Summary
The specific aim of this study was to investigate the effect of chondroitinase ABC treatment on the frictional response of bovine articular cartilage against glass, under creep loading. The hypothesis is that chondroitinase ABC treatment increases the friction coefficient of bovine articular cartilage under creep. Articular cartilage samples (n=12) harvested from two bovine knee joints (1–3 months-old) were divided into a control group (intact specimens) and a treated group (chondroitinase ABC digestion), and tested in unconfined compression with simultaneous continuous sliding (±4 mm at 1 mm/s) under a constant applied stress of 0.5 MPa, for 2,500 s. The time-dependent response of the friction coefficient was measured. With increasing duration of loading, treated samples exhibited a significantly higher friction coefficient than control samples as assessed by the equilibrium value (treated: μeq = 0.19 ± 0.02; control: μeq = 0.12 ± 0.03; p=0.002), though the coefficient achieved immediately upon loading did not increase significantly (treated: μmin = 0.0053 ± 0.0025; control: μmin = 0.037 ± 0.0013; p=0.19). Our results demonstrate that removal of the cartilage glycosaminoglycans using chondroitinase ABC significantly increases the overall time-dependent friction coefficient of articular cartilage. These findings strengthen the motivation for developing chondroprotective strategies by increasing cartilage chondroitin sulfate content in osteoarthritic joints.
Keywords: Cartilage, friction, proteoglycan, GAG, enzymatic digestion
INTRODUCTION
Articular cartilage functions as the bearing material of synovial joints providing very low friction and wear. Experiments have shown that the frictional response of articular cartilage is time-dependent [1–7], with the minimum friction coefficient occurring immediately upon loading (μmin ~ 10−3) and much higher values achieved upon equilibrium (μeq ~ 0.1–0.4). Recent studies have confirmed experimentally [8, 9] that pressurization of the interstitial water of cartilage is the main mechanism regulating this time-dependent frictional behavior [2–4, 6, 7, 10, 11]. According to this mechanism, as the interstitial water pressurizes upon loading it supports most of the articular contact load, leaving only a small fraction to be transmitted across the solid matrix of the tissue, consequently producing a small friction force and friction coefficient. As the fluid pressure decreases with time, the frictional force on the solid matrix increases, along with the friction coefficient, until reaching equilibrium conditions.
The magnitude and duration of interstitial fluid pressurization are dependent upon the tensile and compressive moduli and permeability of the collagen-proteoglycan solid matrix [12–14]. However, the dependence of the equilibrium friction coefficient μeq on tissue composition remains unclear. Degradative enzymes have been widely used to study the role of matrix proteoglycans in the mechanical properties of articular cartilage [15–19], including the effect on the interstitial fluid load support in unconfined compression [20] and the frictional properties [9, 21–23]. In a recent study [9] we showed that an alteration in the mechanism of fluid load support has a detrimental effect on the frictional response of cartilage. Experimental results proved that enzymatic digestion with chondroitinase ABC increased the minimum friction coefficient μmin under stress-relaxation due to a loss of interstitial fluid load support, providing further evidence to the role of interstitial fluid in the transient response of the friction coefficient. The equilibrium friction coefficient μeq also increased significantly after the removal of glycosaminoglycans (GAGs), the side chains of proteoglycans, suggesting that the matrix composition and degree of degradation may play a role in the equilibrium frictional properties.
Other studies have also explored the effects of GAG removal on the frictional response of cartilage under creep loading. Kumar and co-workers [23] found a significant increase in the friction coefficient under constant load after cartilage specimens were treated with chondroitinase ABC. On the other hand, a study by Pickard et al. [22] found no significant change in the frictional response under creep loading, at two different stress levels, after enzymatic treatment with chondroitinase AC. Even though our previous results are consistent with those of Kumar et al. [23], they do not address the conflicting results with Pickard’s study [22] and it may be possible that the testing configuration plays a role on the effect of proteoglycan removal on the friction coefficient.
