Abstract
We have developed a new fabrication technique to create three-dimensional (3D) porous poly(ε-caprolactone fumarate) (PCLF) scaffolds using hydrogel microparticle porogens, as an alternative to overcome certain limitations of traditional scaffold fabrication techniques such as a salt leaching method. Both natural hydrogel, gelatin, and synthetic hydrogel, poly(ethylene glycol) sebacic acid diacrylate, were used as porogens to fabricate 3D porous PCLF scaffolds. Hydrogel microparticles were prepared by a single emulsion technique with the particle size in the range of 100–500 μm after equilibrium in water. The pore size distribution, porosity, pore interconnectivity, and spatial pore heterogeneity of the 3D PCLF scaffolds were assessed using micro-computed tomography and imaging analysis. Scaffolds fabricated with the hydrogel porogens had higher porosity and pore interconnectivity as well as more homogeneous spatial pore distribution, compared to the scaffolds made from the salt leaching process. Compressive moduli of the scaffolds were also measured and showed that lower porosity yielded greater modulus of the scaffolds. Overall, the new fabrication technology using hydrogel porogens may be beneficial for certain tissue engineering applications.
Introduction
The clinical needs for bone regeneration are diverse, and there are roughly one million patients who have skeletal defects each year in the United States alone, which require bone graft procedures to achieve union.1 Toward this end, tissue engineering approaches have received great deal of attention as promising alternatives to conventional autologous bone grafts that are considered the golden standard for repairing critical-sized bone defects.2,3 Numerous biomaterials have been developed for bone tissue engineering applications ranging from naturally derived materials to synthetic biopolymers.4–8 In the past decades, significant progress has been made to fabricate three-dimensional (3D) porous polymeric scaffolds with controlled morphology and micro-architecture for bone tissue engineering.9 However, most of these techniques such as fiber bonding,10 freeze-drying,11 and phase separation12 are not compatible for use with injectable materials.
It is known that porous scaffolds can also be generated by combining a water-leachable porogen (e.g., sodium chloride) in the injectable paste.13,14 However, there are many issues associated with the use of high salt porogen concentrations. These include difficulty of handling and decreased injectability of the composite, nonuniform distribution of the porogen, compromised mechanical strength of the scaffold after porogen leach-out, and potential local toxicity caused by high osmolarity from the leached salt. For example, previous work demonstrated that the solid salt porogen particles will not flow in a homogeneous manner with the polymerizing scaffold during injection when the porosity reaches above 75%.15 Due to the limited amount of porogen that can be incorporated, it is often difficult to achieve high porosity to induce tissue ingrowth and minimize diffusion limitations for certain type of polymers such as injectable polymers. In addition, once the scaffold is injected, the process of porogen diffusion out of the scaffold needs to occur before the scaffold pores being filled with physiological fluids.
In current study, we propose a novel concept of using hydrogel microparticles (MPs) as porogens to reproducibly form well-interconnected pore networks. Hydrogel MPs are incorporated into the injectable paste, they swell during mixing and injection to retain water in the pores, and instead of leaching out, the porogens create pores as they can degrade in physiological condition. The potential advantages of hydrogel porogens include better rheological properties during injection, eventual elimination of the porogen leaching step, and the ability to load and deliver cells, bioactive molecules, or both within the hydrogel phase at the time of scaffold injection into the defective tissue site.
In this study, we chose poly(ɛ-caprolactone fumarate) (PCLF) as a matrix polymer mainly because it is biocompatible, biodegradable, self-crosslinkable without the use of any crosslinkers, and injectable, and it involves relatively simple and straightforward synthesis process.16 Two different hydrogels were chosen for fabrication of hydrogel porogens: one is gelatin, one of the most widely investigated natural hydrogels for tissue engineering applications, and the other is poly(ethylene glycol) sebacic acid diacrylate (PEGSDA), a novel biodegradable synthetic hydrogel developed in our laboratory.17
Materials and Methods
PCLF and PEGSDA macromer synthesis
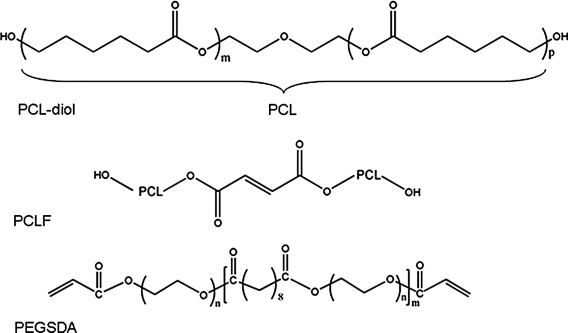
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. PCLF and PEGSDA macromers were synthesized as described previously,16,17 and their chemical structures are shown in Figure 1. Briefly, for PCLF synthesis, poly(ɛ-caprolactone) (PCL) diol (1.0 mol) with nominal molecular weight of 530 Da was dried overnight in a vacuum at 50°C. Dried fumaryl chloride (0.9 mol) and K2CO3 (1.5 mol) were subsequently added in anhydrous methylene chloride. The reaction was run for 12 h at room temperature, and the resulting polymer was obtained after precipitation in petroleum ether. For PEGSDA synthesis, poly(ethylene glycol) (PEGs) with a nominal molecular weight of 3350 Da were dried by azeotropic distillation and removed residual water under reduced pressure. PEG (1.0 mol) was then dissolved in anhydrous methylene chloride. Dried sebacoyl chloride (0.9 mol) and triethylamine (1.5 mol) were subsequently added into the solution with vigorous stirring. The reaction was run for 24 h at room temperature, and the product (PEG sabacate or PEGS) was obtained after precipitation in petroleum ether. Similarly, PEGSDA was synthesized by adding 1.5 mol of acryloyl chloride and triethylamine (TEA) into the PEGS (1.0 mol) solution, the reaction was run for another 24 h, and the resulting polymer (PEGSDA) was obtained after precipitation in petroleum ether and drying in vacuum overnight.
FIG. 1.
