Abstract
Coactivation of spinal α2-adrenergic receptors (ARs) and opioid receptors produces antinociceptive synergy. Antinociceptive synergy between intrathecally administered α2AR and opioid agonists is well documented, but the mechanism underlying this synergy remains unclear. The delta-opioid receptor (DOP) and the α2AARs are coexpressed on the terminals of primary afferent fibers in the spinal cord where they may mediate this phenomenon. We evaluated the ability of the DOP-selective agonist deltorphin II (DELT), the α2AR agonist clonidine (CLON) or their combination to inhibit calcitonin gene-related peptide (CGRP) release from spinal cord slices. We then examined the possible underlying signaling mechanisms involved through coadministration of inhibitors of phospholipase C (PLC), protein kinase C (PKC) or protein kinase A (PKA). Potassium-evoked depolarization of spinal cord slices caused concentration-dependent release of CGRP. Coadministration of DELT and CLON inhibited the release of CGRP in a synergistic manner as confirmed statistically by isobolograpic analysis. Synergy was dependent on the activation of PLC and PKC, but not PKA, whereas the effect of agonist administration alone was only dependent on PLC. The importance of these findings was confirmed in vivo, using a thermal nociceptive test, demonstrating the PKC dependence of CLON-DELT antinociceptive synergy in mice. That inhibition of CGRP release by the combination was maintained in the presence of tetrodotoxin in spinal cord slices suggests that synergy does not rely on interneuronal signaling and may occur within single subcellular compartments. The present study reveals a novel signaling pathway underlying the synergistic analgesic interaction between DOP and α2AR agonists in the spinal cord.
Introduction
Opioid analgesics remain the mainstay for treatment of moderate to severe pain states (American Pain Society, 2008). However, development of adverse side effects such as tolerance, dependence, constipation, addiction liability and opioid-induced hyperalgesia limit their utility (Angst and Clark, 2006). Many approaches have been investigated to bypass these untoward side effects, but use of multimodal analgesic techniques offers distinct advantages as combination therapies may produce analgesia at lower total drug doses. Extensive behavioral (Hylden and Wilcox, 1983; Stevens et al., 1988; Monasky et al., 1990; Ossipov et al., 1990a,b,c; Roerig et al., 1992) and electrophysiological (Sullivan et al., 1987; Wilcox et al., 1987; Omote et al., 1990) studies document that that coactivation of α2-adrenergic receptors (α2ARs) and opioid receptors (ORs) produces synergistic interactions in spinal cord, although characterization of the mechanisms underlying this phenomenon have yet to be elucidated. Therefore, understanding the molecular mechanisms involved in the synergistic interactions of these receptors is of both clinical and theoretical importance in development of more efficacious therapies for pain management, as synergy-enabled decreases in dose may mitigate unwanted side effects.
Both α2ARs and ORs belong to the seven transmembrane-spanning domain G-protein-coupled receptor superfamily and share common signal transduction systems mediated primarily through inhibitory G-proteins, the activation of which inhibits pain transmission. It has been proposed that two receptor populations, acting through common signaling systems, can only synergize if they are anatomically localized to different locations within the pathway (e.g., presynaptic vs postsynaptic) (Honoré et al., 1996). In contrast, previous literature suggests that two analgesic receptor subtypes, α2AARs (Stone et al., 1998) and delta-opioid receptors (DOP) (Dado et al., 1993; Arvidsson et al., 1995; Cheng et al., 1997; Zhang et al., 1998), are extensively colocalized in terminals of capsaicin-sensitive, substance P (SP)-expressing primary afferent fibers in rat (Riedl et al., 2009) and that agonists acting at these receptors are able to produce analgesic synergy in vivo in both mouse (Stone et al., 1997) and rat (Ossipov et al., 1990c).
Given that the mechanisms underlying supra-additive receptor interactions remain unknown, we sought to determine which intracellular signaling pathways are necessary for synergy to occur between α2ARs and DOPs. Because of the striking correspondence of the actions and interactions between α2ARs and DOPs in mouse and rat, we used immunohistochemical and in vivo behavioral studies in mice combined with a more reduced in vitro spinal cord slice preparation in rats to determine whether the observed synergy between agonists acting at α2ARs/DOPs results from something other than multicellular interactions mediated by neuronal circuitry. We then used inhibitors of specific signaling pathways affected by the aforementioned receptor pair to elucidate the mechanisms involved in the synergistic outcome of receptor coactivation. Here, we report that coactivation of α2ARs and DOPs produces a synergistic interaction both in vivo and in vitro, and that this interaction takes place within the terminals of primary afferent neurons in spinal cord. Whereas the analgesic efficacy of both receptors required PLC activation, the synergistic interaction uniquely required PKC activation. These studies are the first to identify a signaling pathway underlying synergy between agonists acting at α2ARs and DOPs and may lead to improved understanding and increased clinical utilization of polyanalgesic therapy.
Materials and Methods
Animals.
Male CD-1 ICR mice (20 ± 5 g; Harlan), male C57BL/6 mice (20 ± 5 g; Charles River) and adult male Sprague Dawley rats (300 ± 25 g; Harlan) were maintained on a 12 h light/dark cycle and food and water were available ad libitum to all animals. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota or the McGill University Animal Care and Ethics Committees.
Immunohistochemistry.
Immunohistochemistry was performed as previously described (Wessendorf and Elde, 1985; Fairbanks et al., 2002; Riedl et al., 2009). In brief, male C57BL/6 mice were anesthetized with a ketamine/xylazine/acepromazine mixture and perfused transcardially with 4% paraformaldehyde and 0.2% picric acid in 0.1 m PBS, pH 6.9. Spinal cords were dissected and stored overnight in 10% sucrose at 4°C. Tissue sections were prepared using a cryostat at a thickness of 10–14 μm, thaw-mounted onto gelatin-coated slides and stored at −20°C. Sections were incubated for 1 h at room temperature in diluent containing 1% normal donkey serum, 0.3% Triton X-100, 0.01% sodium azide and 1% bovine serum albumin in PBS. Sections were then incubated overnight at 4°C in a humid chamber with primary antisera, rinsed 3 × 10 min with PBS, incubated with fluorescently tagged species-specific secondary antisera (Jackson Immunoresearch) for 1 h at room temperature, rinsed 3 × 10 min with PBS and coverslipped using a mixture of glycerol and PBS containing 0.1% p-phenylenediamine. The α2AAR antiserum (1:1000) was prepared in rabbit against a synthetic peptide corresponding to α2AAR436-450 (AFKKILCRGDRKRIV) of the rat sequence and has been previously characterized (Stone et al., 1998; Riedl et al., 2009). The rabbit DOP antiserum (1:1000) was prepared against a synthetic peptide corresponding to anti-DOP3-17 (1:1000; LVPSARAELQSSPLV) and has been previously characterized (Dado et al., 1993; Riedl et al., 2009). SP antibodies raised in two different species and obtained from two different sources were used in these studies and produced similar results: rat anti-SP (1:1000; Accurate Chemical) and guinea pig anti-SP (1:500; Neuromics Antibodies) and have been previously characterized (Cuello et al., 1979; Riedl et al., 2009). Images were collected using a Bio-Rad MRC 1000 confocal microscope (Bio-Rad Microscience Division) or an Olympus BX-51 equipped with a DP-71 camera and assembled in photoshop.
Drug preparation and administration.
Drugs used were clonidine (CLON), chelerythrine, U73122 [1-[6-[((17β)-3-methoxyestra-1,3,5[10]-trien-17-yl)amino]hexyl]-1H-pyrrole-2,5-dione], idazoxan, H89 [N-[2-(4-bromocinnamylamino)ethyl]-5-isoquinoline], tetrodotoxin (TTX) (all from Sigma), deltorphin II (DELT) (Tocris Bioscience), and naltrindole (gift from Dr. Philip Portoghese, University of Minnesota, Minneapolis, MN). All drugs for behavioral experiments were dissolved in 0.9% saline and administered intrathecally (i.t.) in a volume of 5 μl according to the method of Hylden and Wilcox (1980) as modified by Wigdor and Wilcox (1987) in conscious mice. For spinal cord neuropeptide release experiments, U73122 was dissolved in ethanol and diluted in HEPES buffer. All other drugs were dissolved in dH2O and diluted in HEPES buffer. Control experiments with HEPES (shown) and HEPES with ethanol (data not shown) demonstrated that diluted ethanol had no effect on either basal or K+-stimulated CGRP release.
Antinociception.
