Abstract
Objective
To achieve osteochondral regeneration utilizing transplantation of cartilage-lineage cells and adequate scaffolds, it is essential to characterize the behavior of transplanted cells in the repair process. The objectives of this study were to elucidate the survival of mesenchymal cells (MCs). In a polylactic acid (PLA) scaffold and assess the possibility of MC/PLA constructs for osteochondral repair.
Design
Bone marrow from mature male rabbits was cultured for 2 weeks, and fibroblast-like MCs, which contain mesenchymal stem cells (MSCs), were obtained. A cell/scaffold construct was prepared with one million MCs and a biodegradable PLA core using a rotator device. One week after culturing, the construct was transplanted into an osteochondral defect in the medial femoral condyle of female rabbits and the healing process examined histologically. To examine the survivability of transplanted MCs, the male-derived sex-determining region Y (SRY) gene was assessed as a marker of MCs in the defect by polymerase chain reaction (PCR).
Results
In the groups of defects without any treatment, and the transplantation of PLA without cells, the defects were not repaired with hyaline cartilage. The cartilaginous matrix by safranin O staining and type II collagen by immunohistochemical staining were recognized, however the PLA matrix was still present in the defects at 24 weeks after transplantation of the construct. During the time passage, transplanted MCs numbers decreased from 7.8 × 105 at 1 week, to 3.5 × 105 at 4 weeks, and to 3.8 × 104 at 12 weeks. Transplanted MCs were not detectable at 24 weeks.
Conclusions
MCs contribute to the osteochondral repair expressing the cartilaginous matrix, however the number of MCs were decreasing with time (i.e. 24 weeks). These results could be essential for achieving cartilage regeneration by cell transplantation strategies with growth factors and/or gene therapy.
Introduction
Osteoarthritis (OA) is one of the most common diseases, with more than 20 million people affected in the United States. It is characterized as degeneration of articular cartilage and underlying subchondral bone.1 For patients with advanced OA, there are several surgical options, such as chondroplasty, drilling, and microfracture to induce cartilage repair2–5; autologous and allogenic osteochondral transplantations as cartilage replacement procedures6–8; and artificial joint replacement and arthrodesis as salvage procedures.9 Though clinical results are generally positive, there are known problems and these procedures do not involve cartilage regeneration.10–14
Autologous chondrocyte implantation (ACI) has been reported as a cartilage reconstruction trial.15 The clinical procedures have been quite successful, and this is currently one of the best surgical options, especially for young, active patients. However, there are several limitations to this procedure, including the limited availability of harvested cartilage, the dedifferentiation of chondrocytes in vitro, degeneration at the harvest site, flow of cell suspension from the transplanted area, and hypertrophy of the periosteal membrane at the transplanted area.16–20 To resolve these issues, modified trials with various cell sources and scaffolds are being established and additional strategies such as the use of growth factors and/or gene therapy have been attempted. However, the regeneration of genuine, long-lasting, normal cartilage has not yet been successful.
We hypothesized that the survivability and behavior of transplanted cells need to be examined clearly to achieve cartilage regeneration with cell-based treatment. The fate of the cells in the transplanted area has not been explained because of the difficulty in distinguishing transplanted cells from the host cells in vivo. Thus, the ability of transplanted cells to survive and contribute to the repair in the transplanted area has not been well discussed. This Information could provide the most effective timing for performing additional treatments during the repair process.
Chondrocytes are a suitable source for the repair of cartilage defects that do not penetrate the subchondral bone. However, an osteochondral defect involves both cartilage and subchondral bone (Grade 4 by classification of the International Cartilage Repair Society), and thus chondrocytes may not be adequate for osteochondral repair.21
Mesenchymal stem cells (MSCs) have the capacity for self-duplication and the potential to differentiate into several mesenchymal tissues (i.e., bone, cartilage, tendon, muscle, and fat tissues).22,23 MSCs exist in bone marrow and many other tissues and can be harvested from bone marrow by a minor invasive procedure clinically. MSCs can also be cultured and increased to sufficient numbers in vitro while preserving their multipotent differentiation capacity. We previously reported the possibility of using bone marrow–derived mesenchymal cells (MCs), which include MSCs, for osteochondral repair.24,25 Thus, MCs could be used in osteochondral repair without causing injury to the healthy articular cartilage. Polylactic acid (PLA) scaffolds have been used to occupy the space following treatment of teeth and bones clinically and have been suitable carriers for high-density, viable chondrogenic cells.26 For these reasons, the combination of MCs and PLA could overcome the formal problems associated with cartilage repair.27 Thus, the objectives of our study were to elucidate the survivability of MCs in PLA scaffold in an osteochondral defect, and to evaluate the possibility of using MC/PLA constructs for osteochondral repair in vivo.
To evaluate the presence of transplanted cells the SRY study was performed. Matrix metalloproteinase (MMP)-1 is an autosomal gene that exists in both male and female cells. The sex-determining region Y (SRY) gene is located on the short arm of the Y chromosome and exists only in male cells. Thus, when male MCs are transplanted into the osteochondral defect of a female femur it is possible to distinguish the male cells from female tissue by detecting the SRY gene using the PCR technique.28 Because not only the transplanted cells (SRY gene) but also the total number of cells in the tissues (MMP-1 gene) could be calculated by a semi-quantitative method in the SRY study, the relationship between transplanted cells and the surrounding host cells with exact cell numbers could be understood.
Materials and Methods
Animals
Skeletally mature male and female New Zealand White rabbits (9–15 months old and weighing 3.5–4.5 kg) with closed epiphyses were used. All procedures conformed to the guidelines of the University of California Animal Subjects Committee and the American Association for Accreditation of Laboratory Animal Care.