Chemical structures of poly(ɛ-caprolactone) (PCL) diol, poly(ɛ-caprolactone fumarate) (PCLF), and poly(ethylene glycol) sebacic acid diacrylate (PEGSDA).
Preparation of hydrogel MPs
Gelatin MPs
Gelatin MPs were prepared by following previously established procedures.18,19 Briefly, a gelatin solution (10% w/v) was prepared by dissolving 10 g of porcine gelatin powder in 90 mL distilled deionized water (DDW) at 95°C with gentle mixing. The gelatin solution (4 g) was then added dropwise while stirring at 360 rpm into 100 mL mineral oil containing 1.5% of sorbitan monoleate and 0.5% of polyoxyethylene sorbitanmonooleate. After 30 min of MP formation at 10°C, gelatin MPs were filtered and washed with DDW. These MPs were then crosslinked in DDW with 10 mM glutaaldehyde while stirring at 200 rpm at 10°C for 1 h, collected by filtration, washed with DDW three times to remove any unreacted glutaaldehyde, and stored at −4°C until use.
PEGSDA MPs
PEGSDA hydrogel MPs were fabricated similarly using the gelatin MP fabrication method described above (water in oil). Briefly, 1.0 g of PEGSDA macromer was dissolved in 3 mL of DDW (namely, PEGSDA/25%) and centrifuged briefly to remove the trapped air in the solution. Water soluble redox radical initiator system (ammonium persulfate and ascorbic acid) was used to crosslink the macromer.20 About 10 μL of 0.3 M ammonium persulfate and 0.3 M ascorbic acid in DDW was added into the macromer solution and subsequently added dropwise into mineral oil containing 1.5% sorbitan monooleate and 0.5% polyoxyethylene sorbitanmonooleate. The crosslinking reaction was carried out at 40°C with mixing at 260 rpm for 30 min under nitrogen atmosphere. After the reaction, PEGSDA/25% MPs were washed with DDW three times to remove residual mineral oil and stored in a refrigerator until use. To generate different particle size of hydrogel MPs, 33% (w/v) of PEGSDA macromer (namely, PEGSDA/33%) was also used. The surface morphology of hydrogel MPs was analyzed by phase contrast microscopy (Axiovert, Thornwood, NY) equipped with a CCD camera. The particle size distribution was estimated by measuring the particle diameters in at least five randomly chosen microscopy images of the particles using the Analyze™ software (version 7.0) developed by the Mayo Clinic Biomedical Imaging Resource, Rochester, MN.21
Rheological properties of polymer composite formulations
Rheological properties of polymer composite formulations containing melted PCLF (25 wt%) and the porogens (75 wt%) were analyzed using a dynamic mechanical rheometer (TA Instruments, New Castle, DE) with a parallel-plate (20 mm diameter). The dynamic shear strain sweep experiment was first performed at a frequency 10 rad/s to determine the linear stress-strain range of the different samples, which indicated that 0.1% of shear strain was in the linear stress–strain range of tested samples. The complex viscosity, |1;η*|1;, and dynamic shear elastic modulus, G′, of the samples were then obtained by the frequency sweep test with a frequency range from 1 to 100 rad/s at a strain of 0.1%.
Three-dimensional porous PCLF scaffold fabrication
Three-dimensional porous scaffolds were fabricated by free radical polymerization of PCLF using hydrogel MPs or sodium chloride particles (particle size, 300–500 μm) as porogens. In a typical procedure, 0.5 g of PCLF macromer was placed in an oven at 60°C for 10 min and mixed with 1.5 g of hydrogel or salt porogen, corresponding to 75% porosity. About 150 μL benzoyl peroxide solution (200 mg/mL in n-vinyl pyrrolidone, NVP) as an initiator and 20 μL dimethyl toluidine solution (20 μL/mL in NVP) as an accelerator were added to the polymer solution.16 After thorough mixing, the polymerizing solution was transferred to a glass tube mold (1 × 2 cm, diameter × height) and allowed to be crosslinked for 1 h at 37°C.
After crosslinking, the mold was cooled to room temperature and the scaffold was removed from the mold. To quickly generate 3D porous PCLF structure, the hydrogel porogens were leached out from the scaffold by incubating the scaffold in 0.1 N NaOH solution overnight. The salt porogens were also leached out by placing the scaffold in DDW for 7 days with vigorous shaking and water change twice a day to prevent salt saturation. The resulting porous scaffolds were dried at room temperature for 1 day and further dried in a vacuum oven overnight. The 3D porous PCLF scaffolds were then characterized for morphology, pore structures, and mechanical properties.
Characterization of 3D porous PCLF scaffolds
Scanning electron microscopy
Cold-field emission scanning electron microscopy (SEM) (S-4700; Hitachi Instruments, San Jose, CA) was used to examine the morphology of porous PCLF scaffolds. All scaffolds were viewed at 3 kV accelerating voltage and 9500 nA emission current.
X-ray micro-computed tomography
A custom-built micro-computed tomography (micro-CT) was used as described previously.22 All resulting images were reconstructed using a modified Feldkamp cone beam tomographic reconstruction algorithm that resulted in cubic voxels (20 μm on each side).23 After micro-CT scanning, the images were reconstructed using Analyze 3D image display and analysis program (Analyze, version 7.0) developed by the Mayo Clinic Biomedical Imaging Resource.21
Porosity and pore size measurement
Analyze software was utilized to process 3D images of the scaffolds as described previously.22 Briefly, the images were segmented by an operator-selected threshold of intensities to separate voxels representing regions of differing density, and then a 3 × 3 × 3 median filter was applied as a noise reduction operation. Images were then analyzed to obtain void volume fractions of each material and accessible void volume. Volume fractions of air and polymer were obtained by selecting subregions of each volume representing a scaffold and logging counts of voxels representing each material. The porosity of a scaffold was then calculated by Equation (1):
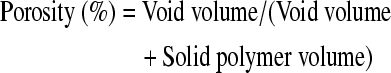 |
(1) |
Pore size distribution throughout the scaffold was also estimated in at least five randomly chosen binary images of the scaffolds by the image analysis using the Analyze software.
