
- 18.De Vos P. De Haan B. Van Schilfgaarde R. Effect of the alginate composition on the biocompatibility of alginate-polylysine microcapsules. Biomaterials. 1997;18:273. doi: 10.1016/s0142-9612(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 19.King A. Lau J. Nordin A. Sandler S. Andersson A. The effect of capsule composition in the reversal of hyperglycemia in diabetic mice transplanted with microencapsulated allogeneic islets. Diabetes Technol Ther. 2003;5:653. doi: 10.1089/152091503322250677. [DOI] [PubMed] [Google Scholar]
- 20.Rokstad A.M. Holtan S. Strand B. Steinkjer B. Ryan L. Kulseng B. Skjak-Braek G. Espevik T. Microencapsulation of cells producing therapeutic proteins: optimizing cell growth and secretion. Cell Transplant. 2002;11:313. [PubMed] [Google Scholar]
- 21.Stabler C. Wilks K. Sambanis A. Constantinidis I. The effects of alginate composition on encapsulated betaTC3 cells. Biomaterials. 2001;22:1301. doi: 10.1016/s0142-9612(00)00282-9. [DOI] [PubMed] [Google Scholar]
- 22.Strand B.L. Ryan T.L. In't Veld P. Kulseng B. Rokstad A.M. Skjak-Brek G. Espevik T. Poly-L-lysine induces fibrosis on alginate microcapsules via the induction of cytokines. Cell Transplant. 2001;10:263. [PubMed] [Google Scholar]
- 23.King A. Sandler S. Andersson A. The effect of host factors and capsule composition on the cellular overgrowth on implanted alginate capsules. J Biomed Mater Res. 2001;57:374. doi: 10.1002/1097-4636(20011205)57:3<374::aid-jbm1180>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Soon-Shiong P. Otterlie M. Skjak-Braek G. Smidsrod O. Heintz R. Lanza R.P. Espevik T. An immunologic basis for the fibrotic reaction to implanted microcapsules. Transplant Proc. 1991;23(1 Pt 1):758. [PubMed] [Google Scholar]
- 25.Strand B.L. Morch Y.A. Skjak-Braek G. Alginate as immobilization matrix for cells. Minerva Biotecnol. 2000;12:223. [Google Scholar]
- 26.Martinsen A. Storro I. Skjakbraek G. Alginate as immobilization material: 3. Diffusional properties. Biotechnol Bioeng. 1992;39:186. doi: 10.1002/bit.260390210. [DOI] [PubMed] [Google Scholar]
- 27.Gugerli R. Cantana E. Heinzen C. von Stockar U. Marison I.W. Quantitative study of the production and properties of alginate/poly-L-lysine microcapsules. J Microencapsul. 2002;19:571. doi: 10.1080/02652040210140490. [DOI] [PubMed] [Google Scholar]
- 28.Kulseng B. Thu B. Espevik T. Skjak-Braek G. Alginate polylysine microcapsules as immune barrier: permeability of cytokines and immunoglobulins over the capsule membrane. Cell Transplant. 1997;6:387. doi: 10.1177/096368979700600405. [DOI] [PubMed] [Google Scholar]
- 29.Orive G. Hernandez R.M. Gascon A.R. Calafiore R. Chang T.M. De Vos P. Hortelano G. Hunkeler D. Lacik I. Shapiro A.M. Pedraz J.L. Cell encapsulation: promise and progress. Nat Med. 2003;9:104. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- 30.van Raamsdonk J.M. Cornelius R.M. Brash J.L. Chang P.L. Deterioration of polyamino acid-coated alginate microcapsules in vivo. J Biomater Sci Polym Ed. 2002;13:863. doi: 10.1163/156856202320401933. [DOI] [PubMed] [Google Scholar]
- 31.Orive G. Tam S.K. Pedraz J.L. Halle J.P. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials. 2006;27:3691. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 32.Purcell E.K., editor; Seymour J.P., editor; Kipke D.R., editor. In Vivo Evaluation of a Neural Stem Cell-Seeded Chronic Probe. San Diego, CA: Society for Neuroscience; 2007. [Google Scholar]
- 33.Reynolds B.A. Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds B.A. Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 35.Martinsen A. Skjak-Baek G. Smidsrod O. Alginate as immobilization material: I. Correlation between chemical and physical properties of alginate gel beads. Biotechnol Bioeng. 1989;33:79. doi: 10.1002/bit.260330111. [DOI] [PubMed] [Google Scholar]
- 36.Strand B.L. Gaserod O. Kulseng B. Espevik T. Skjak-Baek G. Alginate-polylysine-alginate microcapsules: effect of size reduction on capsule properties. J Microencapsul. 2002;19:615. doi: 10.1080/02652040210144243. [DOI] [PubMed] [Google Scholar]