Harvest and culture of bone marrow cells
Mature male rabbits (n = 6) were given intramuscular injections of 35 mg/kg ketamine and 5 mg/kg xylazine. After the medications had taken effect, the rabbits received intravenous injections of 97.8 mg/kg sodium pentobarbital via a lateral ear vein and were sacrificed. Bone marrow was harvested from bilateral femurs and tibias by a sterile surgical technique and suspended in phosphate buffered saline (PBS; Gibco, Grand Island, NY) containing 2% bovine serum albumin (BSA; Serologicals Proteins, Kankakee, IL). After centrifuging for 5 minutes to remove serum ingredients, bone marrow was treated with sterile water for 10 seconds to disrupt red blood cells, resuspended into 2% BSA in PBS, and centrifuged again for 5 minutes. The precipitated bone marrow cells from one rabbit were cultured in eight 100 mm diameter tissue culture dishes (Falcon, Franklin Lakes, NJ) with 10 mL culture medium of 10% fetal bovine serum (FBS) and antibiotic-antimyconic (Gibco) in Dulbecco's modified Eagle's medium (DMEM; Gibco) at 37°C in a humidified atmosphere of 5% CO2. The medium was completely changed, first 24 hours after the seeding of cells, and every three days thereafter, to remove as many floating cells as possible. Two weeks after culturing, the remaining cells adhering to the bottom of the dish were detached by a 5 minutes treatment of 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) solution (Sigma-Aldrich, St. Louis, MO) and counted using a hemocytometer.
Formation of MC/PLA constructs
Two weeks after culturing, the remaining adherent fibroblast-like cells were regarded as MCs (containing MSCs), and these achieved about 80% confluence.
Cylindrical cores (3.7 mm in diameter × 3 mm deep) were prepared from cubes of porous D, D-L, and L-PLA (Drylac Cube, Kensey Nash, Exton, PA) to create MC/PLA constructs. To improve the nutrition supply to the center of the cores, a 1 mm diameter channel was created in the center of the cores.
Cultured MCs were detached and counted as described above. A PLA core and 1 million cells in 500 μL culture medium were rotated at 100 rpm in a glass vial for 2 hours on the rotator device.29 Following that, the constructs were cultured for 1 week at 37°C in a humidified atmosphere of 5% CO2.
Surgical procedure
Female rabbits (n = 52) were given intramuscular injections of 35 mg/kg ketamine and 5 mg/kg xylazine, and a facemask delivering 1–2% isofluorane was used during surgery. An anterior midline incision was made through the skin of the left knee, and the articular surface of the femur was exposed using a medial parapatellar retinacular approach. The knee joint was immobilized in a deeply flexed position, and a cylindrical full-thickness osteochondral defect 3.7 mm in diameter (wider than 2/3 of the condyle) and 3 mm in depth was created in the central weight-bearing surface of the medial femoral condyle using a surgical drill bit. Three groups were then prepared: Group 1 was a defect alone without treatment (n = 12), Group 2 was a defect filled with the PLA core alone without cells (n = 12), and Group 3 was a defect filled with the MC/PLA construct (n = 28). All rabbits were allowed to move freely after these procedures in a temperature-controlled environment with a 12-hour light/dark cycle. Intramuscular buprenorphine was administered for at least 72 hours for postoperative pain control.
Gross morphology
At 1, 4, 12, and 24 weeks after surgery, the rabbits (n = 3 in each group at each time period) were sacrificed, the knee joints were exposed as described above, and the distal portions of the femurs were harvested.
Histology
After evaluating gross morphology, the samples were fixed in 10% buffered neutral formalin for 1 week at room temperature and decalcified with 30% formic acid for approximately 4 weeks under vacuum (20–25 psi) with shaking. The samples were rinsed with water for 1 hour to remove formic acid, dehydrated with ascending alcohol grades, cleared with a xylene substitute at room temperature, infiltrated in paraffin at 60°C under vacuum (15 psi) and pressure (7 psi), and then embedded into paraffin blocks.
The samples were cut into 10 μm sections (2035 Jung Biocut; Leica, IL) and deparaffinized and stained either with Safranin O/Fast Green or Hematoxylin/Eosin. The sections were assessed for surface regularity, existence of PLA matrix, matrix staining of the cartilaginous tissues, reconstruction of the subchondral bone in the deeper layer, and integration of the repair tissue with the adjacent tissues. The extent of inflammatory response and osteophyte formation were also evaluated. Macroscopic and histologic evaluations were performed by two other observers.
Immunohistochemical staining
Sections for staining the slides were deparaffinized by heating at 57°C for 1 hour. The slides were then put into a xylene substitute for 10 minutes twice, followed by placing in 100% ethanol for 1 minute, 70% ethanol for 1 minute, 25% ethanol for 1 minute, and Tris-buffered saline (TBS) for 1 minute to rehydrate serially. For recovering antigenicity, the slides were treated with decal antigen retrieval solution for 30 minutes (Bio Genex, San Ramon, CA). Afterward they were rinsed with 100% methanol twice, then with 70% methanol and TBS, and then treated with 3% tritonX-100 in TBS for 10 minutes. The slides were then treated with 1% H2O2 in water for 30 minutes, rinsed with TBS three times, and reacted with primary monoclonal type II collagen antibody (Medicorp, Montreal, Quebec, Canada) at a dilution of 1:100 of 0.1% tritonX-100 and 1% BSA in TBS overnight at room temperature. The slides were rinsed with TBS thrice and incubated with biotinylated antimouse secondary IgG antibody (Sigma) at a dilution of 1:500 in TBS for 1 hour at room temperature. Following a rinse with TBS three times, the sections were incubated with peroxidase-linked avidin (Sigma) at a dilution of 1:500 in TBS for 1 hour. The slides were then rinsed with TBS three times. Diaminobenzidine (Sigma) and Hematoxylin were used for color development and counter staining.