Pore interconnectivity analysis
Pore interconnectivity (PI) of each scaffold was accessed using a custom Tool Command Language program utilizing operations in the AVW command library (Mayo Foundation, Rochester, MN).24 Interconnectivity of virtual scaffolds was analyzed using a computer algorithm based on the method developed by Camp and colleagues.25 This algorithm first found voxels representing air that maintained connections to other air voxels and labeled regions of connected and isolated air with a connected-components operation. Accessible void volume was computed as the number of air voxels maintaining connections with the outside air surrounding the scaffold as a percentage of the total air voxels as shown in Equation (2):
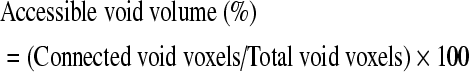 |
(2) |
Next, a larger structuring element was chosen for performing a mathematical morphological operation known as “opening,” and the process was performed again on the original segmented image. By repeating this process using a successively larger structural element for the open operation, the scaffold PI was assessed over a range of minimum sizes that correspond to multiples of the voxel sizes.
Spatial pore heterogeneity
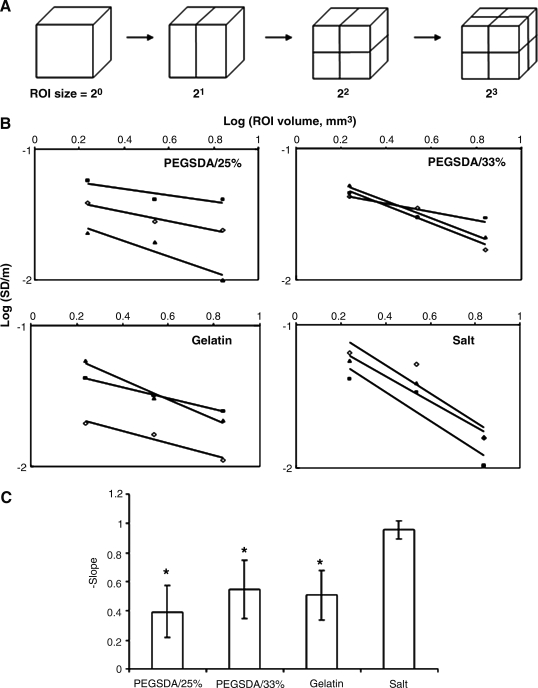
To estimate spatial pore heterogeneity (SPH) in this study, we adopted the method to determined spatial heterogeneity of myocardial perfusion that has been recognized for years.26,27 SPH inside PCLF scaffolds was assessed by calculating porosity distribution at selected scaffold volume of interest as illustrated in Figure 9.28 Figure 9A shows how a scaffold can be subdivided into small regions of interest (ROI). The pore heterogeneity can be quantified by the relative dispersion (RD) of the porosity of the scaffold, which is represented by a standard deviation divided by the mean of all porosity values at specific sample volume of interest. The linear relationship can then be obtained by plotting the log (RD) against the log (size of ROI) (Fig. 9B). If the heterogeneity follows a fractal pattern, each ROI has greater RD with decreasing size of ROI. SPH was then quantified by the slope value of the scaffold and higher value represents higher SPH in the scaffold (Fig. 9C).
FIG. 9.
Quantitative analysis of spatial pore heterogeneity (SPH) of PCLF scaffolds. (A) Schematics show how the porous scaffold can be subdivided into small regions of interest (ROI). (B) Plots of log (relative dispersion (RD) at selected volume of ROI) versus log (sub-volume of ROI) result in a near-linear relationship. RD is the relative dispersion (or coefficient of variation), obtained by dividing the standard deviation by the mean of all values. (C) Average slope values obtained from the plots in (B) for 3D PCLF scaffolds fabricated with different porogens. Greater slope value represents higher spatial heterogeneity. Error bars represent means ± standard deviations for n = 3. *Significant difference (p < 0.05) from the scaffolds fabricated with salt porogens.
Compressive modulus
Compressive mechanical properties of the 3D PCLF scaffolds before and after porogen leaching were measured by a dynamic mechanical analyzer (DMA; TA Instruments). Before testing, initial cross-sectional area and dimension of each sample were measured. All samples were submerged in deionized water and compressed at a loading rate of 2 N/min up to a maximum loading of 15 N. Compressive modulus was determined by dividing applied forces and dimensional changes into initial cross-sectional area and dimension, and then calculating the slope of linear region in the stress versus strain curve.
Statistical analysis
All data are reported as means ± standard deviations. Statistical analysis was performed using single factor analysis of variance (ANOVA) with a significance level of 0.05 for multiple comparison tests.
Results and Discussion
Natural hydrogel, gelatin, and synthetic hydrogel, PEGSDA, were investigated as porogens to fabricate 3D PCLF scaffolds and compared their properties with those of the scaffolds fabricated with salt porogens. PCLF and PEGSDA macromers were successfully synthesized, and the molecular weights of starting PEG precursors and PCL as well as the resulting PEGSDA and PCLF polymers are shown in Table 1.
Table 1.
Number Average Molecular Weight and Polydispersity Index of Synthesized Porous Poly(ɛ-caprolactone Fumarate) and Poly (Ethylene Glycol) Sebacic Acid Diacrylate Macromers
| Sample | PEG or PCL Mn | Mn | PDIa |
|---|---|---|---|
| PCLF530 | PCL530 | 3.050 ± 13 | 2.04 |
| PEGSDA3.4K | PEG3400 | 19.220 ± 280 | 1.85 |
PDI = Mw/Mn.
Mn, number average molecular weight; PDI, polydispersity index.