The long-term objective of this study is to provide evidence in support of the hypothesis that higher proteoglycan content in cartilage promotes a lower friction coefficient. The specific aim is to investigate the effect of chondroitinase ABC treatment on the frictional response of bovine articular cartilage against glass, under creep loading. The specific hypothesis is that chondroitinase ABC treatment increases the friction coefficient of bovine articular cartilage under creep. At the same time, these experiments could help to address the potential contradiction with the results of Pickard et al. [22].
METHODS
Specimen preparation and enzymatic treatment
Twelve cartilage plugs (∅8 mm) were harvested from the femoral condyles of two bovine knee joints (2–4 months old) obtained from a local abattoir and stored in phosphate buffered saline (PBS) at −20°C until testing. On the day of testing the cartilage samples were thawed at room temperature and the deep zone of each plug was removed using a sledge microtome in order to ensure a uniform final thickness (average final thickness: 1.49±0.23 mm), leaving the articular surface intact. Using a cylindrical punch, ∅4.78 mm specimens were further cored out from the ∅8mm plugs. Specimens from each joint were randomly assigned to two testing groups: those in the control group (n=6) received no treatment prior to frictional testing. Based on our previous study [9], specimens in the treated group (n=6) were digested with 0.1 u/ml of chondroitinase ABC from Proteus vulgaris (Sigma, St. Louis, MO) in 3 ml of a buffer solution containing 50 mM Tris-HCl, 60 mM sodium acetate and 0.02% bovine serum albumin (pH 8.0) at 37°C for 24 hours under gentle agitation. This protocol for chondroitinase digestion was motivated by that of Schmidt et al. [17]. After the enzymatic treatment, specimens were thoroughly rinsed with PBS and kept in PBS before friction measurement.
Friction Apparatus and Testing Protocol
The friction measurements were performed in a previously described custom-designed testing apparatus [24]. Sliding motion was provided by a computer controlled translation stage (PM500-1L, Newport Corporation CA), while normal and frictional loads were measured with a multi-axial load cell (20E12A-M25B, JR3 Inc, CA). The friction measurements (glass-on-cartilage) were performed in unconfined compression with continuous reciprocal sliding (±4 mm at 1 mm/s), with the specimen and glass-loading surface immersed in PBS at room temperature. A creep load of 8.9 N (corresponding to a normal stress of 0.5 MPa, consistent with the protocol of Pickard et al.’s study [22]) was applied in 5 seconds and held until near equilibrium was achieved (~2,500 sec). The time-dependent friction coefficient was determined from the ratio of the friction force and normal force, and its minimum and equilibrium values were tabulated for each specimen. The creep displacement response was also monitored using a linear variable differential transformer (HR100, Shaevitz Sensors, VA).
Biochemical Analysis
After the test, specimens were equilibrated in PBS and their wet weights measured (M220, Denver Instruments, CO). They were then lyophilized overnight and reweighed dry to obtain the water content. Following 16-hour digestion with papain (Sigma, MO), the glycosaminoglycan (GAG) content was determined using a 1,9 dymethylmethylene blue assay [25] with chondroitin-6-sulfate (Sigma) as the standard. GAG content was normalized by the wet weight.