Assessment of MC viability in the defect
The rabbits in Group 3 (n = 4 in each time period) were sacrificed and all the tissues inside the defect area were harvested with a curette. Genomic DNA was isolated from the tissues using phenol and chloroform extraction aided by sonication (Sonifier 350; Branson Ultrasonic, Danbury, CT) and ethanol precipitation.
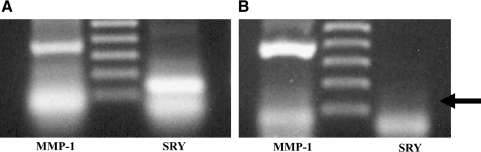
PCR was performed with Taq DNA polymerase (Promega Express, Philadelphia, PA) and oligonucleotide primer pairs (Sigma) based on the sequences of the rabbit MMP-1 cDNA (sense: 5′-GGTACCAAGAGAAAGGGAGGCAAGAC-3′, antisense: 5′-CTCGAGCAGATCCTTCTAATGCCTGGAC-3′) and SRY cDNA (sense: 5′-TGAACGCATTCATGGTGTGGT-3′, antisense: 5′-AGTCTTTGCGCCTCCTGGAA-3′). PCR conditions included an initial denaturation step at 94°C for 3 minutes, followed by 30 cycles of denaturation at 94°C (25 seconds each), annealing at 55°C (1 minute), and extension at 72°C (1 minute) for both MMP-1 and SRY. PCR products were analyzed by 1.5% agarose gel electrophoresis and viewed by ethidium bromide staining. The predicted sizes of PCR-amplified products were 375 bp for MMP-1 and 157 bp for SRY genes (Fig. 1).
FIG. 1.
The polymerase chain reaction (PCR) of matrix metalloproteinase (MMP)-1 (left lanes) and sex-determining region (SRY) (right lanes) genes was performed with male (A) and female (B) mesenchymal cells (MCs). Both MMP-1 and SRY genes exist in male-derived MCs. MMP-1 genes exist in female-derived MCs, but SRY genes do not.
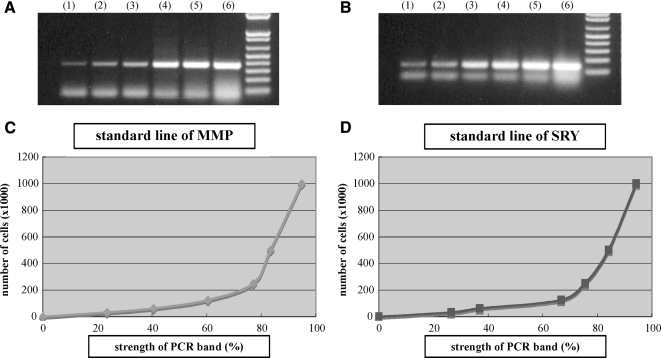
To estimate cell numbers in the samples, PCR was first performed with several known quantities of male-derived MCs, that is, 1 × 106, 5 × 105, 2.5 × 105, 1.25 × 105, 6.13 × 104, and 3.06 × 104. PCR products were electrophoresed through 1.5% agarose gels (Fig. 2A, B), and the strength of these bands was quantified using the National Institutes of Health Image Analysis Software (NIH, Bethesda, MD). Then the linear functions of each two adjacent points (i.e., 1 × 106 and 5 × 105, 5 × 105 and 2.5 × 105) were calculated and the standard lines of PCR-amplified MMP-1 and SRY sequences were generated (Fig. 2C, D). The intensity of the bands from all samples was measured by NIH software, and the cell numbers were estimated using one of the linear functions from the standard lines as a semi-quantitative method.
FIG. 2.
PCR of MMP-1 gene (A) and SRY gene (B) was performed with different concentrations of MCs: (1) 3.06 × 104, (2) 6.13 × 104, (3) 1.25 × 105, (4) 2.5 × 105, (5) 5 × 105, and (6) 1 × 106. The strength of each PCR band from the known cell numbers was calculated using NIH software, and the standard lines of MMP-1 (C) and SRY (D) genes were drawn. The X-axis shows the strength of the PCR bands (%), and the Y-axis shows the number of cells ( × 1000).
Accuracy of PCR semi-quantitative method
In order to evaluate the accuracy of this cell counting method, the cell numbers in the construct (time zero) were calculated by counting the cells directly in cell suspension using a hemocytometer and then subtracted from the original 1 million cells. We also calculated the cell number in the construct by MMP-1 and SRY with PCR method (all the cells were MCs at this time point). These three numbers represented the same measures at this time point, because all cells in the PLA were considered MCs.
Statistical analysis
The variation of cell numbers with time was statistically analyzed using the two-tailed Student's t-test assuming equal variances. All results were presented as means with standard deviations. Significance was defined as a p-value < 0.05.
Results
Gross assessment
The rabbits in all groups showed no symptoms of infection or swelling in the knee joints.