Thermal nociceptive responsiveness was assessed using the warm water (52.5°C) tail-immersion assay, as described previously (Janssen et al., 1963). Briefly, mice were gently wrapped in a soft cloth such that their tails were exposed, and three-quarters of the length of the tail was dipped into the warm water. Tail-flick latencies were obtained before drug application to establish a baseline response. Opioid and adrenergic receptor agonists were injected i.t. as 5 and 10 min pretreatments, respectively. The opioid receptor antagonist was injected concomitant with agonist injection and the adrenergic receptor antagonist was injected i.t. as a 10 min pretreatment before adrenergic receptor agonist injection. PLC and PKC antagonists were injected i.t. as 1 h pretreatments before latency determination, whereas PKA antagonist was injected i.t. as a 30 min pretreatment. A maximum cutoff of 12 s was set to avoid tissue damage. The results were then expressed as a percentage of the maximum possible effect (%MPE) according to the equation: % MPE = Postdrug latency − Predrug latency × 100/Cutoff − Predrug latency.
Neuropeptide release from spinal cord slices.
For determination of calcitonin gene-related peptide (CGRP) release from spinal cord slices, adult male Sprague Dawley rats (275–325 g) were used. Animals were anesthetized with isoflurane and quickly decapitated. Spinal cords were removed by hydraulic extrusion and placed in ice-cold, oxygenated HEPES buffer containing (in mm): 120 NaCl, 5.4 KCl, 0.8 MgCl2, 1.8 CaCl2, 20 HEPES, 0.01 glycine, 15 glucose, and commercial protease inhibitor cocktail (Roche). Two centimeter segments of the lumbar enlargement were removed, divided midsagittally, and chopped into 0.3 × 0.3 mm pieces (McIllwain tissue chopper), and the halves were placed into separate 1 ml perfusion chambers. The tissue was perfused at a flow rate of 0.35–0.4 ml/min in HEPES buffer maintained at 37°C, aerated with 95% O2-5% CO2 and pH adjusted to 7.4. The tissue was allowed a perfusion equilibration period of 30 min to stabilize peptide release and then collected for 6 min periods in 12 × 75 mm glass test tubes. Basal release was assessed by perfusing the tissue with HEPES buffer for 6 min. After this period, peptide release was evoked by perfusing the tissue for an additional 6 min with HEPES buffer containing 60 mm K+. In release inhibition experiments, tissue was perfused for 6 min with HEPES buffer containing DELT, CLON, or the combination in a 1:1 concentration ratio before the K+ stimulation. When PLC inhibitor, PKC inhibitor, PKA inhibitor, or TTX were used, these compounds were present in the superfusate throughout the entire experiment. Immunoreactive CGRP in the collected samples was assayed by enzyme-linked immunosorbent assay (ELISA) (SPI Bio, Catalog No. 589001). No difference in either basal or K+-evoked CGRP release was observed from slices made from either whole cord or cord separated to exclude the ventral horn (data not shown).
Electrophysiological recording.
Adult male Sprague Dawley rats (275–325 g) were anesthetized with isoflurane and quickly decapitated. Spinal cords were removed by hydraulic extrusion and placed in ice-cold, oxygenated artificial CSF (aCSF) with the following composition (in mm): 119 NaCl, 2.5 KCl, 1.0 NaH2PO4, 1.3 MgSO4, 2.5 CaCl2, 26.2 NaHCO3 and 11 glucose. The lumbar enlargement was cut into 1 cm sections, from which dorsal horizontal slices with a thickness of ∼500 μm were taken using a vibratome while the spinal cord was immersed in aCSF. The slicing solution also contained 10 mm kynurenic acid to maintain viability. After recovery, slices were superfused with normal aCSF (22−23°C) containing 100 μm picrotoxin. Field EPSPs (fEPSPs) were evoked every 10 s using a suction electrode placed on the dorsal root entry zone, and recorded using a glass electrode filled with aCSF and placed in the ipsilateral superficial dorsal horn 2–5 mm rostral or caudal of the stimulating electrode. Data were digitized at 5 kHz using a Multiclamp 700A amplifier (Molecular Devices), and analyzed using custom software (Igor Pro, Wavemetrics). fEPSP amplitude following application of TTX (0.1 or 1 μm) is expressed as the percentage of baseline amplitude recorded for 5 min before TTX application.
Data analysis.
The ED50 (nanomoles, in vivo) or EC50 (nanomolar, in vitro) values and 95% confidence intervals (CIs) of both CLON and DELT were calculated using the graded dose–response curve method of Tallarida and Murray (Tallarida, 1992). A minimum of three doses or concentrations were used for each drug or drug combination. In some instances (e.g., multiple doses or concentrations with efficacies approaching 0 or 100%), only the linear portion of the dose/concentration–response curve was included in the ED50/EC50 value calculation. To determine differences in agonist potency between groups, nonoverlapping 95% CIs were considered to represent statistically significant differences. In the experiments testing for synergistic interactions, dose/concentration–response curves, ED50/EC50 values, and 95% CIs were first generated for CLON and DELT administered alone as described above. CLON and DELT were then coadministered at a constant dose/concentration ratio based on the potency ratio of the two drugs given separately. For example, if CLON had an ED50 or EC50 value of 1 nmol or nm (in vivo and in vitro, respectively) and DELT had an ED50 or EC50 value of 1 nmol or nm, the agents were coadministered in a 1:1 ratio and a third dose/concentration–response curve was generated for the combination treatment.
Isobolographic analysis.
Isobolographic analysis is the accepted standard for quantitative evaluation of drug interactions (Tallarida, 1992). Dose/concentration–response curves were first constructed for CLON and DELT administered separately and the ED50/EC50 values were calculated and then used to determine an equieffective dose/concentration ratio between the two as described above. The interaction between the two drugs was tested by comparing the theoretical additive ED50/EC50 value for the combination based on the dose/concentration–response curves of each drug administered separately and the observed experimental ED50/EC50 value of the combination using a t test. These values are based on the total dose of both drugs. An interaction is considered synergistic if the experimental ED50/EC50 value is significantly less (p < 0.05) than the calculated theoretical additive ED50/EC50 values.
Isobolographic analysis allows for graphical representation of drug interactions (see Figs. 2B,D, 4B). This representation depicts the ED50/EC50 value of each agent as the x- or y-intercept. For example, Figure 2B represents the ED50 value of CLON as the y-intercept and the ED50 of DELT as the x-intercept. The line connecting these two points is the theoretical additive line and depicts the dose combinations expected to yield 50% efficacy if the drug interaction is strictly additive. The theoretical additive ED50 value and its confidence interval are determined mathematically and plotted spanning this line. The observed ED50 value for the combination is plotted at the corresponding x,y coordinates along with its 95% confidence interval for comparison to the theoretical additive ED50 value. The same comparisons are made for EC50 values.
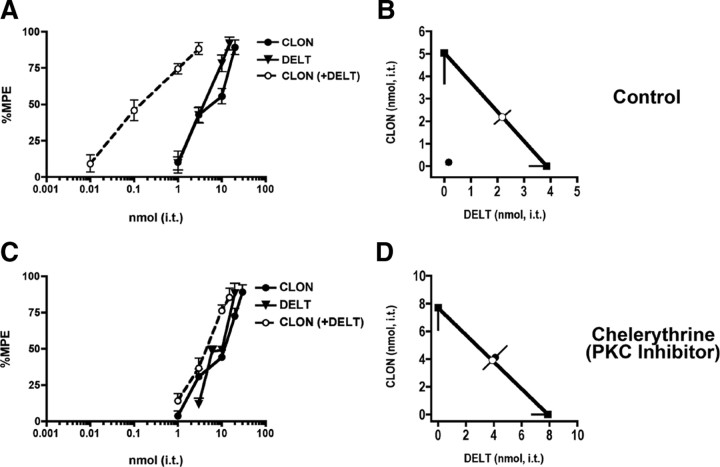
Figure 2.