Hydrogel MP fabrication and characterization
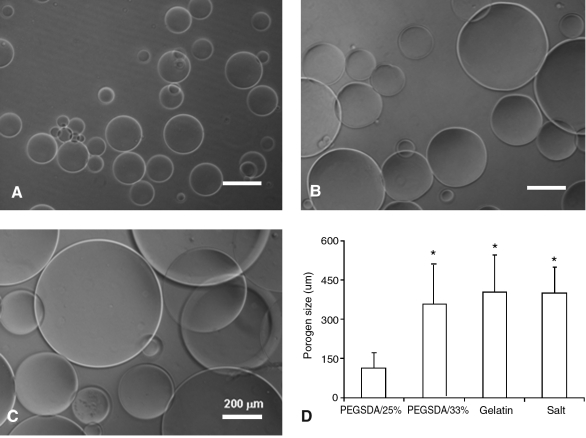
Hydrogel MPs were fabricated using an oil-in-water single emulsion technique. Figure 2 shows microscopic images of hydrogel MPs, and their average particle sizes ranged between 100 and 500 μm (Fig. 2D). In this study, two different hydrogel particle sizes (e.g., ∼100 and ∼400 μm) were chosen to compare pore properties of resulting PCLF scaffolds fabricated with hydrogel MPs. This range of pore sizes have shown most satisfactory outcome especially for bone tissue engineering due to the promotion of tissue ingrowth and distribution of osteoprogenitor cells throughout the matrix as well as the migration of endothelial cells into the matrix, which are crucial to angiogenesis and new bone formation.29
FIG. 2.
Light microscopic images of hydrogel microparticles (MPs): (A) PEGSDA3.4K (25%), (B) PEGSDA3.4K (33%), and (C) gelatin (10%). (D) Particle size distributions of the various porogens. Error bars represent means ± standard deviations for n ≥ 5. *Significant difference (p < 0.05) from PEGSDA/25% porogens.
As expected, to obtain smaller hydrogel MP size, less macromer was needed when the same macromer (e.g., PEGSDA3.4K) was used. When low molecular weight PEGSDA macromer (PEGSDA1.0K) was used, much smaller MPs were formed (average particle size was ∼40 μm) likely due to the decreased viscosity of the resulting polymer solution. These results demonstrated that the particle size of synthetic hydrogel MPs can be varied by controlling the macromer concentration in the polymer solution or the molecular weight of macromers before crosslinking. Gelatin MPs were also successfully fabricated, and their average particle size was similar to PEGSDA/33% MPs (Fig. 2C). The particle size of gelatin MPs could also be controlled by changing initial gelatin concentration in the solution.
Rheological properties of polymer composites
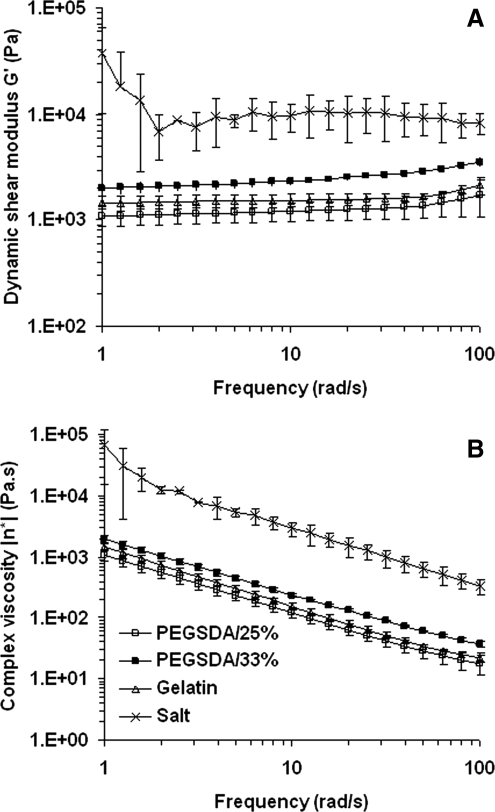
Rheological properties of uncrosslinked PCLF composites containing 75% (w/w) of hydrogel MPs were evaluated and compared to those composites containing the same amount of salt porogens (Fig. 3). Dynamic shear modulus (G') and complex viscosity (|η*|) of the composite containing salts were much higher than those of the composites with hydrogel MPs and less stable throughout the tested frequency ranges, especially at low ranges. The modulus of the composite with PEGSDA/33% was greater than that with PEGSDA/25%, which resulted in greater mechanical strength of the composite likely due to the increased macromer amount in polymer composite. These results demonstrated that salt porogens decreased the injectability of the polymer composite, thus making it difficult for the composite to be injectable. As a result, the distribution of salt porogens in the solution could be much less homogeneous, compared to hydrogel porogens. As the frequency increased, the complex viscosities of polymer solutions decreased, indicating all tested polymer composites have shear thinning property.30 Regarding the effect of hydrogel MP size on an injectability of the composite, the injectability increased (i.e., the viscosity decreased) with decreasing the size of hydrogel MPs.
FIG. 3.
(A) Dynamic shear modulus, G′, and (B) complex viscosity, |1;η*|1;, of hydrogel MPs (75 wt%) dispersed in PCLF composite mixture (25 wt%) at various frequencies. The modulus was determined by frequency sweeping using a torsional rheometer at 0.1% of the strain at room temperature. Complex viscosity was calculated as |η*| = η′ − i · η″ where η′ is the ratio of the loss modulus (G") to the angular frequency (ω) and η″ is the ratio of the storage modulus (G′) to the angular frequency. Error bars represent means ±standard deviations for n = 3.