RESULTS
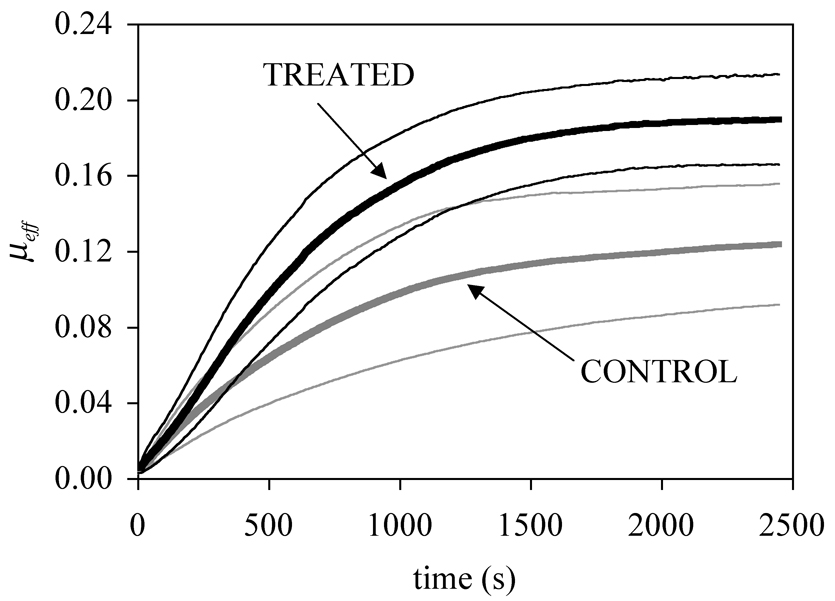
The average and standard deviation of the time-varying friction coefficient (μeff) for all specimens in the control and treated groups are shown in Figure 1, demonstrating a clear difference in the frictional response between both groups. The friction coefficient achieves its minimum value ( μmin ) right after load application, and subsequently increases toward its equilibrium value ( μeq ).
Figure 1.
Average and standard deviation of the time-dependent friction coefficient μeff for the chondroitinase treated (solid line) and control (dotted line) groups (n=6 per group).
Averages and standard deviations of the minimum and equilibrium friction coefficients are reported in Table 1. One-way ANOVA and Bonferroni post-hoc testing of the results (performed with SAS statistical software; SAS Institute INC., NC) showed that the increase in μmin after treatment with chondroitinase ABC was not statistically significant (p=0.19). However, a highly significant increase was found in μeq between control and treated groups (p=0.002). The characteristic time constant for the rise of μeff with time (defined as the time τμ needed for μeff to rise from μmin by (1−e−1)(μeq − μmin)) of the treated specimens was found to be 751±162 s, compared to 678±128 s for the control specimens, although this difference was not statistically significant (p=0.41).
Table 1.
Means and standard deviations of outcome measures in the control (intact cartilage) and treatment (chondroitinase ABC-digested cartilage) groups (n=6 per group).
| Control | Treated | p-value | |||
|---|---|---|---|---|---|
| Mean | St. Dev. | Mean | St. Dev. | ||
| μmin | 0.0037 | 0.0013 | 0.0053 | 0.0025 | 0.191 |
| μeq | 0.12 | 0.03 | 0.19 | 0.02 | 0.002 |
| τμ (s) | 751 | 162 | 678 | 128 | 0.408 |
| μmin/μeq | 0.031 | 0.011 | 0.027 | 0.011 | 0.542 |
| εeq | 0.55 | 0.03 | 0.55 | 0.05 | 0.945 |
| τε (s) | 434 | 201 | 187 | 38 | 0.014 |
| H2O (%w/w) | 81.9 | 0.8 | 81.9 | 1.7 | 0.962 |
| GAG (%w/w) | 2.96 | 0.32 | 2.15 | 0.62 | 0.017 |
Biochemical analyses (Table 1) showed that the enzymatic treatment did not change the water content (81.9 ± 0.8% for the control group; 81.9 ± 1.7 % for the treated group, p=0.96). GAG content was significantly lower in the chondroitinase treatment group (2.15±0.62%) when compared to the control group (2.96±0.32%) (p=0.017).