Coadministration of DELT and CLON is synergistic in the tail flick test. A, Nociceptive thermal responses were challenged by intrathecal administration of DELT, CLON, and their combination. DELT (filled triangles) and CLON (filled circles) inhibited the behavior in a dose-dependent manner with similar potency and efficacy. When both DELT and CLON were coadministered at a constant dose ratio of 1:1 (open circles), the resulting potency was ∼30-fold higher than either drug given alone, suggesting that the interaction was synergistic. Error bars represent mean ± SEM for each dose point (n = 6 animals/dose). B, Isobolographic analysis of the data in Figure 1A. The y-intercept represents the CLON ED50 (5 nmol; 95% CI = 3.6–6.4), and the x-intercept represents the DELT ED50 (3.9 nmol; 95% CI = 3.2–4.5) when each was administered alone. The heavy line connecting the intercepts is the theoretical additive line and the open circle represents the theoretical additive combined ED50. Coordinates for drug combinations falling below this line and outside the confidence limits indicate a synergistic interaction. When the two compounds were coadministered at a 1:1 dose ratio, the resultant ED50 (closed circle) (0.17 nmol; 95% CI = 0.11–0.23) of DELT in the presence of CLON fell well below the additive line, indicating that the interaction was synergistic. Error bars parallel to each axis represent the lower 95% CI for each compound given alone. The error bars on the combined dose points represent the upper and lower 95% CIs. C, Coadministration of DELT and CLON show additivity in the presence of the PKC inhibitor chelerythrine. Nociceptive thermal responses were challenged by intrathecal administration of DELT, CLON, and their combination in the presence of an inhibitor of PKC. DELT (filled triangles) and CLON (filled circles) inhibited the responses in a dose-dependent manner with similar potency and efficacy. Coadministration of DELT and CLON at a 1:1 dose ratio (open circles) was ∼1.9-fold more potent than either drug given alone, compared with ∼30-fold potency shift in the absence of chelerythrine (see Fig. 1A). Error bars represent mean ± SEM for each dose point (n = 6 animals/dose). D, Isobolographic analysis was applied to the data in Figure 1C. The y-intercept represents the ED50 (7.7 nmol; 95% CI = 6.1–9.3) for CLON, and the x-intercept represents the ED50 (7.9 nmol; 95% CI = 6.8–9.0) for DELT when each was administered alone. Coadministration at a 1:1 dose ratio resulted in an ED50 (closed circle) (4.1 nmol; 95% CI = 3.2–5.0) for DELT in the presence of CLON that fell on the theoretical additive line, indicating a strictly additive interaction in the presence of the PKC inhibitor.
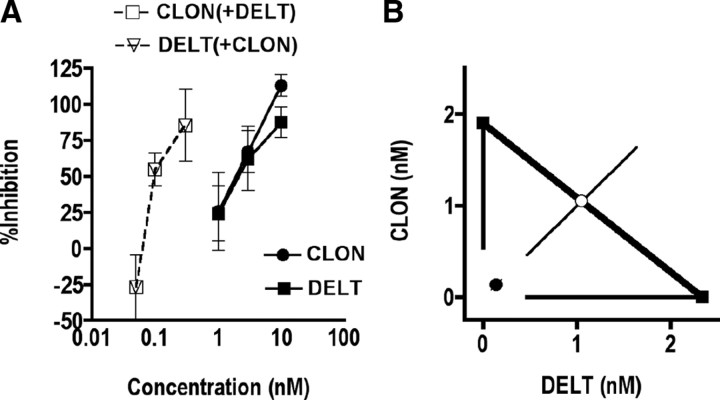
Figure 4.
Coadministration of DELT and CLON inhibits K+-evoked release of CGRP from spinal cord slices in a synergistic manner. A, K+-evoked release of CGRP was challenged by administration of DELT, CLON and their combination. DELT (filled squares) and CLON (filled circles) inhibited the release of CGRP in a concentration-dependent manner with similar potency and efficacy. Coadministration of DELT and CLON at constant concentration ratio of 1:1 (open squares and open triangles) was ∼30-fold more potent than either drug given alone, suggesting that the interaction was synergistic. Error bars represent mean ± SEM for each concentration point (n = 3–9 samples/concentration). B, Isobolographic analysis of the data in Figure 3A. The y-intercept represents the CLON EC50 (1.9 nm; 95% CI = 0.5–3.3), and the x-intercept represents the DELT EC50 (2.3 nm; 95% CI = 0.5–4.2) when each was administered alone. The heavy line connecting the intercepts is the theoretical additive line and the open circle represents the theoretical additive combined EC50. Coordinates for drug combinations falling below this line and outside the confidence limits indicate a synergistic interaction. Coadministration of CLON and DELT at a 1:1 concentration ratio resulted in an EC50 (0.06; 95% CI = 0.01–0.1) of DELT in the presence of CLON that fell well below the additive line, indicating that the interaction was synergistic. Error bars parallel to each axis represent the lower 95% CI for each compound given alone. The error bars on the combined concentration points represent the upper and lower 95% CIs.
The magnitude of drug synergism can also be expressed in terms of an Interaction Index (γ) (Tallarida, 2002). The index is defined by the equation: a/A + b/B = γ, where A and B are the doses/concentrations of drugs A and B administered separately that give a specified level of effect and (a,b) is the combination dose/concentration that produces this same level of effect (the ED50/EC50 values are commonly used for this calculation). In the absence of a drug interaction, γ = 1. If the interaction is synergistic, γ < 1. The interaction index is used here as a quantitative measure to characterize the magnitude of synergism by the CLON-DELT combination between treatment groups.
All dose–response and concentration–response and isobolographic analyses were performed with the FlashCalc 4.5.3 pharmacological statistics software package generously supplied by Dr. Michael Ossipov (Department of Pharmacology, University of Arizona College of Medicine, Tucson, AZ).
Results
Colocalization of α2AR, DOP, and SP immunoreactivity in the dorsal horn of mouse spinal cord
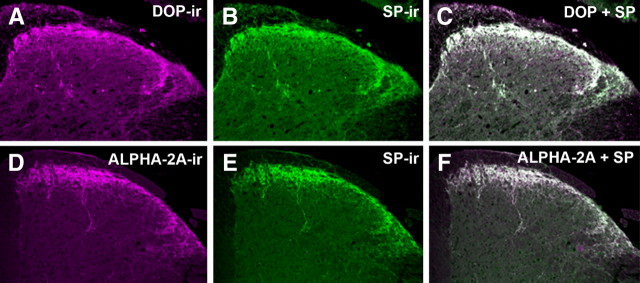
Previous reports have demonstrated that both α2AAR and DOP are expressed in the peptidergic population of primary afferent sensory neurons in rat (Dado et al., 1993; Arvidsson et al., 1995; Stone et al., 1998; Zhang et al., 1998; Riedl et al., 2009). However, anatomical characterization of α2AAR and DOP has not been fully investigated in mice. Therefore, we double-labeled mouse spinal cord sections with antibodies directed against α2AAR, DOP, and the neuropeptide SP (Fig. 1). On merging of the digital images of sections, double-labeled elements appear white (Fig. 1C,F). Colocalization of α2AAR immunoreactivity (α2AAR-IR) and SP-IR was observed with the rabbit-derived α2AAR and both rat- and guinea pig-derived SP antibodies obtained from independent sources (Fig. 1D–F; data not shown). Similarly, rabbit-derived anti-DOP labeling colocalized with both the rat- and the guinea pig-derived SP antibodies (Fig. 1A–C; data not shown). The independent colocalization of α2AAR-IR and DOP-IR with multiple SP antibodies is entirely consistent with anatomical localization in rat and strongly suggests that the colocalization observed is not artifactual. The extensive colocalization observed between both α2AAR and DOP with SP suggests that α2AAR and DOP colocalize in SP-containing fibers in the mouse spinal cord and may be positioned to mediate the antinociceptive effects of spinally delivered agonists for these receptors.
Figure 1.
Colocalization of α2AAR and DOP with SP in mouse spinal cord. A–C, Representative images of the dorsal horn of mouse spinal cord double-labeled with DOP (A, magenta) and SP (B, green) antisera. When images A and B are digitally merged (C), instances of colocalization appear as white. D–F, Representative images of mouse spinal cord double-labeled with α2AAR (D, magenta) and SP (E, Green) antisera. When images D and E are digitally merged (F), instances of colocalization appear white. The extensive colocalization observed between both α2AAR and DOP with SP suggests that α2AAR and DOP colocalize on SP-containing fibers in the mouse spinal cord. This extensive colocalization, already well characterized in rats (Riedl et al., 2009), appears to generalize to mice.
Intrathecal CLON-DELT: Behavioral antinociceptive synergy
Intrathecal administration of either CLON or DELT produced dose-dependent antinociception at 10 and 5 min postinjection, respectively (Fig. 2A); these pretreatment times were chosen to match the time of peak effect of each agent given alone (data not shown). Comparison of the respective ED50 values revealed a potency ratio between CLON and DELT of ∼1:1. Coadministration of the drugs (CLON at 10 min and DELT at 5 min) at a constant dose ratio equal to the potency ratio (1:1) yielded a third antinociceptive dose–response curve shown in Figure 2A. This combination dose–response curve is expressed in terms of the doses of CLON (0.01, 0.1, 1, 3) given in the presence of the same doses of DELT (0.01, 0.1, 1, 3) as opposed to total drug (e.g., 0.02, 0.2, 2, 6) to facilitate visual appreciation of the potency shifts of each drug in the presence of the other. The potency of each drug was increased ∼30-fold in the presence of the other, suggesting that the interaction was synergistic. The dose–response data from Figure 2A are represented graphically as an isobologram in Figure 2B, which shows that the ED50 value of the combination(closed circle) is significantly lower than the theoretical additive ED50 value (open circle). This interaction was confirmed as synergistic by statistical comparison (t test) between the observed combined ED50 value and the theoretical additive ED50 value. The interaction index, γ, was 0.04; this small γ value indicates that the synergistic interaction between CLON and DELT is of a high magnitude.