Scaffold fabrication and characterization
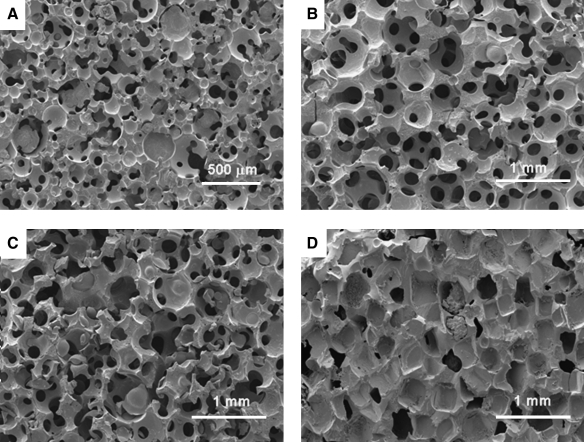
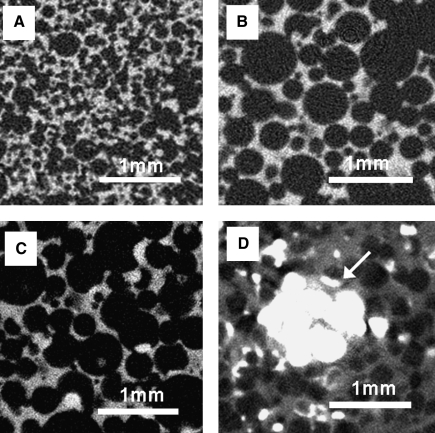
Three-dimensional porous PCLF scaffolds were fabricated using different hydrogel MPs or sodium chloride porogens (Fig. 4). When salt particles were used for 3D scaffold fabrication, pore morphology of the resulting PCLF scaffolds was mostly cubical, whereas spherical shapes were dominant in the scaffolds fabricated with hydrogel porogens. Pores in the scaffold prepared with salt particles also appeared to be less interconnected compared to the scaffolds with hydrogel porogens. To create pores in the scaffold, salt particles had to be leached out from the scaffold for up to 7 days using numerous rinses of DDW with vigorous shaking. However, some salt particles still remained in the scaffold, especially in the core section even after 7 days of salt leaching, compared to the other scaffolds made of hydrogel-based porogens, which showed complete porogen removal (Fig. 5).
FIG. 4.
SEM micrographs of 3D porous PCLF scaffolds prepared with different porogens: (A) PEGSDA/25%, (B) PEGSDA/33%, (C) gelatin, and (D) salt particles, at 75 wt% initial porogen content.
FIG. 5.
Two-dimensional micro-computed tomography (CT) images of PCLF scaffolds prepared with different porogens: (A) PEGSDA/25%, (B) PEGSDA/33%, (C) gelatin, and (D) salt particles at 75 wt% initial porogen content. White arrow indicates remaining salt particles.
Three-dimensional image analysis
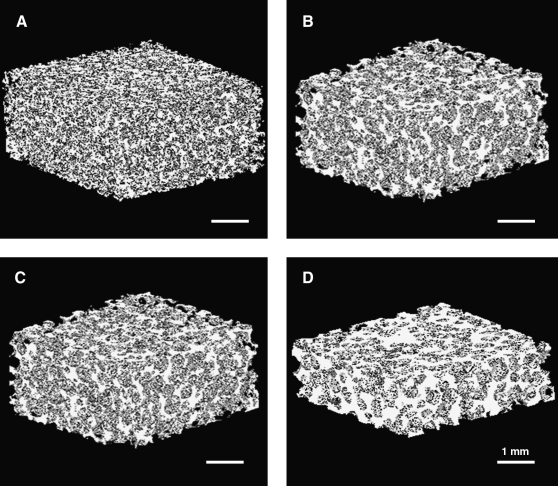
Until recently, 3D porous scaffolds have experienced lack of information about quantitative PI and SPH analysis despite its significance for specific tissue regeneration such as bone. However, as advanced image analysis has become available, digitalization of PI has been realized.22,31 In the current study, micro-CT analysis was utilized to assess PI and SPH as well as 3D pore morphology of the PCLF scaffolds fabricated with salts or hydrogel MPs. Three-dimensional images of porous PCLF scaffolds were reconstructed by scanning the scaffolds and analyzing 3D images as described in the Materials and Methods section (Fig. 6). Highly porous interconnected and well-controlled internal micro-architecture can be found in hydrogel porogen-based PCLF scaffolds (Fig. 6A–C), compared to salt leaching–based scaffold (Fig. 6D) whose pores appeared to be less interconnected and poorly distributed. There was no macroscopic difference in scaffold morphology between the scaffolds with similar particle sizes (e.g., PEGSDA/33% and gelatin MP–based scaffolds).
FIG. 6.
Three-dimensional micro-CT images of PCLF scaffolds prepared with different porogens: (A) PEGSDA/25%, (B) PEGSDA/33%, (C) gelatin, and (D) salt particles at 75 wt% initial porogen content. Micro-CT scanning data sets were reconstructed with 20 μm voxels.
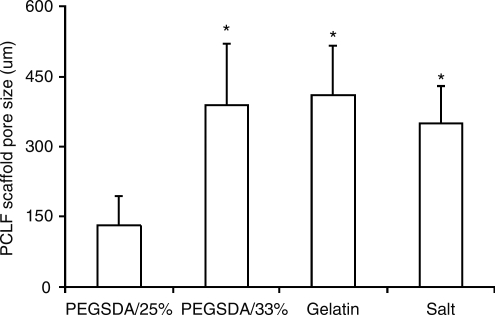
Pore size distribution of PCLF scaffolds was calculated using image analysis (Fig. 7). Using hydrogel MPs, we obtained highly porous PCLF scaffolds with pore sizes in the range of 100–500 μm, indicating that PCLF pore size distribution well corresponded to initial porogen size distribution (Fig. 2D).
FIG. 7.
Average pore sizes of PCLF scaffolds fabricated with different porogens. Error bars represent means ± standard deviations for n ≥ 5. *Significant difference (p < 0.05) from the scaffolds fabricated with PEGSDA/25% porogens.
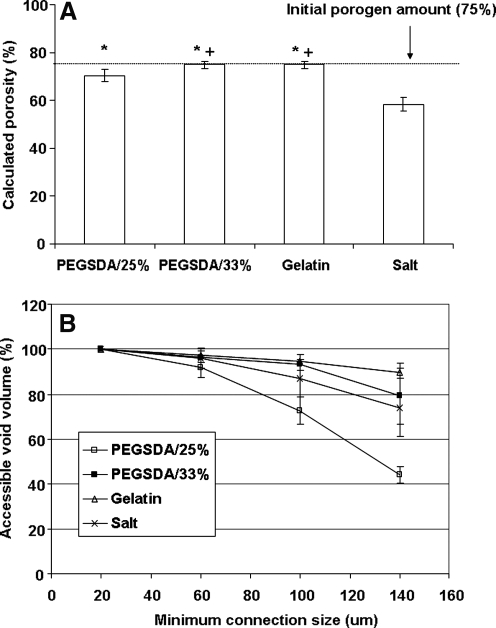
Calculated porosity of each scaffold was shown in Figure 8A. It is important to note that the calculated porosity does include dead pores or isolated pores inside a scaffold that are often ignored by other conventional techniques for measuring the porosity of the scaffold such as mercury intrusion porosimetry.32 Therefore, micro-CT technique may provide more accurate assessment of scaffold's porosity because the mercury intrusion porosimetry only measures pores accessible to the pressurized mercury. The results showed that calculated porosity was different from initial porogen amounts incorporated in scaffolds, especially when salt porogen was used.