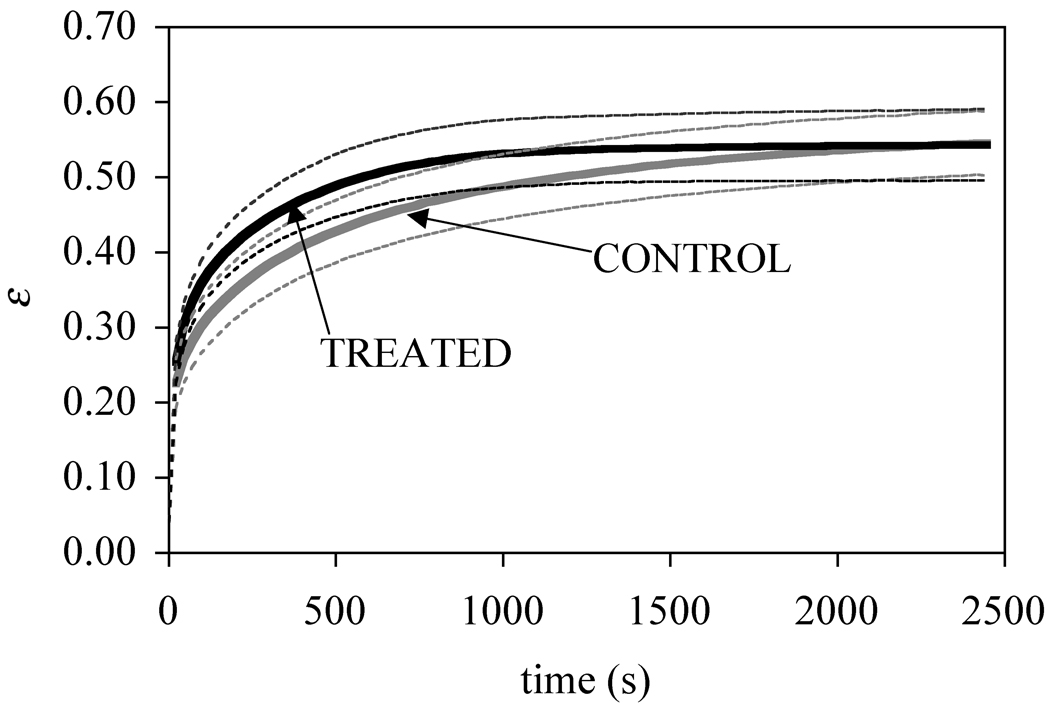
The creep deformation response, normalized by the specimen thickness to produce an engineering strain ε, is presented in Figure 2, showing that treated specimens reached equilibrium faster than their respective control. The corresponding time constant τε for the control group was 434±201 s, while for the treatment group it was 187±38 s (p=0.014) (Table 1). No difference was observed in the equilibrium creep strain between the control group (0.55 ± 0.03) and the treated group (0.55 ± 0.05) (p=0.95).
Figure 2.
Average and standard deviation of the response of the creep strain for the chondroitinase treated and control groups (n=6 per group).
DISCUSSION
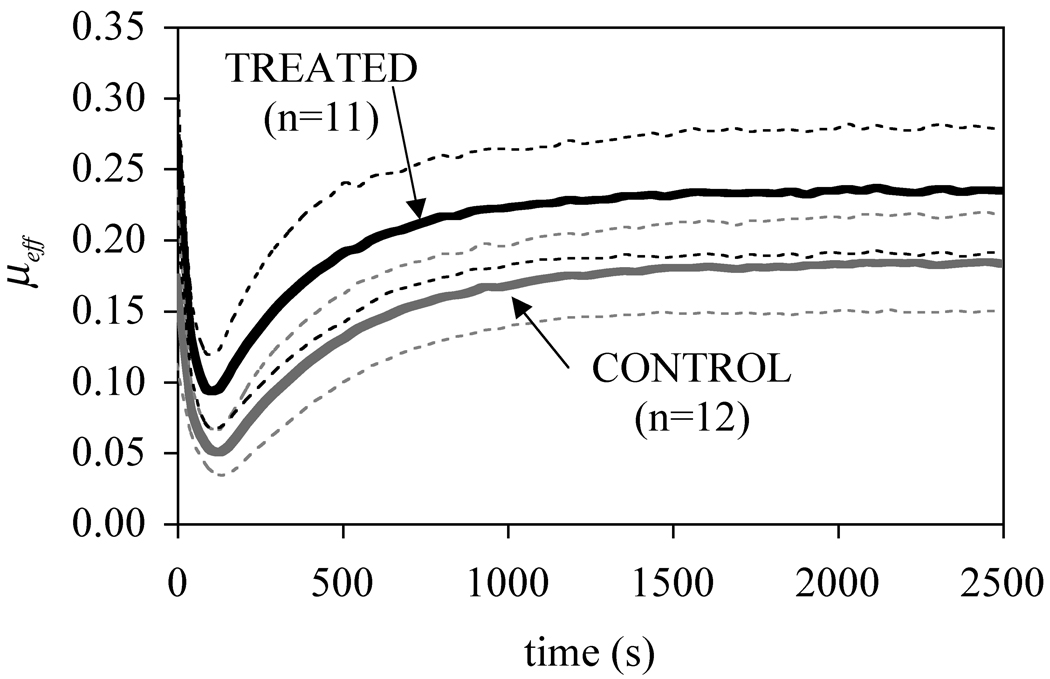
The main objective of this study was to investigate the effect of chondroitinase ABC digestion on the frictional response of articular cartilage under creep loading. It is evident from these results (Figure 1), along with those of our earlier study (Figure 3) [9], that removal of the GAG side chains from proteoglycans using chondroitinase ABC significantly increases the overall time-dependent friction coefficient of articular cartilage, regardless of the testing configuration. This finding thus supports the specific hypothesis that chondroitinase ABC treatment increases the friction coefficient of bovine articular cartilage under creep loading.
Figure 3.
Average friction coefficient obtained under a stress-relaxation testing configuration (10% compressive strain after an application of a tare load of 1.8 ± 0.4 N), from the study of Basalo et al. [9].
Chondroitinase ABC lyase degrades mostly chondroitin sulfate, which is the most abundant GAG in the matrix proteoglycans. (According to the manufacturer, the enzyme used in this study has a much slower rate of degradation of hyaluronan.) Even though proteoglycans are present in the extracellular matrix of articular cartilage in a relatively small amount, they are responsible for many characteristics of the tissue. The mechanical properties of cartilage are related to the bulk properties of the proteoglycans. The highly concentrated fixed negative charges brought on by the GAG side chains produce an inflow of counterions that create a Donnan osmotic pressure effect which contributes significantly to the compressive modulus of the tissue. The transport of interstitial fluid and electrolytes is also affected by the presence of the negative fixed charges [26, 27].