Inhibition of PKC completely and selectively reverses CLON-DELT synergistic inhibition of nociceptive responses in the tail flick test
To assess the signaling mechanisms mediating the observed behavioral antinociceptive synergy between α2AR and DOP agonists, we evaluated the effect of intrathecally coadministered CLON and DELT in the tail flick assay when mice were pretreated with selective inhibitors of PLC, PKC and PKA (U73122, 3 nmol, i.t.; chelerythrine, 1 nmol, i.t.; and H89, 6 nmol, i.t., respectively). PKC inhibition was selected because of its activation downstream of diacylglycerol produced and Ca2+ mobilized by PLC, previous behavioral work (Roerig, 1998) and recent evidence in trigeminal nociceptors showing that application of the inflammatory mediator bradykinin (BK) rapidly induces functional DOP competence through a PKC-dependent signaling mechanism (Patwardhan et al., 2005). PKA inhibition was chosen as a negative control to show specificity of the PKC effect.
After administration of the PKC inhibitor, CLON (50 min) and DELT (55 min) were administered separately or coadministered at a constant dose ratio equal to the potency ratio (1:1), and three antinociceptive dose–response curves were generated (Fig. 2C). The dose–response data from Figure 2C and the resulting isobologram (Fig. 2D) show that the ED50 value of the combination did not differ significantly from the theoretical additive ED50. This interaction was confirmed as additive by statistical comparison (t test) between the observed combined ED50 value and the theoretical additive ED50 value. The interaction index, γ, was 1.05, indicating an absence of a supra-additive drug interaction in the presence of the PKC inhibitor. Although pretreatment with PKC inhibitor completely abolished the synergy between CLON and DELT, it did not significantly change the relative potency of the agonists given separately.
In contrast to the PKC inhibitor, pretreatment with U73122 (a PLC inhibitor) (Fig. 3A, dark bars) reversed or reduced the inhibitory effects of high-efficacy doses of both CLON and DELT as well as a synergistic combination dose. This result permits speculation that PLC-mediated vesicle translocation to the plasma membrane contributes to both the inhibitory effects of single agonist administration and their synergistic interaction, whereas PKC is unique to the synergistic interaction. Inhibition of PKA with H89 (Fig. 3B, vertical striped bars) had no effect on CLON administered alone or the synergistic CLON-DELT combination, indicating that PKA, unlike PKC, is not required for CLON-DELT synergy. Pretreatment with H89 consistently reduced the inhibitory effect of DELT administered alone (Fig. 3B).
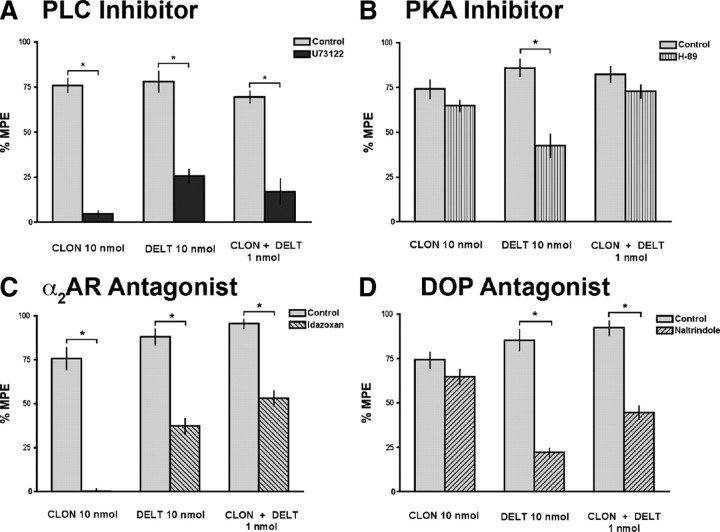
Figure 3.
Effect of the PLC inhibitor U73122, the PKA inhibitor H89, the α2AR antagonist idazoxan, and the DOP antagonist naltrindole on the ability of CLON, DELT, and the CLON-DELT combination to inhibit nociceptive thermal responses in the tail flick test. High-efficacy doses of CLON and DELT as well as the low-dose CLON-DELT combination were tested under control conditions (light bars) and in the presence of U73122 (3 nmol i.t., dark bars), H89 (6 nmol i.t., vertical striped bars), idazoxan (10 nmol i.t., downward diagonal stripes), and naltrindole (8.8 nmol i.t., upward diagonal stripes). A, The upstream inhibitor of PLC, U73122, abolished the inhibitory effects of the agonists administered alone (76.0 ± 3.9% vs 4.6 ± 1.6% inhibition for CLON and 78.1 ± 5.7% vs 25.7 ± 3.7% inhibition for DELT in the presence of U73122) as well as the synergistic effect of their low-dose combination (69.6 ± 3.3% vs 17.0 ± 7.1% inhibition in the presence of U73122). B, Pretreatment with the PKA inhibitor, H89, had no effect on either CLON administered alone (74.1 ± 5.1% vs 64.9 ± 3.0% inhibition in the presence of H89) or the low-dose combination (82.5 ± 4.5% vs 72.9 ± 3.8% inhibition for in the presence of H89). Pretreatment with H89 reduced the effect of DELT administered alone (88.4 ± 4.2% vs 40.8 ± 6.2% inhibition for DELT in the presence of H89). C, Pretreatment with the α2AR antagonist, idazoxan, completely reversed the effect of CLON administered alone (75.7 ± 6.3% vs 0.2 ± 1.5% inhibition in the presence of idazoxan), and reduced the effects of both DELT alone (87.9 ± 4.4% vs 37.3 ± 4.5% inhibition in the presence of idazoxan) and the CLON-DELT combination (95.4 ± 2.6% vs 53.0 ± 4.4% inhibition in the presence of idazoxan). D, Pretreatment with the DOP antagonist, naltrindole, had no effect on CLON administered alone (74.2 ± 4.5% vs 64.7 ± 4.4% inhibition in the presence of naltrindole), but reduced the inhibitory effect of DELT administered alone (85.3 ± 5.9% vs 22.1 ± 2.4% inhibition in the presence of naltrindole). Pretreatment with naltrindole also reduced the effect of the CLON-DELT combination (92.3 ± 4.0% vs 44.6 ± 3.7% inhibition in the presence of naltrindole). Error bars represent mean ± SEM (n = 6 animals/dose). *p < 0.05; Student's t test.
It is known that there is a low basal level of adrenergic tone in the form of norepinepherine release from descending noradrenergic neurons that terminate in the dorsal horn of the spinal cord (Pertovaara, 2006). Therefore, we hypothesized that the observed inhibitory effect of DELT in the tail flick assay was partially mediated through α2-adrenergic receptor activation by endogenous NE. PKA has been implicated in modulation of neurotransmitter release dynamics from nerve terminals (Trudeau et al., 1996); thus, inhibiting PKA with H89 could be interfering with the aforementioned descending noradrenergic tone, thus reducing the inhibitory effect of DELT administration.
To test this hypothesis, the effects of CLON, DELT, and the CLON-DELT combination were challenged with i.t. pretreatment of the α2AR antagonist, idazoxan (10 nmol, i.t.) (Fig. 3C, downward diagonal stripes). As expected, i.t. pretreatment with idazoxan completely reversed the inhibitory effect of CLON administered alone. Pretreatment with idazoxan also reduced the inhibitory effect of the CLON-DELT combination. In support of the hypothesized ongoing noradrenergic tone, i.t. pretreatment with idazoxan also reduced the inhibitory effect of DELT, supporting a role for endogenous α2AR activation in the effect of DELT administration alone. Activation of α2ARs by endogenous NE has previously been reported to be involved in opioid receptor-mediated antinociception, as mice lacking functional α2AARs or wild-type mice treated with idazoxan showed decreased morphine potency in inhibiting nocifensive responses to i.t. administration of SP (Stone et al., 1997). We also challenged both agonists administered separately and in combination with the DOP antagonist, naltrindole (8.8 nmol, i.t.) (Fig. 3D, upward diagonal stripes). The inhibitory effects of both DELT alone and the CLON-DELT combination were reduced with DOP antagonist pretreatment. However, i.t. pretreatment with the DOP antagonist had no effect on CLON administered alone. These data suggest that, although the effect of CLON administered alone in vivo is mediated solely through α2ARs, the effect of i.t. DELT administration is primarily mediated through DOP but partially mediated through α2AR activation by endogenous NE.