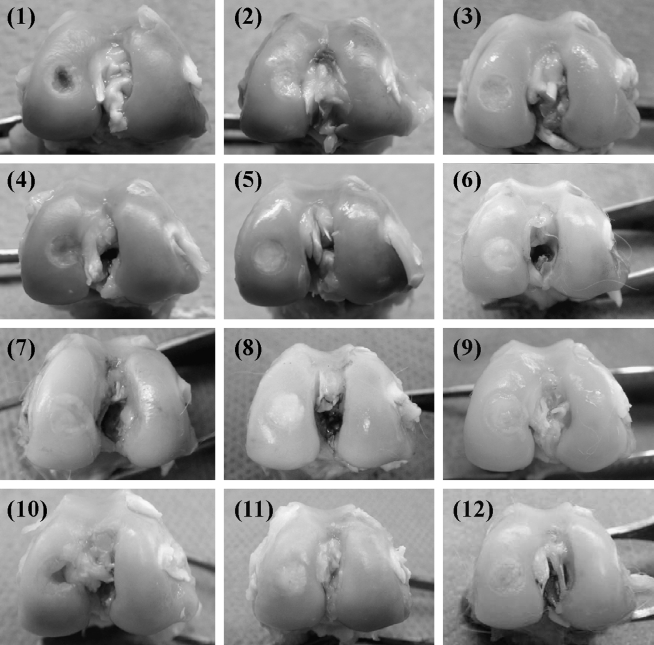
Group 1: At 1 week postsurgery, the margins of the defects could be recognized and the surfaces depressed. At 4 weeks the margins were detectable and the surfaces depressed; however, the defects were filled with white opaque tissue. At 12 weeks the surfaces did not depress and the defects were filled with white opaque tissue. At 24 weeks the surfaces were depressed in two of the three samples, and the defects were filled with white opaque tissue (Fig. 3-1).
FIG. 3.
Macroscopic findings of the defects: Group 1 (1, 4, 7, and 10) are defect alone, Group 2 (2, 5, 8, and 11) are PLA core alone without MCs, and Group 3 (3, 6, 9, and 12) are MC/PLA construct. (1–3) are at 1 week, (4–6) at 4 weeks, (7–9) at 12 weeks, and (10–12) at 24 weeks after surgery.
Group 2: At 1 week the defect areas were defined and the surfaces slightly depressed. At 4 weeks the surfaces were still slightly depressed and the defects covered with white opaque tissue. At 12 weeks the surfaces did not depress but were covered with white opaque tissue. At 24 weeks the margin of the defects was recognizable; however, the surfaces did not depress and the defects were still covered with white opaque tissue (Fig. 3-2).
Group 3: At 1 week the margins were recognized and the surfaces slightly depressed. At 4 weeks the surfaces did not depress and the defects were covered with white opaque tissue. At 12 weeks the surfaces did not depress and the color of the tissues covering the defects became similar to the surrounding tissue. At 24 weeks the margins became unclear, the surfaces did not depress, and the color of the surface was similar to that of the surrounding tissue (Fig. 3-3).
Histological evaluation
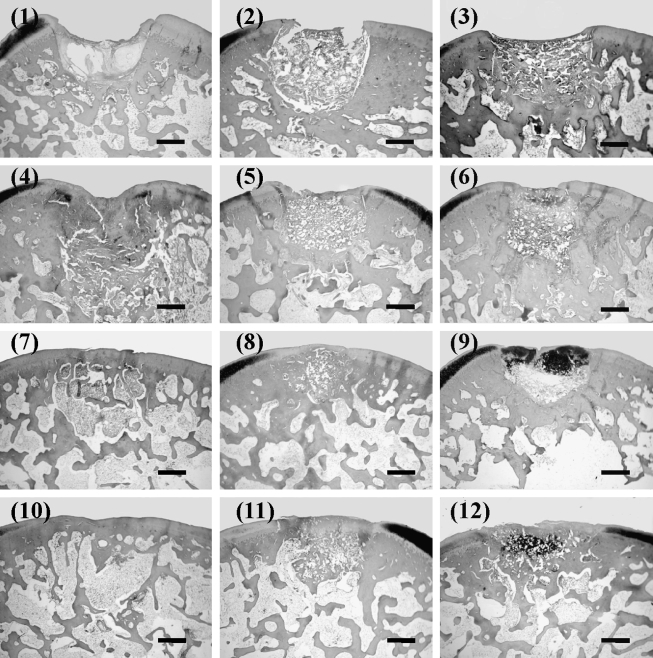
Group 1: At 1 week postsurgery the margins could be recognized and the surfaces depressed and irregular. Some areas of the defects appeared to be filled with fibrous tissue; however, the defects were not repaired with cartilaginous and/or osseous tissue. At 4 weeks the surfaces were still depressed. Most parts of the defects were filled with fibrous tissue. The repaired tissue was not stained with Safranin O. At 12 weeks the surface became smooth and the deeper layer of the defects was reconstructed with bone tissue, but the superficial layer was not repaired with cartilage tissue. At 24 weeks the deeper layer of the defects was remodeled with bone, though the defects were not repaired with cartilage tissue. There was integration between the defect area and the host tissue (Fig. 4-1).
FIG. 4.
Histological findings of the defects stained with Safranin O/Fast Green (scale bar: 1 mm). (1), (4), (7), and (10) correspond to Group 1; (2), (5), (8), and (11) correspond to Group 2; and (3), (6), (9), and (12) correspond to Group 3. (1)–(3) are at 1 week, (4)–(6) at 4 weeks, (7)–(9) at 12 weeks, and (10)–(12) are at 24 weeks after surgery.
Group 2: At 1 week the margins were discernable and the surfaces slightly depressed. The defects were occupied with PLA matrix, but the defect areas were not repaired with cartilage tissue. At 4 weeks the surfaces were still slightly depressed and PLA was confirmed to exist in the defect areas. The repaired tissues slightly increased compared with that of the 1-week model, but the defects were not repaired with cartilage and bone tissue. The integration of PLA with the host was not achieved. At 12 weeks the surfaces became smooth and PLA still existed in the defects. The deeper layer of the defects was remodeled with bone, however the defects were not repaired with cartilage tissue. At 24 weeks PLA still existed in the defects, but the defects were not repaired with cartilage tissue. The integration between the defect area and the host tissue was present (Fig. 4-2).