- 37.van Raamsdonk J.M. Chang P.L. Osmotic pressure test: a simple, quantitative method to assess the mechanical stability of alginate microcapsules. J Biomed Mater Res. 2001;54:264. doi: 10.1002/1097-4636(200102)54:2<264::aid-jbm14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Bunger C.M. Jahnke A. Stange J. de Vos P. Hopt U.T. MTS colorimetric assay in combination with a live-dead assay for testing encapsulated L929 fibroblasts in alginate poly-L-lysine microcapsules in vitro. Artif Organs. 2002;26:111. doi: 10.1046/j.1525-1594.2002.06853.x. [DOI] [PubMed] [Google Scholar]
- 39.Cowell R.M. Plane J.M. Silverstein F.S. Complement activation contributes to hypoxic-ischemic brain injury in neonatal rats. J Neurosci. 2003;23:9459. doi: 10.1523/JNEUROSCI.23-28-09459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene L.A. Tischler A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campos L.S. Leone D.P. Relvas J.B. Brakebusch C. Fassler R. Suter U. ffrench-Constant C. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 2004;131:3433. doi: 10.1242/dev.01199. [DOI] [PubMed] [Google Scholar]
- 42.Kolb B. Gorny G. Cote S. Ribeiro-da-Silva A. Cuello A.C. Nerve growth factor stimulates growth of cortical pyramidal neurons in young adult rats. Brain Res. 1997;751:289. doi: 10.1016/s0006-8993(96)01410-2. [DOI] [PubMed] [Google Scholar]
- 43.Nicole O. Ali C. Docagne F. Plawinski L. MacKenzie E.T. Vivien D. Bulsson A. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci. 2001;21:3024. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkins A. Compston A. Trophic factors attenuate nitric oxide mediated neuronal and axonal injury in vitro: roles and interactions of mitogen-activated protein kinase signalling pathways. J Neurochem. 2005;92:1487. doi: 10.1111/j.1471-4159.2004.02981.x. [DOI] [PubMed] [Google Scholar]
- 45.Han B.H. Holtzman D.M. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20:5775. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu B. Pang P.T. Woo N.H. The yin and yang of neurotrophin action. Nat Rev. 2005;6:603. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 47.Peters M.C. Isenberg B.C. Rowley J.A. Mooney D.J. Release from alginate enhances the biological activity of vascular endothelial growth factor. J Biomater Sci Polym Ed. 1998;9:1267. doi: 10.1163/156856298x00389. [DOI] [PubMed] [Google Scholar]
- 48.Nunamaker E.A. Purcell E.K. Kipke D.R. In vivo stability and biocompatibility of implanted calcium alginate disks. J Biomed Mater Res A. 2007;83:1128. doi: 10.1002/jbm.a.31275. [DOI] [PubMed] [Google Scholar]
- 49.Benson J.P. Papas K.K. Constantinidis I. Sambanis A. Towards the development of a bioartificial pancreas: effects of poly-L-lysine on alginate beads with BTC3 cells. Cell Transplant. 1997;6:395. doi: 10.1177/096368979700600406. [DOI] [PubMed] [Google Scholar]
- 50.Wang X. Spencer H.G. Calcium alginate gels: formation and stability in the presence of an inert electrolyte. Polymer. 1998;39:2759. [Google Scholar]
- 51.Thu B. Bruheim P. Espevik T. Smidsrod O. Soon-Shiong P. Skjak-Braek G. Alginate polycation microcapsules. II. Some functional properties. Biomaterials. 1996;17:1069. doi: 10.1016/0142-9612(96)85907-2. [DOI] [PubMed] [Google Scholar]
- 52.De Castro M. Orive G. Hernandez R.M. Gascon A.R. Pedraz J.L. “Comparative study of microcapsules elaborated with three polycations (PLL, PDL, PLO) for cell immobilization.”. J Microencapsul. 2005;22:303. doi: 10.1080/026520405000099893. [DOI] [PubMed] [Google Scholar]
- 53.Wang M.S. Childs R.F. Chang P.L. A novel method to enhance the stability of alginate-poly-L-lysine-alginate microcapsules. J Biomater Sci Polym Ed. 2005;16:91. doi: 10.1163/1568562052843302. [DOI] [PubMed] [Google Scholar]
- 54.Desai N.P. Sojomihardjo A. Yao Z. Ron N. Soon-Shiong P. Interpenetrating polymer networks of alginate and polyethylene glycol for encapsulation of islets of langerhans. J Microencapsul. 2000;17:677. doi: 10.1080/02652040050161675. [DOI] [PubMed] [Google Scholar]
- 55.Canaple L. Rehor A. Hunkeler D. Improving cell encapsulation through size control. J Biomater Sci. 2002;13:783. doi: 10.1163/156856202760197410. [DOI] [PubMed] [Google Scholar]