CLON-DELT: Synergistic inhibition of K+-stimulated CGRP release from spinal cord slices
To determine whether the antinociceptive interaction observed in vivo was attributable to activation of receptors at the level of primary afferent terminals, we determined the ability of α2AR and DOP agonists to inhibit K+-stimulated release of the neuropeptide CGRP in vitro using the spinal cord slice preparation. Measurement of CGRP release was chosen because, although K+ stimulation causes depolarization of all cells in the slice preparation, CGRP in the spinal cord is exclusively released by primary afferent terminals (Franco-Cereceda et al., 1987; Plenderleith et al., 1990). Stimulation of spinal cord slices with 60 mm K+ significantly increased the concentration of immunoreactive CGRP (iCGRP) in the superfusate from 44.7 ± 5.2 pg/ml (basal level) to 221 ± 44.4 pg/ml (data not shown). This increase was inhibited in a concentration-dependent manner by pretreatment with either the α2AR agonist CLON (closed circles) or the DOP agonist DELT (closed squares) (Fig. 4A). To determine whether a synergistic interaction exists between these receptors in this preparation, slices were superfused with both drugs in combination. The resultant concentration–response curve (Fig. 4A, open squares and open triangles) shows the effect of fixed-ratio combinations of the two agents administered simultaneously. The potency of each drug was increased ∼30-fold in the presence of the other, similar to results obtained in vivo, suggesting that the interaction is synergistic. The concentration–response data from Figure 4A are represented graphically as an isobologram in Figure 4B, which shows that the EC50 of the combination (closed circle) is significantly lower than the theoretical additive EC50 (open circle). Statistical comparison (t test) between the observed combined EC50 value and the theoretical additive EC50 value demonstrates that this interaction is synergistic. The interaction index, γ, for the combination was 0.06, indicating a substantial synergistic interaction between α2AR and DOP agonists at the level of primary afferent terminals. Pretreatment of spinal cord slices with the nonselective OR antagonist naloxone (1 μm) or the α2AR antagonist idazoxan (1 μm) abolished the inhibitory action of DELT and CLON, respectively, confirming that the observed effects were OR- and α2AR-mediated (data not shown).
Inhibition of PKC completely and selectively reverses CLON-DELT synergistic inhibition of CGRP release from spinal cord slices
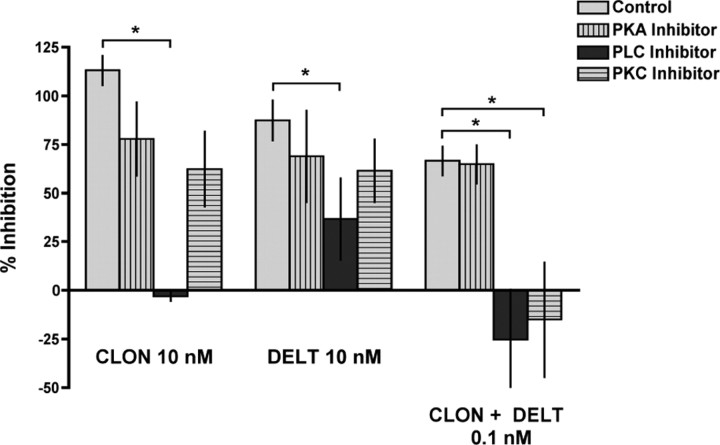
To determine whether the reduction of antinociceptive synergy with PKC inhibition observed in vivo generalized to the level of primary afferent terminals, we tested whether the PKC inhibitor could similarly affect the synergistic interaction between CLON and DELT in reducing inhibition of K+-stimulated release of the neuropeptide CGRP in vitro using the spinal cord slice preparation. To determine whether there are differential signaling mechanisms mediating the effects of these agonists administered alone and in the synergistic combination, we challenged the ability of CLON, DELT and the combination to inhibit K+-stimulated CGRP release from rat spinal cord slices with selective inhibitors of PLC, PKC and PKA (Fig. 5). High-efficacy concentrations of CLON and DELT were tested under control conditions (light bars) and in the presence of the PKA inhibitor H89 (1 μm), the PLC inhibitor U73122 (10 μm), or the PKC inhibitor chelerythrine (2.5 μm) (vertical striped bars, dark bars, and horizontal striped bars, respectively). Only the PLC inhibitor significantly reversed the inhibitory effects of CLON and DELT when administered alone, consistent with behavioral results. Furthermore, neither inhibition of PKC nor inhibition of PKA with H89 administration reversed the effect of either CLON or DELT administered alone in vitro.
Figure 5.
Effect of the PKC inhibitor chelerythrine, the PLC inhibitor U73122 and the PKA inhibitor H89 on the ability of CLON, DELT, and the CLON-DELT combination to inhibit CGRP release from spinal cord slices. (Note: ordinate represents percentage inhibition of release; values near 0 indicate blockade of release inhibition.) High-efficacy concentrations of CLON and DELT, as well as the low-concentration CLON-DELT combination were tested under control conditions (light bars) and in the presence of H89 (1 μm, vertical striped bar), U73122 (10 μm, dark bars), or chelerythrine (2.5 μm, horizontal striped bars). Chelerythrine did not significantly affect the ability of the individual agonists to inhibit CGRP release (113 ± 7.6% vs 62.3 ± 19.3% inhibition for CLON in the presence of chelerythrine and 87.4 ± 10.4% vs 61.5 ± 16.2% inhibition for DELT in the presence chelerythrine); however, chelerythrine abolished the synergistic effect of the low-concentration combination (61.5 ± 8.2% vs −15.0 ± 29.5% inhibition in the presence of chelerythrine). In contrast, U73122 blocked the inhibition of release by either agent alone (113 ± 7.6% vs −3.1 ± 2.5% inhibition for CLON in the presence of U73122 and 87.4 ± 10.4% vs 36.7 ± 21.0% inhibition for DELT in the presence U73122) as well as the synergistic inhibition of release (66.6 ± 7.5% vs −25.3 ± 25.8% inhibition in the presence of U73122), indicating that PLC activation is required for inhibition of release. H89 did not significantly affect the ability of the individual agonists to inhibit CGRP release (113 ± 7.6% vs 77.9 ± 18.9% inhibition for CLON in the presence of H89 and 87.4 ± 10.4% vs 68.9 ± 23.6% inhibition for DELT in the presence H89). Treatment with H89 also had no effect on CLON-DELT synergism (66.6 ± 7.5% vs 64.8 ± 10.0% inhibition in the presence of H89), supporting the specific requirement of PKC activation for synergy. Error bars represent mean ± SEM (n = 3–8 samples/group). *p < 0.05; Student's t test.
When the effect of inhibitors of PLC, PKC and PKA on DELT-CLON synergy were evaluated, inhibition of both PLC and PKC, but not PKA, blocked the synergistic interaction. These data suggest that, although the effect of both agonists administered separately and together requires activation of the PLC pathway, only the synergistic effect of both agonists coadministered relies on activation of PKC. PKC, but not PKA dependence in the spinal cord slice preparation is consistent with the in vivo data in Figures 2 and 3.
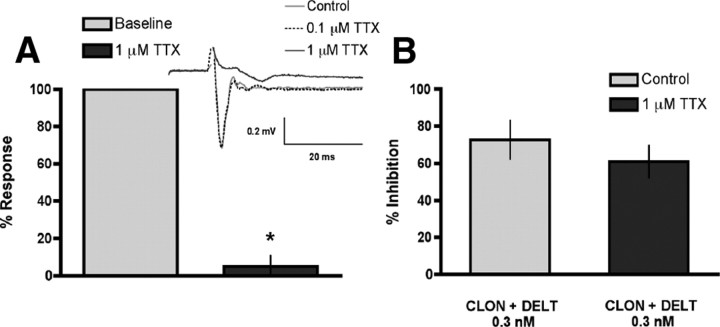
CLON-DELT synergy is maintained in the presence of tetrodotoxin
The similar results reported in (Riedl et al., 2009) for greater-than-additive inhibition of CGRP release from spinal cord synaptosomes suggests that this interaction takes place in subcellular compartments cocontaining the receptors. To determine whether the synergistic interaction between the two agonists requires colocalization within subcellular compartments rather than through interneuronal circuitry, we evaluated whether CLON-DELT synergy in the spinal cord slice preparation is maintained in the presence of the sodium channel blocker, TTX, which inhibits neural transmission. We chose a concentration of 1 μm TTX based on (1) a literature survey of rat (Murase and Randic, 1983; Ryu et al., 1988; Yoshimura and Jessell, 1989; Yoshimura and Jessell, 1990) and mouse (Han et al., 2007) studies, and (2) a positive control experiment showing that 1 μm TTX, but not 0.1 μm, completely blocked fEPSPs in rat spinal cord slices (4.9 ± 5.9% response in the presence of TTX vs baseline) (Fig. 6A). The presence of TTX (1 μm) in the superfusate throughout the experiment did not affect the synergistic inhibition of the CLON-DELT combination (0.1 nm, 1:1 concentration ratio) of K+-evoked CGRP release from spinal cord slices (72.7 ± 10.4% vs 60.9 ± 8.9% inhibition in the presence of TTX) (Fig. 6B). Together, these results support and extend recent findings in spinal cord synaptosomes (Riedl et al., 2009) that the observed synergy between agonists acting at these two receptors occurs within single subcellular compartments (i.e., the terminals of primary afferent nociceptive fibers in the dorsal horn of the spinal cord).