Group 3: At 1 week the surfaces were slightly depressed, the defects were occupied with PLA including fibrous tissues, and the defects were not repaired with cartilage tissues. At 4 weeks the surfaces were slightly depressed and PLA existed in the defects. The deeper layer of the defects was not repaired with bone tissue; however, the superficial layer of the defects was stained with Safranin O, indicating the existence of cartilaginous matrix. At 12 weeks the surface became smooth and PLA existed in the defects. The layer of cartilaginous tissue became deeper than that of the 4-week model, and the integration of PLA with the host was not achieved. Integration between the defect area and the host tissue was developing. At 24 weeks the surface was smooth, and PLA still existed in the defects. The deeper layer of the defects was repaired with bone tissue and the superficial layer was filled with cartilaginous tissue. Integration between the defect area and the host tissue was achieved (Fig. 4-3).
Immunohistochemical staining
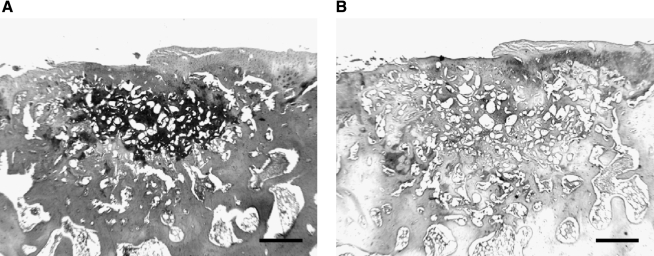
The sections at 24 weeks in Group 3 were used for immunohistochemical staining. The cartilage layer was stained with type II collagen, but the subchondral layer did not stain in the surrounding host area. Some areas of the superficial layer, which were not stained with Safranin O, stained with type II collagen similar to the surrounding cartilage layer. A few areas of the deeper layer stained with Safranin O also stained with type II collagen (Fig. 5).
FIG. 5.
Higher magnification of the section of Group 3 stained with Safranin O/Fast Green at 24 weeks (A), and immunohistochemical staining of type II collagen (B). Scale bar: 500 μm.
Accuracy of PCR semi-quantitative method
At time zero, just after the creation of the construct, all the cells in the construct were considered MCs. The cells were counted directly by MMP-1 and SRY with PCR method. These three methods showed no significant differences, and therefore the accuracy of the PCR semi-quantitative method was confirmed.
Variation of MC numbers in PLA from in vitro to in vivo conditions
The repaired tissues were harvested from the transplanted areas in Group 3. These tissues were soft and easy to separate with curette from the host tissues in the 1-, 4-, and 12-week groups. In the 24-week group, the repaired tissues connected tightly to the adjacent tissues, and it was impossible to harvest all the repaired tissues in the defect. In order not to contaminate the host tissues, the repaired tissues were harvested only from the center area of the defects in this group.
PCR was performed for harvested tissues at different time periods in vivo, as well as on the day of MC/PLA construct creation (time zero), and 1 week after culturing of the construct in vitro. The strength of all PCR bands were calculated using NIH imaging software and applied to the standard lines of MMP-1 and SRY genes. Then the number of total cells and transplanted MCs in the harvested tissues were estimated.
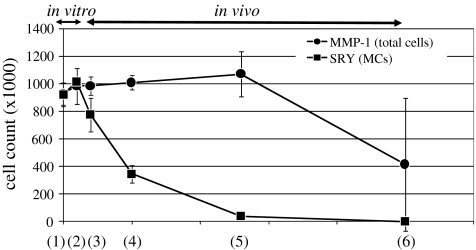
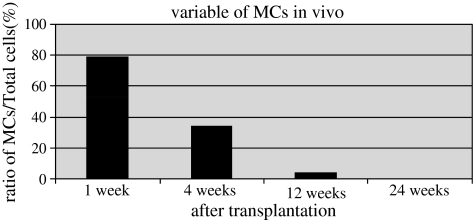
At time zero, total number of cells was 9.3 × 105 and increased up to 9.8 × 105 during 1 week culturing in vitro. The constructs were transplanted and the total cell numbers were 9.8 × 105 at 1 week, 1.0 × 106 at 4 weeks, 1.1 × 106 at 12 weeks, and 4.2 × 105 at 24 weeks. MC numbers were 7.8 × 105 at 1 week after transplantation, 3.5 × 105 at 4 weeks, and 3.8 × 104 at 12 weeks. MCs were not detected at 24 weeks (Fig. 6). The ratio of the number of MCs divided by the total number of cells in the defect was 79% at 1 week after transplantation, 34% at 4 weeks, and 4% at 12 weeks. The ratio at 24 weeks was not calculated, because transplanted MCs were not detected in the defects at this time (Fig. 7).
FIG. 6.
The varying cell numbers of MCs (line with squares) and total cells (line with circles) in the defects from in vitro to in vivo conditions. The data was presented as mean ± SD. The X-axis shows the time periods: (1) time zero, (2) the construct 1 week after culturing, (3) 1 week after transplantation, (4) 4 weeks after transplantation, (5) 12 weeks after transplantation, and (6) 24 weeks after transplantation. (1) and (2) represent in vitro, and (3)–(6) represent in vivo conditions. The Y-axis shows the cell numbers (×1000).
FIG. 7.
The varying ratio of transplanted MCs to total cells with time after transplantation of the construct in vivo. The Y-axis shows the ratio of MCs to total number of cells. The ratio at 24 weeks was zero, because transplanted MCs were not detected in the defects.