In the present study, the difference in μeff between control and treated specimens became more significant as time increased (Figure 1), whereas the increase in the friction coefficient μmin achieved immediately upon loading was not statistically significant. Similarly, chondroitinase digestion did not significantly decrease the characteristic time constant τμ of the friction coefficient μeff. These results suggest that the influence of GAG degradation was more significant on μeq, which represents the frictional response after interstitial fluid pressurization has subsided [8].
When examining the creep deformation, the equilibrium strain εeq remained unaffected by chondroitinase digestion, whereas the time constant τε for reaching strain equilibrium significantly decreased. The implication from these results is that the equilibrium compressive modulus of cartilage did not change whereas the transient response was significantly affected. Earlier studies have shown that the removal of the GAG side chains of matrix proteoglycans causes a decrease in compressive modulus when the applied strain is small [16, 19], but an increase when the applied strain is large [9, 20], showing a complex interaction between the nonlinear equilibrium stress-strain response under large strains and proteoglycan content. Thus the observation that the equilibrium modulus did not change significantly with chondroitinase digestion falls within the range of observations reported in the prior literature. Interestingly, in light of our earlier observation that μeq decreases significantly with increasing compressive strain [28], it is fortunate that εeq did not change following digestion, or else another confounding factor might have significantly affected the testing of our hypothesis. In light of these findings, it is possible that the discrepancy with Pickard’s results [22] is related to the amount of compressive strain. Their study achieved complete proteoglycan removal, which may have caused larger equilibrium compressive strains (whose magnitude is not reported unfortunately) than the current study.