Figure 6.
CLON-DELT combination synergy in the spinal cord slice preparation is maintained in the presence of the sodium channel blocker, TTX. A, Positive control for the efficacy of 1 μm TTX to eliminate interneuronal signaling: TTX completely blocked evoked fEPSPs in rat spinal cord slice preparations [4.9 ± 5.9% response in the presence of TTX (dark bar) vs baseline (light bar)]. A (Inset), Representative traces of evoked fEPSPs in the presence or absence of TTX (0.1 or 1 μm). B, The inhibitory action of a synergistic CLON-DELT combination was challenged by the addition of TTX (1 μm) to the superfusate. TTX did not alter the synergistic inhibition of K+-evoked (60 mm) CGRP release invoked by the CLON-DELT combination (0.1 nm, 1:1 concentration ratio) (72.7 ± 10.4% vs 60.9 ± 8.9% inhibition in the presence of TTX), supporting that synergy between α2ARs and DOPs does not rely on multicellular circuitry. Error bars represent mean ± SEM (n = 3–4 slices or samples/group). *p < 0.05 compared with baseline; Student's t test.
CLON-DELT combination causes significant CGRP release in the absence of K+ stimulation
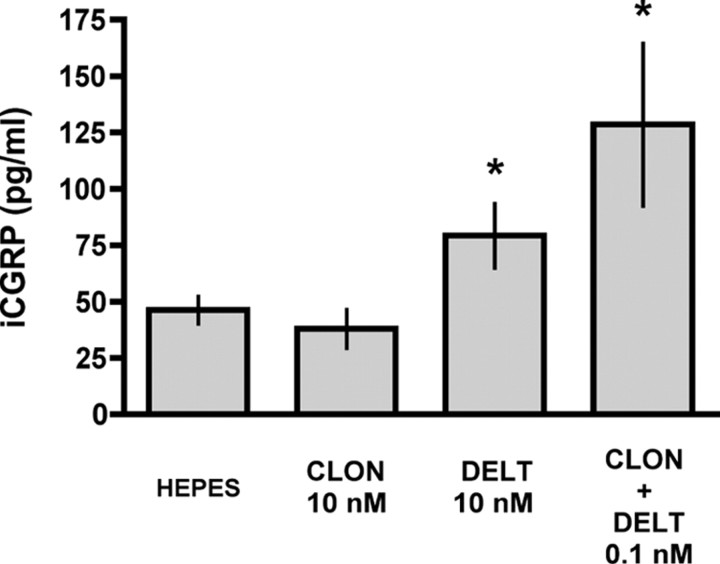
Since DOP agonist-induced CGRP release from cultured DRG neurons has been shown to correlate with functional DOP insertion into the plasma membrane (Bao et al., 2003), we sought to determine whether the low-concentration CLON-DELT combination could also cause CGRP release in the absence of K+ stimulation (Fig. 7). Spinal cord slices were superfused with CLON, DELT, and the CLON-DELT combination (10 nm, 10 nm and 0.1 nm, respectively) to test for CGRP release in the absence of K+ depolarization. Stimulation of spinal cord slices with CLON (10 nm) failed to cause significant release of CGRP. In agreement with (Bao et al., 2003), however, stimulation of spinal cord slices with DELT (10 nm) caused significant release of CGRP. Stimulation of spinal cord slices with a 100-fold lower concentration of the CLON-DELT combination also caused significant release of CGRP in the absence of K+ stimulation. These data suggest that the CLON-DELT combination may act synergistically at DOP and α2AR to externalize large dense-core vesicles containing CGRP, and thus may act to insert functional receptors into the membrane in the same manner as DOP agonist alone.
Figure 7.
Low-concentration CLON-DELT combination causes significant CGRP release in the spinal cord slice preparation in the absence of K+ stimulation. The ability of CLON, DELT, and the CLON-DELT combination to cause release of CGRP in spinal cord slices was investigated in the absence of K+ depolarization. CLON did not significantly increase CGRP levels above baseline levels [46.3 ± 5.9 pg/ml (basal level) vs 37.9 ± 8.5 pg/ml (CLON, 10 nm)]. In contrast, both DELT [46.3 ± 5.9 pg/ml (basal level) vs 79.2 ± 14.3 pg/ml (DELT, 10 nm)] and a 100-fold lower concentration of the CLON-DELT combination [46.3 ± 5.9 pg/ml (basal level) vs 128.5 ± 35.9 pg/ml (CLON-DELT, 0.1 nm)] were able to stimulate significant CGRP release without K+ stimulation, suggesting that the CLON-DELT combination may act synergistically at DOPs and α2ARs to externalize large dense-core vesicles. Error bars represent mean ± SEM (n = 3–11 samples/group). *p < 0.05 compared with HEPES; Student's t test.
Discussion
The results from this study indicate that the synergistic interaction between agonists acting at α2ARs and DOPs can occur at the level of primary afferent terminals to inhibit release of nociceptive neuropeptides from spinal cord slices and that this inhibition translates to synergistic antinociception in vivo. Whereas PLC activation is required for both antinociception and inhibition of neuropeptide release by α2AR and DOP agonists given singly or together, PKC activation is specifically required for the synergistic interaction between coadministered agonists. In contrast, PKA is not involved in the effects of α2AR and DOP agonists administered separately or in combination, reinforcing the unique ability of PKC to mediate α2AR/DOP synergy. That the synergistic interaction observed in vitro is maintained in the presence of TTX indicates that α2AR/DOP synergy can take place in single subcellular compartments in the absence of multicellular circuitry. These in vivo and in vitro results confirm and extend a recent report showing that these receptors inhibit neuropeptide release in a greater-than-additive manner from spinal cord synaptosomes (Riedl et al., 2009).
Behavioral and in vitro synergy between agonists acting at α2AR and DOP
Synergistic interactions between classes of analgesic agonists have been frequently reported in the literature, although the mechanisms underlying this phenomenon remain to be fully defined. It has been suggested, for example, that synergy will be observed between agonists acting at the following receptor pairs: δ-opioid/α2A-adrenergic (Stone et al., 1997), δ-opioid/α2C-adrenergic (Fairbanks et al., 2002), μ-opioid/α2A-adrenergic (Stone et al., 1997) and μ-opioid/α2C-adrenergic (Fairbanks et al., 2000). We and others have previously demonstrated that both α2AARs and DOPs are localized on the terminals of capsaicin-sensitive, SP-expressing primary afferent neurons in the dorsal horn of the spinal cord in rat (Dado et al., 1993; Arvidsson et al., 1995; Stone et al., 1998; Zhang et al., 1998) where they are highly colocalized (Riedl et al., 2009). The present study demonstrates that these two receptors colocalize identically in mouse, underscoring the congruence between species. The localization of the α2AAR subtype together with previous studies showing that the effect of CLON is eliminated in mice lacking functional α2AARs (Stone et al., 1997) suggests that the CLON effect seen in the CLON-DELT synergistic interaction is mediated through this particular α2AR subtype. Because α2AARs and DOPs colocalize in the terminals of primary afferent neurons (Riedl et al., 2009), we sought to use selective agonists for the α2AR and DOP in both a behavioral model and a more reduced spinal cord slice preparation to determine whether the synergistic interaction between these agonists to colocalized receptors could take place within a single subcellular compartment. This study shows that the selective α2AR and DOP agonists CLON and DELT are each able to dose-dependently inhibit nociceptive responses when administered i.t. in the tail flick assay and to synergize in producing this antinociceptive effect. These results confirm previous findings using a different α2AR agonist (brimonidine, also known as UK-14,304) (Stone et al., 1997). Furthermore, this interaction appears to take place within the terminals of primary afferent neurons: CLON and DELT inhibit K+-evoked release of CGRP in a greater-than-additive manner from both spinal cord synaptosomes (Riedl et al., 2009) and superfused spinal cord slices (this study). That this synergy is maintained in the presence of the sodium channel blocker, TTX, further indicates that this interaction does not rely on multineuronal circuitry.