Discussion
The survivability of MCs in a PLA scaffold in an osteochondral defect and the possibility of osteochondral repair with MC/PLA construct were evaluated in the in vivo conditions. Transplanted MCs survived and contributed to the osteochondral repair by expressing cartilaginous matrix. The MCs, however, were replaced by host cells with time and disappeared within 24 weeks after the transplantation.
Several chondrogenic lineage cell sources have been reported, such as chondrocytes, MCs, periosteal cells, and perichondrial cells.15,30–32 Periosteum was used to affix transplanted cells in the transplanted area originally. The scaffolds commonly used are PLA, polylactic-glycolic acid (PLGA), collagen gel, fibrin glue, and atelocollagen gel.28,33–36 Although chondrogenic cell/scaffold constructs could facilitate transplantation of a large number of cells in the defect, regeneration of cartilage had not been fully achieved. Additional growth factors and gene therapies have been described to improve repair tissue close to hyaline cartilage. But it is essential to elucidate the behavior and survivability of transplanted cells when cell-based treatment is performed. Therefore, we tried to evaluate the role of transplanted cells without advanced treatments in this study.
MCs and a PLA core have been applied for osteochondral reconstruction. MCs including MSCs have the capacity to differentiate into both cartilage and bone under adequate conditions. PLA is a biocompatible, biodegradable, and nonimmunologic reactive material in vivo.29 The benefits of chondrogenic cell/PLA constructs for cell survival, increase in cell number, and expression of cartilaginous matrix in vitro were demonstrated.26
Articular cartilage tissues have a limited intrinsic healing potential.37 The natural healing capacity of a full-thickness osteochondral defect was, however, demonstrated in a rabbit model with a small-size defect.38 The defect was 3.7 mm in diameter and 3.0 mm in depth in our experiment, and could be applied as an unrepaired defect model as the defect was not repaired with hyaline cartilage in Group 1.
Recently, the potential of an immunoresponse by small polymeric particles released from the PLA has been described.39 However, no inflammatory reaction was observed in the knee joints of Group 2. The PLA was also confirmed to not have the capacity to repair an osteochondral defect.
Twenty-four weeks after transplantation of the MC/PLA construct in Group 3, the defect was filled with PLA matrix, glycosaminoglycan (GAG), and type II collagen. When compared with serial sections having positive areas of GAG and type II collagen, there was coexistence in some areas. Thus, it is assumed that MCs could maintain their capacity to differentiate into cartilage lineage cells in the PLA in vitro, and once MCs are transplanted into the defect some MCs could express one or both of the cartilage matrices in vivo.
To detect the presence of transplanted cells, MSC/gelatin constructs were transplanted into osteochondral defects, and MSCs were detected 2 weeks later by labeling with fluorescent dye.40 The chondrocytes were also detected in the defect up to 14 weeks after ACI using fluorescent dye.41 Emans et al.42 demonstrated transplantation of chondrocyte or periosteal cell/scaffold constructs into an osteochondral defect and examined cell viability by fluorescent labeling 5 days after transplantation. However, the intensity of the fluorescent dye may decrease with time and may not be adequate for a long-term study. Mierisch43 performed the transfection of a fluorescent protein gene to chondrocytes and traced the transplanted cells in the defect 4 weeks after ACI. This method, however, includes difficulty with the ratio of transfection efficiency.
By contrast, we previously succeeded in viewing transplanted cells using transgenic rats in vivo. Transgenic rat–derived MCs were transplanted into osteochondral defects of wild-type rats, and the fate of transplanted cells was evaluated by in situ hybridization technique24 or with a confocal laser scanning microscope.25 The cells could be traced as long as they survived. Nishimori et al.44 also demonstrated transplantation of MSCs into osteochondral defects using a transgenic rat model and reported the existence of transplanted cells up to 4 weeks after transplantation. In our models, however, osteochondral defects were repaired with hyaline-like cartilage and the transplanted cells were detected up to 24 weeks after transplantation. The number of cells in the transplanted area was not determined because cell viability was assessed with two-dimensional sections.
Ostrander et al.28 demonstrated the SRY study for examining the fate of donor cells in their rabbit model. They transplanted a perichondrial cell/PLA construct, and the survivability of the transplanted cells was evaluated up to 4 weeks after transplantation. The results showed that more than 87% of the transplanted cells survived during this time in the defect and, though the number of transplanted cells decreased, the total number of cells increased with time. They concluded that a decline in the percentage of donor cells was possible only with an influx of host cells. Even though our results showed that MCs stayed longer than the perichondrial cells, MCs disappeared within 24 weeks. However, a decline in donor cell numbers does not necessarily mean failure of the treatment.32 These authors preincubated the construct before transplantation and demonstrated enhanced survivability of perichondrial cells in the PLA. Based on their result, in our study the construct was cultured 1 week before transplantation and number of MCs increased in vitro. They discussed the importance of a marginal influx of host cells into the implanted construct and a marginal efflux of donor cells into surrounding tissues for integration between host and donor. They concluded that donor cells decreased and host cells increased at the same time in the defect with integration.
We focused on the total number of cells in the defect, detected by MMP-1, as well as the transplanted cells, detected by SRY, and thus the cell movement between donor and host could be assessed in this model. Total cell number did not change significantly up to 12 weeks; however, MCs were decreasing in number. PLA was confirmed to provide a suitable condition for cell survival in the defect.28 The efflux of the transplanted MCs into surrounding tissue was also viewed histologically.25 Based on these results, MCs were thought to survive in the PLA and to be replaced by an influx of host cells, with an assumed efflux of MCs into surrounding tissue. Because of this process, the integration of repair tissue with the adjacent tissue was well developed at 24 weeks after transplantation, even though the tissues in the defect area were easily harvested by curettage until 12 weeks. In order not to contaminate MCs in the host tissue, the tissues in the center area of the defects were harvested at this time period. MCs could not be detected in the 24-week samples, and therefore MCs were thought to have differentiated into cartilage and bone lineages in the defect in the in vivo condition, efflux to the surrounding tissues, and be replaced by host cells while expressing cartilage and bone matrices.