FIG. 8.
(A) Porosity of PCLF scaffolds prepared with hydrogel MPs and salt particles, calculated from micro-CT data analysis. *,+Significant (p < 0.05) differences from the scaffolds fabricated with salt and PEGSDA/25% porogen, respectively. (B) Accessible void volume of the scaffolds at various minimum connection sizes indicated. Error bars represent means ± standard deviations for n = 3.
All hydrogel porogen–based scaffolds showed significantly higher porosity compared to the salt porogen–based scaffold (calculated porosity, 58.5%). Possible explanation for this result follows: First, there was substantial amount of remaining salt particles inside the scaffolds despite extensive leaching processes, especially core section of the scaffold (Fig. 5D). Second, porogen shape is another reason contributing to the difference in porosity because salt particles are mostly cubic in shape, and it is difficult to be interconnected with each other, leading to poor PI. Finally, nonuniform distribution of the salt porogen, especially at high salt content (75% in this study) may contribute to lower porosity due to poor injectability as previously reported elsewhere.15 Among hydrogel porogen–based scaffolds, the scaffolds with larger hydrogel MPs (PEGSDA/33% and gelatin) had significantly higher porosities (75.0% and 75.1%, respectively) compared to the scaffold with smaller hydrogel MPs (e.g., PEGSDA/25%, 70%). Again, there was no significant difference between the scaffolds fabricated with similar sizes of hydrogel porogens, regardless of the hydrogel origins (e.g., PEGSDA/33% and gelatin).
To digitalize PI of the scaffolds, accessible void volumes were calculated as a fraction of the total void volumes and plotted against minimum connection sizes (Fig. 8B) as previously illustrated in our laboratory.22 Initially, 100% of air space maintained connections with the outside air so that PI was almost 100% through connections of 20 μm or greater for all scaffolds. As the minimal connection size increased, more regions of air were isolated and less air space remained connected to the outside air. In general, the PI (accessible void volumes) decreased with increasing minimum connection size. When connections of 140 μm or greater were used, the accessible void volume of the scaffold prepared with PEGSDA/25% porogen significantly decreased to about 31%, whereas other scaffolds showed no significant difference among the scaffolds. The scaffold prepared with salt porogen showed higher PI, compared to that with smaller hydrogel porogen (e.g., PEGSDA/25%), although the porosity of salt-based scaffold was significantly lower than that of PEGSDA/25%-based scaffold. These results suggest that PI may be dependent primarily on the pore size and then secondly on the porosity of the scaffold.
Finally, the pore distribution inside a scaffold was quantified by adopting a method that was used to calculate spatial heterogeneity of myocardial perfusion.28 As illustrated in the Materials and Methods section, we calculated the porosity of each sub-ROI for a scaffold (n = 3) and plotted the log (RD = standard deviation/mean) versus the log (volume of ROI) as shown in Figure 9B. In general, linear relationship was found in those plots (the range of R2 was 0.75–0.99), and slope value of each plot was shown in Figure 9C. The SPH of the scaffold fabricated with salt was significantly higher than the scaffolds with hydrogel porogens due to the difference in porogen shape and poorer porogen distribution as mentioned earlier in this section. There was no significant difference among hydrogel porogen–based scaffolds regardless of pore size although the interconnectivity of the scaffold with smaller porogens was significantly lower than that with larger porogens (Fig. 8B).
In summary, these results demonstrate that the hydrogel porogen fabrication technique produces more homogeneous and well-distributed pore architecture throughout the scaffold, leading to higher porosity and PI, compared to the salt leaching method when pore size is similar. When pore size is different, larger pore results in higher PI than smaller pore does as discussed above.
Mechanical properties
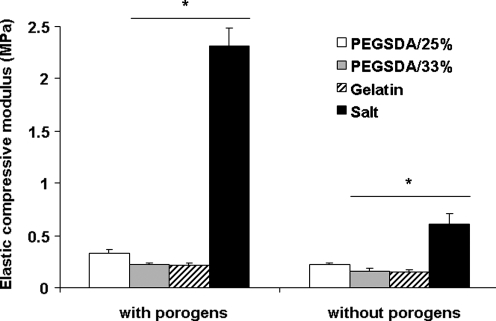
Elastic compressive moduli of 3D PCLF scaffolds before and after leaching the porogens were measured using DMA (Fig. 10). Before porogen removal, the moduli of PCLF scaffolds fabricated with hydrogel porogens were in the range of 0.2–0.4 MPa, whereas the scaffold with salt porogens showed the highest modulus, due to the substantial amount of salt present in the scaffold. The modulus was significantly reduced after removing all salts (73.5% decrease of initial mechanical strength). These results indicate that salt leaching method significantly compromised mechanical strength of the scaffold much greater than hydrogel MP method (33% decrease of initial mechanical strength) after the porogen leach-out step. Further, although PCLF scaffolds fabricated with salt leaching method showed higher mechanical strength compared with hydrogel porogen method, those scaffolds may not be ideal due to substantial amount of remaining salt inside the scaffolds that significantly compromise the PI as discussed earlier in this section. Among hydrogel porogen–based PCLF scaffolds, the scaffold fabricated with PEGSDA/25% had significantly greater compressive modulus compared to the scaffolds prepared with PEGSDA/33% or gelatin porogen at all time points.