Signaling mechanisms mediating α2AR and DOP synergy in the spinal cord
Because we observed that the analgesic synergy between agonists acting at α2ARs and DOPs in spinal cord occurs within the terminals of primary afferent neurons, we sought to address the signaling mechanisms involved in this interaction. In small dorsal root ganglion (DRG) neurons, DOPs are known to predominantly localize to the cytoplasm and have been shown through immunofluorescence (Dado et al., 1993; Arvidsson et al., 1995), electron microscopy (Cheng et al., 1995; Zhang et al., 1998) and biochemical evidence (Wang et al., 2008) to often associate with the membrane of large dense-core vesicles that contain neuropeptides (e.g., CGRP, SP), with only a limited number of DOPs distributed in the plasma membrane. Because, under basal conditions, DOPs are mainly localized to the cytoplasm, it has been suggested that the majority are “reserve” receptors that are then targeted to and inserted in the plasma membrane in response to physiological changes (Zhang et al., 1998; Cahill et al., 2001a,b; Bao et al., 2003; Cahill et al., 2003; Gendron et al., 2006). Activation of DOPs through agonist binding has been shown to trigger a slow but long-lasting exocytosis of large dense core vesicles (Wang et al., 2008), leading to an increase in cell surface area, insertion of functional DOPs, and CGRP release in a PLC- and Ca2+-dependent manner, presumably through activation of Gq (Bao et al., 2003). It is also known that activation of either the DOP (Yoon et al., 1999) or α2AAR (Dorn et al., 1997) can mobilize IP3-sensitive Ca2+ stores through a signal transduction pathway that involves activation of PLC by Gβγsubunits released from agonist-induced dissociation of the Gi heterotrimer. That DOPs and α2ARs share common signaling pathways through similar G-proteins and can both mobilize intracellular Ca2+ through activation of PLC suggests that trafficking mechanisms for α2ARs in primary afferent terminals are similar to those of DOPs.
Whereas GPCR-mediated activation of PLC and subsequent release of Ca2+ from internal stores is often associated with receptors coupling to stimulatory G-proteins (e.g., Gs and Gq), α2ARs and DOPs preferentially couple through inhibitory G-proteins. However, evidence exists in the literature suggesting that ORs can couple not only to Gi/Go, but to a variety of G-proteins. For instance, opioids can produce analgesia through activation of PLA2 and have been shown to act through various G-proteins to activate phospholipase C (PLC), thereby mobilizing Ca2+, activating PKC and enhancing presynaptic voltage-gated, ATP-gated and Ca2+-gated K+-channel activity (for review, see Aantaa et al., 1995; Connor and Christie, 1999; Millan, 1999, 2002; Law et al., 2000).
In support of the mechanism of DOP agonist-induced receptor externalization via PLC (Bao et al., 2003), the results from this study show that the effect of α2AR and DOP agonists administered separately or in combination is blocked by an inhibitor of PLC both behaviorally and in vitro. This outcome suggests that agonist-driven externalization via PLC is involved in the analgesic effects of CLON, DELT, and the CLON-DELT combination. Therefore, the possibility exists that each agonist also promotes the externalization of the other receptor, presenting an opportunity for mutual enhancement of receptor number. This mechanism is further supported by the present results showing that administration of the CLON-DELT combination results in significant release of CGRP (thus enabling/signaling externalization of “reserve” receptors) from spinal cord slices in the absence of K+ stimulation at a 100-fold lower concentration than is needed to produce release with agonist alone.
In contrast to PLC, however, downstream activation of PKC seems to be uniquely involved in the synergistic interaction between CLON and DELT. We have shown that inhibition of PKC completely blocked the synergistic combination, but did not significantly blunt the action of either agonist alone in either the tail flick test or inhibition of K+-evoked CGRP release from spinal cord slices. This involvement of PKC in the synergistic interaction is consistent with the role of PKC in enhancing DOP “competence” (Patwardhan et al., 2005). Furthermore, the failure of PKA inhibition to block the synergistic effect underscores the specificity of PKC's involvement. These data are also consistent with previous antinociception results indicating that PKC, but not PKA, activity regulates the synergistic interaction between morphine and CLON in inhibiting nocifensive responses to i.t. administration of SP (Wei and Roerig, 1998), suggesting that PKC may mediate multiple opioid and α2AR subtype interactions in the spinal cord.
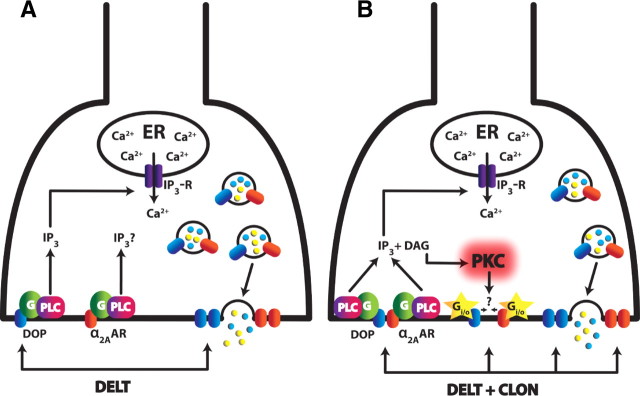
In the proposed model (Fig. 8), we postulate that agonist-induced receptor insertion via PLC is necessary for the spinal analgesic effects of CLON and DELT by allowing “reserve” receptors to be trafficked to the plasma membrane. We further speculate that, when α2ARs and DOPs are coactivated in the primary afferent terminal, PKC is activated, presumably through increased levels of diacylglycerol (DAG) downstream of PLC, is translocated to the plasma membrane and functions to mediate/facilitate the synergistic interaction.
Figure 8.
Proposed mechanism of CLON-DELT analgesic synergy localized to primary afferent terminals in the dorsal horn of the spinal cord. A, Model of DOP agonist-induced DOP insertion coinciding with neuropeptide release (adapted from Bao et al., 2003). Activation of DOPs through administration of DELT causes activation of PLC (presumably through Gq), thereby increasing intracellular Ca2+ concentrations via IP3 receptors. This spike in Ca2+ mediates exocytosis of LDCVs, thus releasing neuropeptides and inserting intracellular “reserve” DOPs to the plasma membrane. B, Proposed model of CLON-DELT synergy mediated by PKC through coactivation of α2ARs and DOPs. Coactivation of α2ARs and DOPs causes neuropeptide release at a 100-fold lower concentration than DELT administration alone via the same mechanism as A. In contrast to DOP agonist-induced DOP insertion, however, coactivation of α2ARs and DOPs causes activation of PKC through increased levels of DAG. Activation of PKC, in turn, mediates the synergistic interaction of α2ARs and DOPs. One of several hypotheses for this mechanism is that the phosphorylation target(s) of PKC allow enhanced Gi/o coupling of both the α2AAR and DOP (yellow stars). An alternative hypothesis is that activation of PKC favors the formation of α2AR/DOP heterodimers with an enhanced inhibitory mode of action.
One of several possible explanations for the differential signaling following receptor coactivation is the formation of heterodimeric complexes between α2AARs and DOPs. The emergence of novel pharmacological properties from heterodimer activation distinct from either component receptor alone has been previously investigated for dopamine receptors (Rashid et al., 2007). In the case of ORs, in vitro evidence indicates synergistic binding and coupling potentiation of coactivated κ-ORs and DOPs (Jordan and Devi, 1999) and conformational changes via crosstalk that modulate receptor function between α2ARs and μ-ORs (Vilardaga et al., 2008). Furthermore, α2AR-DOP synergy is lost in mice lacking functional α2AARs (Stone et al., 1997), whereas it is retained in μ-OR KO mice (Guo et al., 2003), supporting the specific involvement of DOPs in the synergistic interaction. Together these data demonstrate that α2AR-DOP synergy is dependent on the presence of both receptors, but, to this point, α2AR-DOP heterodimer formation in vivo is purely theoretical. An alternative hypothesis is that the phosphorylation target(s) of PKC allow enhanced Gi/o coupling of both the α2AAR and DOP. Evidence for a specific role for PKC is a first step in elucidating the pathways involved in α2AR/DOP synergy, but further work is needed to determine the contribution of downstream targets of PKC.
Conclusion
Synergy is important in clinical pain management as much lower doses of each drug can be administered to produce analgesia, thus reducing unwanted side effects and improving treatment outcomes. These results provide strong evidence that synergy between analgesic agonists acting at anatomically colocalized receptor populations in spinal cord can occur, and that this interaction is not dependent on multineuronal circuitry. In the case of α2AR and DOP agonist combinations, the synergistic interaction appears to be mediated through the activation of PKC. The phosphorylation target(s) of PKC mediating the enhanced potency remain unknown, as does the mechanism of enhancement. Identifying the molecular basis of spinal analgesic synergy may contribute to improved therapeutic strategies to control chronic pain and understand mechanisms of chronic pain induction.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant R01DA015438 to G.L.W., and Canadian Institutes of Health Research Grant MOP-86691 and Fonds de la Recherche en Santé du Québec Chercheur Boursier #14312 to L.S.S; NIH Training Grant T32DA07234 supported A.C.O. We thank Dr. Walter Bowles for providing perfusion equipment and Cicely Schramm for thoughtful reading of this manuscript.