In this study we succeeded in examining the variation of MCs in vivo by a semi-quantitative method. Cartilage reconstruction trials with cell/scaffold construct transplantation for cartilage repair have been performed in the clinical area. However, it is still unclear how many cells survive in the defects and how many cells should be transplanted. The results of this study could provide the answers to these questions, as it seems that transplanted cells decrease in the early time period. If further treatments are added to improve regenerated tissue, it is suggested they be done in vitro or in the early stage in vivo. The SRY study is a useful method to evaluate the survivability of transplanted cells in vivo, and MC/PLA constructs could be clinically used for osteochondral repair.
Conclusion
Transplanted MCs can survive in PLA scaffolds in an osteochondral defect and promote repair of the defect. However, the number of cells decrease with time in vivo. These results are essential to understanding the behavior of autologous cell transplantation for tissue engineering strategies.
Footnotes
This paper is based on the Osteoarthritis Research Society International (OARSI) award presentation at the 2006 Prague Meeting.
Acknowledgments
We gratefully acknowledge support from NIH Training Grant AR07484, Malcolm and Dorothy Coutts Institute for Joint Reconstruction and Research, San Diego, CA, and Sharp Healthcare, San Diego, CA.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lawrence R.C. Helmick C.G. Arnett F.C. Deyo R.A. Felson D.T. Giannini E.H. Heyse S.P. Hirsch R. Hochberg M.C. Hunder G.G. Liang M.H. Pillemer S.R. Steen V.D. Wolfe F. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Johnson L.L. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy. 1986;2:54. doi: 10.1016/s0749-8063(86)80012-3. [DOI] [PubMed] [Google Scholar]
- 3.Steadman J.R. Rodkey W.G. Rodrigo J.J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop. 2001;391(Suppl):S362. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 4.Pridie K.H. A method of resurfacing osteoarthritic knee joints. Proc & Reports of Councils & Assoc; J Bone Joint Surg Br. 1959;41B:618. [Google Scholar]
- 5.Mitchell N. Shepard N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg Am. 1976;58:230. [PubMed] [Google Scholar]
- 6.Matsusue K. Yamamuro T. Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9:318. doi: 10.1016/s0749-8063(05)80428-1. [DOI] [PubMed] [Google Scholar]
- 7.Hangody L. Kish G. Kárpáti Z. Szerb I. Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Arthroscopy. 1997;5:262. doi: 10.1007/s001670050061. [DOI] [PubMed] [Google Scholar]
- 8.Gortz S. Bugbee W.D. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Gidwani S. Fairbank A. The orthopaedic approach to managing osteoarthritis of the knee. BMJ. 2004;329:1220. doi: 10.1136/bmj.329.7476.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa T. Eyre D.R. Koide S. Glimcher M.J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62:79. [PubMed] [Google Scholar]
- 11.Hangody L. Feczkó P. Bartha L. Bodó G. Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop. 2001;391(Suppl):S328. doi: 10.1097/00003086-200110001-00030. Review. [DOI] [PubMed] [Google Scholar]
- 12.Jamali A.A. Emmerson B.C. Chung C. Convery F.R. Bugbee W.D. Fresh osteochondral allografts. Clin Orthop. 2005;437:176. [PubMed] [Google Scholar]
- 13.Buechel F.F. A sequential three-step lateral release for correcting fixed valgus knee deformities during total knee arthroplasty. Clin Orthop. 1990;260:170. [PubMed] [Google Scholar]
- 14.Jordan L.R. Olivo J.L. Voorhorst P.E. Survivorship analysis of cementless meniscal bearing total knee arthroplasty. Clin Orthop. 1997;338:119. doi: 10.1097/00003086-199705000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 16.Driesang I.M. Hunziker E.B. Delamination rates of tissue flaps used in articular cartilage repair. J Orthop Res. 2000;18:909. doi: 10.1002/jor.1100180609. [DOI] [PubMed] [Google Scholar]
- 17.Henderson I. Gui J. Lavigne P. Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy. 2006;22:1318. doi: 10.1016/j.arthro.2006.07.057. [DOI] [PubMed] [Google Scholar]
- 18.Kreuz P.C. Steinwachs M. Erggelet C. Krause S.J. Ossendorf C. Maier D. Ghanem N. Uhl M. Haag M. Classification of graft hypertrophy after autologous chondrocyte implantation of full-thickness chondral defects in the knee. Osteoarthritis Cartilage. 2007;15:1339. doi: 10.1016/j.joca.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Barlic A. Drobnic M. Malicev E. Kregar-Velikonja N. Quantitative analysis of gene expression in human articular chondrocytes assigned for autologous implantation. J Orthop Res. 2008;26:847. doi: 10.1002/jor.20559. [DOI] [PubMed] [Google Scholar]
- 20.Temenoff J.S. Mikos A.G. Review: tissue engineering for articular cartilage. Biomaterials. 2000;21:431. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G. Liu W. Cui L. Wang X. Liu T. Cao Y. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 2006;12:3209. doi: 10.1089/ten.2006.12.3209. [DOI] [PubMed] [Google Scholar]
