Summary
The importance of stromal cells and the factors that they express on cancer initiation and progression has been highlighted by recent literature. The cellular components of the stroma of epithelial tissues are well recognized to have a supportive role in carcinogenesis, where the initiating mutations of the tumor originate in the epithelial cells. The use of xenograft and mouse models suggest that mutations in the stromal fibroblasts can also initiate epithelial tumors. Many of these tumors are a result of the alteration of paracrine growth factor pathways that act on the epithelia. However, the tissue specificity of the responses to the growth factors is a mystery not yet solved.
Keywords: paracrine signalling, stroma, cancer, fibroblast
Carcinomas are malignant neoplasms derived from epithelial cells and represent the most common form of human cancer. A specialized stroma accompanies carcinomas, and this stroma is characterized by modifications in the non-epithelial cell types that secret extracellular matrix (ECM) proteins and growth factors. Just as in the normal epithelial tissue the stromal cells include those comprising the vasculature, inflammatory cells (lymphocytes, macrophages, mast cells), and fibroblasts. However, as genetic mutations in the epithelia result in altered rates of apoptosis and proliferation as well as changes in morphogenesis, the carcinoma cells in turn promote angiogenesis, increased inflammatory cell recruitment, altered ECM expression, and accelerated fibroblast proliferation [1–4]. Recent reports suggest a role for inflammation, ECM, and stromal fibroblasts in the initiation and progression of carcinomas. In this review, we will examine the concept of the co-evolution of the epithelial cancer cells with its stroma. Although it is understood that there is reciprocal relationship between the epithelia and stroma in normal tissues, the mechanisms for this interaction in cancer have been elucidated more recently.
Epithelial Interactions With The Stroma
The role of the ECM in tumorigenesis includes effects on epithelial polarity and angiogenesis [2]. The cells of the stroma and epithelia together regulate the expression and remodeling of the ECM [5–7]. The basement membrane separates the epithelial and endothelial cells from the stromal components. Inflammatory cells and fibroblasts express the stromal ECM proteins. However, the basal epithelia, myoepithelia, and fibroblasts express the major components of the basement membrane (collagen VI, laminin, entactin, and heparan-sulphate proteoglycans) in a tissue specific manner [8]. Epithelial integrins confer polarity through binding to the basement membrane [9]. Interestingly the specificity of the integrins expressed and the matrix with which it is associated influence epithelial and endothelial proliferation and migration. Intact type VI collagen interacting with α1β1 and α2β1 integrins can be mitogenic to endothelia in tumor angiogenesis; however, when the same matrix is digested by matrix metalloproteinases (MMPs), the binding to β1 integrins diminishes [10]. Instead β3 integrins bind denatured collagen VI and its activation can signal to inhibit proliferation and migration of endothelial cells [11]. Recently White et al. [12**] found that the disruption of β1 integrin expression in mammary epithelial cells did not influence mammary glandular development, however this knockout did significantly inhibit mammary tumorigenesis. The authors also found that β1 integrin expression is involved in maintaining the proliferative capacity of late-stage tumor cells. The data suggest the possibility that the balance of activated β1 and β3 integrins on the epithelial cell surface, in conjunction with the expression of MMPs that regulate ECM and growth factor activation (discussed below), influences the development of mammary carcinomas.
Impact of Stromal Cells On The Epithelia
The genetic basis of carcinogenesis involves a process of acquisition of multiple genetic mutations in epithelial cells [13] resulting in an activated stroma [3,4]. This specialized stroma has an abundance of inflammatory cells and activated fibroblasts that express ECM and growth factors to support the survival and proliferation of carcinoma cells in a paracrine fashion.
The mechanism of the long recognized relationship between inflammation and cancer is emerging [1]. Chronic inflammation can be a precursor to the development of carcinomas. For example chronic pancreatitis, ulcerative colitis, and inflammatory bowel disease are associated with pancreatic, stomach, and colon carcinomas, respectively [1]. Inflammatory cells are also a key component of the stroma of carcinomas arising independent of chronic inflammation. Inflammatory cells secrete numerous cytokines, growth factors and chemokines, and produce reactive oxygen species that stimulate proliferation, prevent apoptosis, induce morphogenesis, or mediate DNA damage in the epithelial cells [1]. One potential mechanism identified in at least inflammation-associated cancers of the stomach and colon involve the activation of the NF-kB signalling pathways. In a recent study the inhibitor of NF-kB, IKKβ, was conditionally deleted in enterocytes and macrophages [14**]. The cell-specific loss of IKKβ expression reduced the incidence of inflammation-associated cancer in both the stomach and the colon by interestingly different mechanisms. In the enterocytes, IKKβ contributes to tumor initiation by suppressing apoptosis. However, in myeloid cells IKKβ enhances growth factor expression promoting tumor proliferation [14**].