References
- Aantaa R, Marjamäki A, Scheinin M. Molecular pharmacology of alpha 2-adrenoceptor subtypes. Ann Med. 1995;27:439–449. doi: 10.3109/07853899709002452. [DOI] [PubMed] [Google Scholar]
- American Pain Society. Ed 6. Glenview, IL: American Pain Society; 2008. Principles of analgesic use in the treatment of acute pain and cancer pain. [Google Scholar]
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW. delta-Opioid receptor immunoreactivity: distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci. 1995;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hökfelt T, Zhou Z, Zhang X. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001a;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat CNS: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001b;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, Inturrisi CE, Pickel VM. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of delta-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci. 1995;15:5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Res. 1997;778:367–380. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- Connor M, Christie MD. Opioid receptor signalling mechanisms. Clin Exp Pharmacol Physiol. 1999;26:493–499. doi: 10.1046/j.1440-1681.1999.03049.x. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Galfre G, Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979;76:3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dado RJ, Law PY, Loh HH, Elde R. Immunofluorescent identification of a delta (delta)-opioid receptor on primary afferent nerve terminals. Neuroreport. 1993;5:341–344. doi: 10.1097/00001756-199312000-00041. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Oswald KJ, McCluskey TS, Kuhel DG, Liggett SB. Alpha 2A-adrenergic receptor stimulated calcium release is transduced by Gi-associated G(beta gamma)-mediated activation of phospholipase C. Biochemistry. 1997;36:6415–6423. doi: 10.1021/bi970080s. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Posthumus IJ, Kitto KF, Stone LS, Wilcox GL. Moxonidine, a selective imidazoline/alpha(2) adrenergic receptor agonist, synergizes with morphine and deltorphin II to inhibit substance P-induced behavior in mice. Pain. 2000;84:13–20. doi: 10.1016/S0304-3959(99)00171-2. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. Alpha(2C)-Adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J Pharmacol Exp Ther. 2002;300:282–290. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A, Henke H, Lundberg JM, Petermann JB, Hökfelt T, Fischer JA. Calcitonin gene-related peptide (CGRP) in capsaicin-sensitive substance P-immunoreactive sensory neurons in animals and man: distribution and release by capsaicin. Peptides. 1987;8:399–410. doi: 10.1016/0196-9781(87)90117-3. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XH, Fairbanks CA, Stone LS, Loh HH. DPDPE-UK14,304 synergy is retained in mu opioid receptor knockout mice. Pain. 2003;104:209–217. doi: 10.1016/s0304-3959(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Han SK, Park JR, Park SA, Chun SW, Lee JC, Lee SY, Ryu PD, Park SJ. Noradrenaline inhibits substantia gelatinosa neurons in mice trigeminal subnucleus caudalis via alpha(2) and beta adrenoceptors. Neurosci Lett. 2007;411:92–97. doi: 10.1016/j.neulet.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Honoré P, Chapman V, Buritova J, Besson JM. To what extent do spinal interactions between an alpha-2 adrenoceptor agonist and a mu opioid agonist influence noxiously evoked c-Fos expression in the rat? A pharmacological study. J Pharmacol Exp Ther. 1996;278:393–403. [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Hylden JLK, Wilcox GL. Pharmacological characterization of Substance P-induced nociception in mice: modulation by opioid and noradrenergic agonists at the spinal level. J Pharmacol Exp Ther. 1983;226:398–404. [PubMed] [Google Scholar]
- Janssen PA, Niemegeers CJ, Dony JG. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittelforschung. 1963;13:502–507. [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu Rev Pharmacol Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Monasky MS, Zinsmeister AR, Stevens CW, Yaksh TL. Interaction of intrathecal morphine and ST-91 on antinociception in the rat: dose-response analysis, antagonism and clearance. J Pharmacol Exp Ther. 1990;254:383–392. [PubMed] [Google Scholar]
- Murase K, Randiæ M. Electrophysiological properties of rat spinal dorsal horn neurones in vitro: calcium-dependent action potentials. J Physiol. 1983;334:141–153. doi: 10.1113/jphysiol.1983.sp014485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote K, Kitahata LM, Collins JG, Nakatani K, Nakagawa I. The antinociceptive role of mu- and delta-opiate receptors and their interactions in the spinal dorsal horn of cats. Anesth Analg. 1990;71:23–28. doi: 10.1213/00000539-199007000-00004. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Harris S, Lloyd P, Messineo E. An isobolographic analysis of the antinociceptive effect of systemically and intrathecally administered combinations of clonidine and opiates. J Pharmacol Exp Ther. 1990a;255:1107–1116. [PubMed] [Google Scholar]
- Ossipov MH, Harris S, Lloyd P, Messineo E, Lin BS, Bagley J. Antinociceptive interaction between opioids and medetomidine: systemic additivity and spinal synergy. Anesthesiology. 1990b;73:1227–1235. doi: 10.1097/00000542-199012000-00022. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lozito R, Messineo E, Green J, Harris S, Lloyd P. Spinal antinociceptive synergy between clonidine and morphine, U69593, and DPDPE: isobolographic analysis. Life Sci. 1990c;47:PL71–PL76. doi: 10.1016/0024-3205(90)90530-5. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Plenderleith MB, Haller CJ, Snow PJ. Peptide coexistence in axon terminals within the superficial dorsal horn of the rat spinal cord. Synapse. 1990;6:344–350. doi: 10.1002/syn.890060406. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl MS, Schnell SA, Overland AC, Chabot-Doré AJ, Taylor AM, Ribeiro-da-Silva A, Elde RP, Wilcox GL, Stone LS. Coexpression of alpha(2A)-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. J Comp Neurol. 2009;513:385–398. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerig SC, Lei S, Kitto K, Hylden JK, Wilcox GL. Spinal interactions between opioid and noradrenergic agonists in mice: multiplicativity involves delta and alpha-2 receptors. J Pharmacol Exp Ther. 1992;262:365–374. [PubMed] [Google Scholar]
- Ryu PD, Gerber G, Murase K, Randic M. Actions of calcitonin gene-related peptide on rat spinal dorsal horn neurons. Brain Res. 1988;441:357–361. doi: 10.1016/0006-8993(88)91414-x. [DOI] [PubMed] [Google Scholar]
- Stevens CW, Monasky MS, Yaksh TL. Spinal infusion of opiate and alpha-2 agonists in rats: tolerance and cross-tolerance studies. J Pharmacol Exp Ther. 1988;244:63–70. [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17:7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hökfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AF, Dashwood MR, Dickenson AH. Alpha 2-adrenoceptor modulation of nociception in rat spinal cord: location, effects and interactions with morphine. Eur J Pharmacol. 1987;138:169–177. doi: 10.1016/0014-2999(87)90430-4. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Statistical analysis of drug combinations for synergism. Pain. 1992;49:93–97. doi: 10.1016/0304-3959(92)90193-F. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. The interaction index: a measure of drug synergism. Pain. 2002;98:163–168. doi: 10.1016/s0304-3959(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Emery DG, Haydon PG. Direct modulation of the secretory machinery underlies PKA-dependent synaptic facilitation in hippocampal neurons. Neuron. 1996;17:789–797. doi: 10.1016/s0896-6273(00)80210-x. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat Chem Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- Wang HB, Guan JS, Bao L, Zhang X. Distinct subcellular distribution of delta-opioid receptor fused with various tags in PC12 cells. Neurochem Res. 2008;33:2028–2034. doi: 10.1007/s11064-008-9678-9. [DOI] [PubMed] [Google Scholar]
- Wei ZY, Roerig SC. Spinal morphine/clonidine antinociceptive synergism is regulated by protein kinase C, but not protein kinase A activity. J Pharmacol Exp Ther. 1998;287:937–943. [PubMed] [Google Scholar]
- Wessendorf MW, Elde RP. Characterization of an immunofluorescence technique for the demonstration of coexisting neurotransmitters within nerve fibers and terminals. J Histochem Cytochem. 1985;33:984–994. doi: 10.1177/33.10.2413102. [DOI] [PubMed] [Google Scholar]
- Wigdor S, Wilcox GL. Central and systemic morphine antinociception in the mouse: contribution of descending noradrenergic and serotonergic pathways. J Pharmacol Exp Ther. 1987;242:90–95. [PubMed] [Google Scholar]
- Wilcox GL, Carlsson KH, Jochim A, Jurna I. Mutual potentiation of antinociceptive effects of morphine and clonidine on motor and sensory responses in rat spinal cord. Brain Res. 1987;405:84–93. doi: 10.1016/0006-8993(87)90992-9. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Lo TM, Loh HH, Thayer SA. Delta-opioid-induced liberation of Gbetagamma mobilizes Ca2+ stores in NG108-15 cells. Mol Pharmacol. 1999;56:902–908. doi: 10.1124/mol.56.5.902. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. J Neurophysiol. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Arvidsson U, Elde R, Hökfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82:1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]