- 22.Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 23.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Oshima Y. Watanabe N. Matsuda K. Takai S. Kawata M. Kubo T. Fate of transplanted bone-marrow-derived mesenchymal cells during osteochondral repair using transgenic rats to simulate autologous transplantation. Osteoarthritis Cartilage. 2004;12:811. doi: 10.1016/j.joca.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Oshima Y. Watanabe N. Matsuda K. Takai S. Kawata M. Kubo T. Behavior of transplanted bone marrow-derived GFP mesenchymal cells in osteochondral defect as a simulation of autologous transplantation. J Histochem Cytochem. 2005;53:207. doi: 10.1369/jhc.4A6280.2005. [DOI] [PubMed] [Google Scholar]
- 26.Chu C.R. Monosov A.Z. Amiel D. In situ assessment of cell viability within biodegradable polylactic acid matrices. Biomaterials. 1995;16:1381. doi: 10.1016/0142-9612(95)96873-x. [DOI] [PubMed] [Google Scholar]
- 27.Yan H. Yu C. Repair of full-thickness cartilage defect with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Ostrander R.V. Goomer R.S. Tontz W.L. Khatod M. Harwood F.L. Maris T.M. Amiel D. Donor cell fate in tissue engineering for articular cartilage repair. Clin Orthop. 2001;389:228. doi: 10.1097/00003086-200108000-00032. [DOI] [PubMed] [Google Scholar]
- 29.Chu C.R. Coutts R.D. Yoshioka M. Harwood F.L. Monosov A.Z. Amiel D. Articular cartilage repair using allogeneic perichondrocyte-seeded biodegradable porous polylactic acid (PLA): a tissue-engineering study. J Biomed Mater Res. 1995;29:1147. doi: 10.1002/jbm.820290915. [DOI] [PubMed] [Google Scholar]
- 30.Wakitani S. Imoto K. Yamamoto T. Saito M. Murata N. Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 31.Mardones R.M. Reinholz G.G. Fitzsimmons J.S. Zobitz M.E. An K.N. Lewallen D.G. Yaszemski M.J. O'Driscoll S.W. Development of a biologic prosthetic composite for cartilage repair. Tissue Eng. 2005;11:1368. doi: 10.1089/ten.2005.11.1368. [DOI] [PubMed] [Google Scholar]
- 32.Ball S.T. Goomer R.S. Ostrander R.V. Tontz W.L., Jr. Williams S.K. Amiel D. Preincubation of tissue engineered constructs enhances donor cell retention. Clin Orthop. 2004;420:276. doi: 10.1097/00003086-200403000-00039. [DOI] [PubMed] [Google Scholar]
- 33.Caterson E.J. Li W.J. Nesti L.J. Albert T. Danielson K. Tuan R.S. Polymer/alginate amalgam for cartilage tissue engineering. Ann N Y Acad Sci. 2002;961:134. doi: 10.1111/j.1749-6632.2002.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 34.Uematsu K. Hattori K. Ishimoto Y. Yamauchi J. Habata T. Takakura Y. Ohgushi H. Fukuchi T. Sato M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273. doi: 10.1016/j.biomaterials.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Dragoo J.L. Carlson G. McCormick F. Khan-Farooqi H. Zhu M. Zuk P.A. Benhaim P. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13:1615. doi: 10.1089/ten.2006.0249. [DOI] [PubMed] [Google Scholar]
- 36.Agung M. Ochi M. Adachi N. Uchio Y. Takao M. Kawasaki K. Osteochondritis dissecans of the talus treated by the transplantation of tissue-engineered cartilage. Arthroscopy. 2004;20:1075. doi: 10.1016/j.arthro.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 37.Mankin H.J. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460. [PubMed] [Google Scholar]
- 38.Shapiro F. Koide S. Glimcher M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Spain T.L. Agrawal C.M. Athanasiou K.A. New technique to extend the useful life of a biodegradable cartilage implant. Tissue Eng. 1998;4:343. doi: 10.1089/ten.1998.4.343. [DOI] [PubMed] [Google Scholar]
- 40.Quintavalla J. Uziel-Fusi S. Yin J. Boehnlein E. Pastor G. Blancuzzi V. Singh H.N. Kraus K.H. O'Byrne E. Pellas T.C. Fluorescently labeled mesenchymal stem cells (MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002;23:109. doi: 10.1016/s0142-9612(01)00086-2. [DOI] [PubMed] [Google Scholar]
- 41.Dell'Accio F. Vanlauwe J. Bellemans J. Neys J. De Bari C. Luyten F.P. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res. 2003;21:123. doi: 10.1016/S0736-0266(02)00090-6. Erratum in: J Orthop Res 21, 572, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Emans P.J. Pieper J. Hulsbosch M.M. Koenders M. Kreijveld E. Surtel D.A.M. van Blitterswijk C.A. Bulstra S.K. Kuijer R. Riesle J. Differential cell viability of chondrocytes and progenitor cells in tissue-engineered constructs following implantation into osteochondral defects. Tissue Eng. 2006;12:1699. doi: 10.1089/ten.2006.12.1699. [DOI] [PubMed] [Google Scholar]
- 43.Mierisch C.M. Wilson H.A. Turner M.A. Milbrandt T.A. Berthoux L. Hammarskjöld M.L. Rekosh D. Balian G. Diduch D.R. Chondrocyte transplantation into articular cartilage defects with use of calcium alginate: the fate of the cells. J Bone Joint Surg Am. 2003;85A:1757. doi: 10.2106/00004623-200309000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Nishimori M. Deie M. Kanaya A. Exham H. Adachi N. Ochi M. Repair of chronic osteochondral defects in the rat: a bone marrow-stimulating procedure enhanced by cultured allogenic bone marrow mesenchymal stromal cells. J Bone Joint Surg Br. 2006;88B:1236. doi: 10.1302/0301-620X.88B9.17810. [DOI] [PubMed] [Google Scholar]