FIG. 10.
Elastic compressive moduli of 3D porous PCLF scaffolds prepared with different porogens before and after porogen removal. The porous 3D scaffolds were obtained after 1 day of incubation in 0.1 N NaOH solution to remove all hydrogel porogens or 7 days of incubation in distilled deionized water (DDW) for salt porogens. Error bars represent means ± standard deviations for n = 4. *Significant difference (p < 0.05) from the scaffolds fabricated with PEGSDA/25% porogens.
Previous work suggested that mechanical strength of porous scaffolds prepared with smaller porogens is significantly lower than that of the scaffold fabricated with larger porogens when the porosity of the scaffolds is the same.33 This indicates that the mechanical strength of porous scaffolds are mainly dependent on PI; that is, smaller porogens likely lead to nonhomogeneous pore distribution and thus lower interconnectivity when compared to larger porogens. In our study, we also demonstrated that scaffolds prepared with smaller porogens had lower interconnectivity compared to larger porogens. However, the compressive modulus of the scaffold prepared with smaller hydrogel MPs was significantly higher than that with larger hydrogel MPs. Therefore, it is possible to assume that lower PI and porosity resulted in higher mechanical strength of the scaffolds when the heterogeneity of pore distribution is similar. Therefore, the actual porosity and PI of the scaffolds may be main contributors to the mechanical strength of the porous scaffolds. Many techniques have been developed to improve the mechanical strength of a scaffold to meet the minimum requirement for load-bearing bone tissue engineering applications, such as incorporation of bone fibers34 or crosslinking molecules such as NVP or poly(propylene fumarate) diacrylates.15
Future outlook
Preliminary in vitro degradation study showed that these hydrogel MPs were biodegradable and that degradation rate was dependent on the type of hydrogels (data not shown). Degradation rate could be accelerated in vivo as seen in previous investigations with other biodegradable macromers likely due to the presence of enzymes such as collagenase and/or cellular interactions.35,36 In this case, hydrogel porogens may not need to be leached out because they contain large amount of water, are cytocompatible, degrade in a relatively short time period compared to the matrix polymer (e.g., PCLF), and can serve as delivery carriers of drugs such as grow factors and/or even progenitor cells such as mesenchymal stem cells, both of which are known to be beneficial for eventual tissue regeneration.37 However, further studies yet to be executed to validate that the current technique is useful to fabricate truly injectable 3D porous polymer network. These include characterization of real 3D scaffolds including the porosity and morphology analysis during the degradation process under physiological condition without leaching hydrogel porogens. In addition, further investigations are also needed to determine the biological performance of the 3D PCLF scaffolds fabricated using the hydrogel MPs as porogens, regarding in vitro and in vivo studies of these scaffolds in terms of cell infiltration, tissue ingrowth, and host integration with the scaffolds to assess real benefits of current fabrication technology over other traditional methods.
Conclusions
We have developed a new technique to prepare 3D porous injectable PCLF scaffolds using biodegradable hydrogel MPs as porogens. Hydrogel MPs were successfully fabricated with appropriate particle sizes. When compared with the salt leaching method, hydrogel porogen method renders more uniform and well-distributed pore architecture, higher porosity, and higher PI. PCLF scaffolds fabricated with larger hydrogel porogens showed higher porosity, PI, and lower mechanical strength, compared to the ones with smaller hydrogel porogens. This technique may be advantageous over the conventional salt leaching technique, and it can be readily extended to the use of other materials for 3D porous scaffold fabrication.
Acknowledgments
This study was funded by the Mayo Foundation and the National Institutes of Health (R01 AR45871 and R01 EB003060). The authors would like to acknowledge James A. Gruetzmacher for performing GPC analysis, Steven M. Anderson for manufacturing glass molds, and Scott I. Gamb for scanning SEM samples. The authors also thank Dr. Erik L. Ritman's laboratory for 3D image analysis, especially Patricia E. Beighley and Andrew J. Vercnocke for micro-CT scanning and image processing.
Disclosure Statement
No competing financial interests exist.
References
- 1.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Winn S.R. Uludag H. Hollinger J.O. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Adv Drug Delivery Rev. 1998;31:303. doi: 10.1016/s0169-409x(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 3.Lutolf M.R. Weber F.E. Schmoekel H.G. Schense J.C. Kohler T. Muller R. Hubbell J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 4.Langer R. Tirrell D.A. Designing materials for biology and medicine. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 5.Ratner B.D. Hoffman A.S. Schoen J.F. Lemon J.E. Biomaterials Science; an Introduction to Materials in Medicine. 2nd. San Diego: Academic Press; 2004. [Google Scholar]
- 6.Hubbell J.A. Biomaterials in Tissue Engineering. Biotechnology. 1995;13:565. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 7.Ratner B.D. Bryant S.J. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 8.Langer R. Peppas N.A. Advances in biomaterials, drug delivery, and bionanotechnology. Aiche J. 2003;49:2990. [Google Scholar]
- 9.Hollister S.J. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]
- 10.Mikos A.G. Bao Y. Cima L.G. Ingber D.E. Vacanti J.P. Langer R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J Biomed Mater Res. 1993;27:183. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- 11.Whang K. Thomas C.H. Healy K.E. Nuber G. A novel method to fabricate bioabsorbable scaffolds. Polymer. 1995;36:837. [Google Scholar]