The change in the characteristic time τε can be attributed to an increase in hydraulic permeability [18], or an alteration in the intrinsic, fluid-flow-independent viscoelasticity of the collagen-proteoglycan matrix following chondroitinase ABC digestion [15, 17]. Since the time constant τμ of the interstitial fluid pressure-dependent friction coefficient did not significantly change, this would suggest that digestion had a greater influence on the intrinsic flow-independent properties of the tissue in this study.
Though the protocol used for enzymatic digestion was consistent with that of our previous stress-relaxation study [9], the decrease in GAG content in the current study was smaller (from 3.0% to 2.2% here, versus 3.3% to 1.7% in the earlier study). The difference in the amount of degradation may be explained by tissue variability and lot-to-lot enzyme variability. This may explain why μmin did not increase significantly after the enzymatic treatment, in contrast to our stress-relaxation study. The fact that μeq did increase very significantly suggests that the equilibrium frictional properties are more susceptible to relatively small changes in matrix composition and the degree of degradation. In future studies, the use of different levels of digestion would help elucidate this issue and would give better insight into how sensitive the mechanical and frictional properties are to different levels of tissue degradation.
The observation that μeq increases following GAG digestion is consistent with our recent study which found that μeq is higher at the articular surface than right underneath it or at the deep zone [24]. Both studies imply that μeq increases with decreasing GAG content, even though the current study altered GAG content enzymatically while the earlier study employed mechanical means (microtoming) to expose regions of cartilage with increasing GAG content. Neither approach is ideal, since both enzymatic degradation and microtoming may arguably alter other factors which regulate the frictional response of cartilage. However, combined with our earlier observation that higher compressive strains (which lead to increased GAG concentrations) reduce μeq [28], there is now a preponderance of evidence in support of the hypothesis that higher proteoglycan content in cartilage promotes a lower friction coefficient.
A potential limitation of the current study is that cartilage samples in the control group were tested immediately after specimen preparation, whereas samples in the treatment group underwent incubation at 37°C for 24 hours prior to testing. However, in our previous stress-relaxation study [9], we included an additional control group in which cartilage samples were incubated in PBS for 24 hours under the same conditions as the chondroitinase treated group. It was found that incubation in PBS did not reduce the GAG content and did not affect the minimum and equilibrium friction coefficients.
Osteoarthritis is characterized by a progressive degradation of the joint’s cartilage that involves loss of proteoglycans, increased hydration and fibrillation of the matrix [29]. The results of this study indicate that a relatively small loss of proteoglycans will significantly degrade the frictional properties of cartilage. These findings strengthen the motivation for developing chondroprotective strategies by increasing cartilage chondroitin sulfate content in osteoarthritic joints [30–32]. Future studies would naturally involve testing of normal and enzymatically degraded articular cartilage plugs that have been incubated either in chondroitin sulfate or PBS control solutions, to investigate the effect of chondroitin sulfate supplementation on the frictional properties of cartilage [33].
ACKNOWLEDGMENTS
This study was supported by a grant from the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the National Institutes of Health (AR43628).
REFERENCES
- 1.Walker PS, Dowson D, Longfield MD, Wright V. "Boosted Lubrication" in Synovial Joints by Fluid Entrapment and Enrichment. Ann Rheum Dis. 1968;27:512–520. doi: 10.1136/ard.27.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCutchen CW. Sponge-Hydrostatic and Weeping Bearings. Nature. 1959:1284. doi: 10.1038/1841284a0. [DOI] [PubMed] [Google Scholar]
- 3.McCutchen CW. The Frictional Properties of Animal Joints. Wear. 1962;5:1–17. [Google Scholar]
- 4.Forster H, Fisher J. The Influence of Loading Time and Lubricant on the Friction of Articular Cartilage. Proc Inst Mech Eng [H] 1996;210:109–119. doi: 10.1243/PIME_PROC_1996_210_399_02. [DOI] [PubMed] [Google Scholar]
- 5.Forster H, Fisher J. The Influence of Continuous Sliding and Subsequent Surface Wear on the Friction of Articular Cartilage. Proc Inst Mech Eng [H] 1999;213:329–345. doi: 10.1243/0954411991535167. [DOI] [PubMed] [Google Scholar]
- 6.Ateshian GA, Wang H, Lai WM. The Role of Interstitial Fluid Pressurization and Surface Porosities on the Boundary Friction of Articular Cartilage. J Tribology. 1998;120:241–251. [Google Scholar]
- 7.Malcom LL. An Experimental Investigation of the Frictional and Deformational Response of Articular Cartilage Interfaces to Static and Dynamic Loading. 1976 [Google Scholar]
- 8.Krishnan R, Kopacz M, Ateshian GA. Experimental Verification of the Role of Interstitial Fluid Pressurization in Cartilage Lubrication. J Orthop Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basalo IM, Raj D, Krishnan R, Chen FH, Hung CT, Ateshian GA. Effects of Enzymatic Degradation in the Frictional Response of Articular Cartilage in Stress-Relaxation. Journal of Biomechanics. 2005 doi: 10.1016/j.jbiomech.2004.05.045. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macirowski T, Tepic S, Mann RW. Cartilage Stresses in the Human Hip Joint. J Biomech Eng. 1994;116:10–18. doi: 10.1115/1.2895693. [DOI] [PubMed] [Google Scholar]
- 11.Ateshian GA. A Theoretical Formulation for Boundary Friction in Articular Cartilage. J Biomech Eng. 1997;119:81–86. doi: 10.1115/1.2796069. [DOI] [PubMed] [Google Scholar]
- 12.Park S, Krishnan R, Nicoll SB, Ateshian GA. Cartilage Interstitial Fluid Load Support in Unconfined Compression. J Biomech. 2003;36:1785–1796. doi: 10.1016/s0021-9290(03)00231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soltz MA, Ateshian GA. Experimental Verification and Theoretical Prediction of Cartilage Interstitial Fluid Pressurization at an Impermeable Contact Interface in Confined Compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 14.Soltz MA, Ateshian GA. A Conewise Linear Elasticity Mixture Model for the Analysis of Tension-Compression Nonlinearity in Articular Cartilage. J Biomech Eng. 2000;122:576–586. doi: 10.1115/1.1324669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic Shear Properties of Articular Cartilage and the Effects of Glycosidase Treatments. J Orthop Res. 1993;11:771–781. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 16.Lyyra T, Arokoski JP, Oksala N, Vihko A, Hyttinen M, Jurvelin JS, Kiviranta I. Experimental Validation of Arthroscopic Cartilage Stiffness Measurement Using Enzymatically Degraded Cartilage Samples. Phys Med Biol. 1999;44:525–535. doi: 10.1088/0031-9155/44/2/017. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of Proteoglycan Extraction on the Tensile Behavior of Articular Cartilage. J Orthop Res. 1990;8:353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- 18.Lotke PA, Granda JL. Alterations in the Permeability of Articular Cartilage by Proteolytic Enzymes. Arthritis Rheum. 1972;15:302–308. doi: 10.1002/art.1780150312. [DOI] [PubMed] [Google Scholar]