- 12.Ma P.X. Zhang R.Y. Synthetic nano-scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46:60. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Yaszemski M.J. Payne R.G. Hayes W.C. Langer R. Mikos A.G. In vitro degradation of a poly(propylene fumarate)-based composite material. Biomaterials. 1996;17:2127. doi: 10.1016/0142-9612(96)00008-7. [DOI] [PubMed] [Google Scholar]
- 14.Hedberg E.L. Tang A. Crowther R.S. Carney D.H. Mikos A.G. Controlled release of an osteogenic peptide from injectable biodegradable polymeric composites. J Control Release. 2002;84:137. doi: 10.1016/s0168-3659(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 15.Peter S.J. Kim P. Yasko A.W. Yaszemski M.J. Mikos A.G. Crosslinking characteristics of an injectable poly(propylene fumarate)/beta-tricalcium phosphate paste and mechanical properties of the crosslinked composite for use as a biodegradable bone cement. J Biomed Mater Res. 1999;44:314. doi: 10.1002/(sici)1097-4636(19990305)44:3<314::aid-jbm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Jabbari E. Wang S.F. Lu L.C. Gruetzmacher J.A. Ameenuddin S. Hefferan T.E. Currier B.L. Windebank A.J. Yaszemski M.J. Synthesis, material properties, and biocompatibility of a novel self-cross-linkable poly(caprolactone fumarate) as an injectable tissue engineering scaffold. Biomacromolecules. 2005;6:2503. doi: 10.1021/bm050206y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J. Lee K.W. Hefferan T.E. Currier B.L. Yaszemski M.J. Lu L. Synthesis and evaluation of novel biodegradable hydrogels based on poly(ethylene glycol) and sebacic acid as tissue engineering scaffolds. Biomacromolecules. 2008;9:149. doi: 10.1021/bm700924n. [DOI] [PubMed] [Google Scholar]
- 18.Payne R.G. Yaszemski M.J. Yasko A.W. Mikos A.G. Development of an injectable, in situ crosslinkable, degradable polymeric carrier for osteogenic cell populations Part 1. Encapsulation of marrow stromal osteoblasts in surface crosslinked gelatin microparticles. Biomaterials. 2002;23:4359. doi: 10.1016/s0142-9612(02)00184-9. [DOI] [PubMed] [Google Scholar]
- 19.Park H. Temenoff J.S. Holland T.A. Tabata Y. Mikos A.G. Delivery of TGF-beta 1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26:7095. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 20.Temenoff J.S. Shin H. Conway D.E. Engel P.S. Mikos A.G. In vitro cytotoxicity of redox radical initiators for cross-linking of oligo(poly(ethylene glycol) fumarate) macromers. Biomacromolecules. 2003;4:1605. doi: 10.1021/bm030056w. [DOI] [PubMed] [Google Scholar]
- 21.Robb R.A. Hanson D.P. Karwoski R.A. Larson A.G. Workman E.L. Stacy M.C. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989;13:433. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- 22.Moore M.J. Jabbari E. Ritman E.L. Lu L.C. Currier B.L. Windebank A.J. Yaszemski M.J. Quantitative analysis of interconnectivity of porous biodegradable scaffolds with micro-computed tomography. J Biomed Mater Res A. 2004;71A:258. doi: 10.1002/jbm.a.30138. [DOI] [PubMed] [Google Scholar]
- 23.Feldkamp L.A. Goldstein S.A. Parfitt A.M. Jesion G. Kleerekoper M. The direct examination of three-dimensional bone architecture in vitro by computed tomography. J Bone Miner Res. 1989;4:3. doi: 10.1002/jbmr.5650040103. [DOI] [PubMed] [Google Scholar]
- 24.Hanson D.P. Robb R.A. Aharon S. Augustine K.E. Cameron B.M. Camp J.J. Karwoski R.A. Larson A.G. Stacy M.C. Workman E.L. New software toolkits for comprehensive visualization and analysis of three-dimensional multimodal biomedical images. J Digit Imaging. 1997;10:229. doi: 10.1007/BF03168711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camp J.J. Hann C.R. Johnson D.H. Tarara J.E. Robb R.A. Three-dimensional reconstruction of aqueous channels in human trabecular meshwork using light microscopy and confocal microscopy. Scanning. 1997;19:258. doi: 10.1002/sca.4950190402. [DOI] [PubMed] [Google Scholar]
- 26.Bassingthwaighte J.B. King R.B. Roger S.A. Fractal nature of regional myocardial blood flow heterogeneity. Circ Res. 1989;65:578. doi: 10.1161/01.res.65.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falsetti H.L. Carroll R.J. Marcus M.L. Temporal heterogeneity of myocardial blood flow in anesthetized dogs. Circulation. 1975;52:848. doi: 10.1161/01.cir.52.5.848. [DOI] [PubMed] [Google Scholar]
- 28.Ritman E.L. Temporospatial heterogeneity of myocardial perfusion and blood volume in the porcine heart wall. Ann Biomed Eng. 1998;26:519. doi: 10.1114/1.98. [DOI] [PubMed] [Google Scholar]
- 29.Karageorgiou V. Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Ferry J.D. Viscoelastic Properties of Polymers. 3rd. New York: Wiley; 1980. [Google Scholar]
- 31.Otsuki B. Takemoto M. Fujibayashi S. Neo M. Kokubo T. Nakamura T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials. 2006;27:5892. doi: 10.1016/j.biomaterials.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Lu L. Peter S.J. Lyman M.D. Lai H.L. Leite S.M. Tamada J.A. Uyama S. Vacanti J.P. Langer R. Mikos A.G. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21:1837. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 33.Ma P.X. Choi J.W. Biodegradable polymer scaffolds with well-defined interconnected spherical pore network. Tissue Eng. 2001;7:23. doi: 10.1089/107632701300003269. [DOI] [PubMed] [Google Scholar]
- 34.Zhu G.Z. Mallery S.R. Schwendeman S.P. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat Biotechnol. 2000;18:52. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y.D. Kim Y.M. Langer R. In vivo degradation characteristics of poly(glycerol sebacate) J Biomed Mater Res A. 2003;66A:192. doi: 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- 36.Anderson J.M. Perspectives on the in vivo responses of biodegradable polymers. In: Hollinger J.O., editor. Biomedical Applications of Synthetic Biodegradable Polymers. Boca Raton: CRC; 1995. pp. 223–227. [Google Scholar]
- 37.Mikos A.G. Herring S.W. Ochareon P. Elisseeff J. Lu H.H. Kandel R. Schoen F.J. Toner M. Mooney D. Atala A. Van Dyke M.E. Kaplan D. Vunjak-Novakovic G. Engineering complex tissues. Tissue Eng. 2006;12:3307. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]