- 19.Bonassar LJ, Frank EH, Murray JC, Paguio CG, Moore VL, Lark MW, Sandy JD, Wu JJ, Eyre DR, Grodzinsky AJ. Changes in Cartilage Composition and Physical Properties Due to Stromelysin Degradation. Arthritis Rheum. 1995;38:173–183. doi: 10.1002/art.1780380205. [DOI] [PubMed] [Google Scholar]
- 20.Basalo IM, Mauck RL, Kelly TN, Nicoll SB, Chen FH, Hung CT, Ateshian GA. Cartilage Interstitial Fluid Load Support in Unconfined Compression Following Enzymatic Degradation. Journal of Biomechanical Engineering. 2004;126:779–786. doi: 10.1115/1.1824123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickard JE, Fisher J, Ingham E, Egan J. Investigation into the Effects of Proteins and Lipids on the Frictional Properties of Articular Cartilage. Biomaterials. 1998;9:1807–1812. doi: 10.1016/s0142-9612(98)00147-1. [DOI] [PubMed] [Google Scholar]
- 22.Pickard J, Ingham E, Egan J, Fisher J. Investigation into the Effect of Proteoglycan Molecules on the Tribological Properties of Cartilage Joint Tissues. Proc Inst Mech Eng [H] 1998;212:177–182. doi: 10.1243/0954411981533953. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Oka M, Toguchida M, Kobayashi E, Uchida T, Nakamura T. Role of Uppermost Superficial Layer of Articular Cartilage in the Lubrication Mechanism of Joints. J. Anat. 2001:241–250. doi: 10.1046/j.1469-7580.2001.19930241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan R, Caligaris M, Mauck RL, Hung CT, Costa KD, Ateshian GA. Removal of the Superficial Zone of Bovine Articular Cartilage Does Not Increase Its Frictional Coefficient. Osteoarthritis Cartilage. 2004;12:947–955. doi: 10.1016/j.joca.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farndale RW, Sayers CA, Barrett AJ. A Direct Spectrophotometric Microassay for Sulfated Glycosaminoglycans in Cartilage Cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 26.Maroudas A. Physicochemical Properties of Articular Cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Kent, England: Pitman Medical; 1979. pp. 215–290. [Google Scholar]
- 27.Mow VC, Ratcliffe A. Structure and Function of Articular Cartilage and Meniscus. In: Mow VC, Hayes W, editors. Basic Orthopaedic Biomechanics. Philadelphia: Lippincot-Raven Publishers; 1997. pp. 113–177. [Google Scholar]
- 28.Ateshian GA, Soltz MA, Mauck RL, Basalo IM, Hung CT, Lai WM. The Role of Osmotic Pressure and Tension-Compression Nonlinearity in the Frictional Response of Articular Cartilage. Transport in Porous Media. 2003;50:5–33. [Google Scholar]
- 29.Freeman MAR, Meachim G. Ageing and Degeneration. In: Freeman MAR, editor. Adult Articular Cartilage. Pitman Medical; 1979. pp. 487–543. [Google Scholar]
- 30.Das A, Jr, Hammad TA. Efficacy of a Combination of Fchg49 Glucosamine Hydrochloride, Trh122 Low Molecular Weight Sodium Chondroitin Sulfate and Manganese Ascorbate in the Management of Knee Osteoarthritis. Osteoarthritis Cartilage. 2000;8:343–350. doi: 10.1053/joca.1999.0308. [DOI] [PubMed] [Google Scholar]
- 31.Lippiello L. Glucosamine and Chondroitin Sulfate: Biological Response Modifiers of Chondrocytes under Simulated Conditions of Joint Stress. Osteoarthritis Cartilage. 2003;11:335–342. doi: 10.1016/s1063-4584(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 32.Lippiello L, Woodward J, Karpman R, Hammad TA. In Vivo Chondroprotection and Metabolic Synergy of Glucosamine and Chondroitin Sulfate. Clin Orthop Relat Res. 2000:229–240. doi: 10.1097/00003086-200012000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Basalo IM, Chahine NO, Chen FH, Hung CT, Ateshian GA. Chondroitin Sulfate Reduces the Friction Coefficient of Articular Cartilage. Arthritis Rheum. doi: 10.1016/j.jbiomech.2006.07.007. In Review. [DOI] [PubMed] [Google Scholar]