One approach to examining the role of stromal fibroblasts in the carcinogenic process has been by inducing sub-lethal DNA damage to fibroblasts through irradiation. Initially, the more general effect of the mammary stroma on epithelial cancer was tested by allografting non-transformed mammary epithelial cells into irradiated or non-irradiate cleared mammary fat pads of mice. The radiation-induced alterations of the stroma produced a greater number of mammary carcinomas compared to the control, non-irradiated stroma [15*]. These studies suggest that the stromal cells have an inductive role in transformation of epithelial cells. Higher expression of active transforming growth factor-beta1 (TGF-β1) by the irradiated mammary stroma was attributed a causative role for corresponding changes in epithelia to a spindle morphology, elevation of collagen III expression, and suppressed immune system [15*]. More recently, in a xenograft experiment, pancreatic carcinoma cells were recombined with irradiated pancreatic fibroblasts. This resulted in an elevated incidence of a more aggressive and invasive cancer compared to the same epithelial cells recombined with non-irradiated fibroblasts [16*]. In this circumstance the fibroblasts were the mediators of the more invasive behavior of the carcinoma. These observations were associated with elevated expression of the activated hepatocyte growth factor (HGF) receptor, c-Met, in epithelial cells and increased TGF-β1 expression by the irradiated pancreatic fibroblasts. These reports suggest a trans-activating mechanism for fibroblasts on the epithelia where alterations in stromal fibroblasts enhanced carcinoma progression. Our findings argue against a mechanism in which silencing of genes by binding PcG proteins to PREs gives rise to repositioning of silenced loci inside compact chromatin domains, that is, away from the perichromatin compartment. Clearly, the state of chromatin after silencing by PcG proteins is different from tha
Mediators of the Stromal Signals
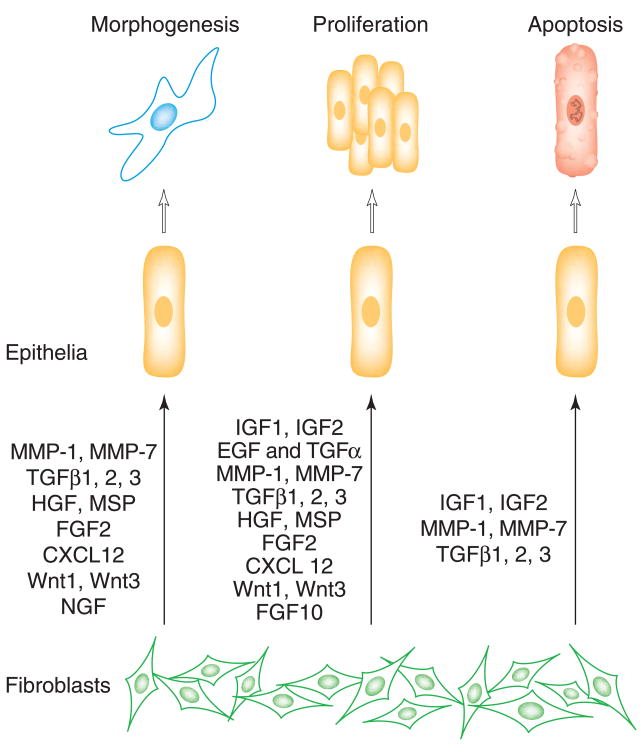
Carcinoma cells often show enhanced expression of growth factors and the regulators of growth factor effects. The stromally expressed matrix metalloproteinase (MMPs), elevated in tumors, have diverse roles in growth-regulation, angiogenesis, metastasis, and promotion of epithelial transformation [17]. Some of the signals are mediated through the activation of growth factors [17,18]. Additionally, since many growth factor pathways have common signaling pathways, a mutation involving a signaling protein can potentially affect several growth factor pathways. Multiple families of growth factors implicated as autocrine and paracrine mediators of stromal-epithelial interactions have been identified to be altered in carcinomas. These include the fibroblast growth factor (FGF) family, the insulin-like growth factor (IGF) family, epidermal growth factor (EGF), transforming growth factor alpha (TGFα), and HGF all of which are predominantly stimulators of proliferation (Figure 1). Under normal circumstances, these growth factors are expressed predominantly by fibroblasts with elevated expression in many carcinomas. However, a confounding event in the tumors can involve the epithelial expression of the normally fibroblast-derived growth factors to facilitate autocrine and paracrine stimulation of proliferation.
Figure 1. Epithelial growth, differentiation, and apoptosis are regulated by fibroblast derived soluble factors [37–40].
The abbreviations are EGF, epidermal growth factor; FGF, fibroblast growth factor; HGF, hepatocyte growth factor; IGF, insulin growth factor; IL6, interleukin 6; KGF, keratinocyte growth factor; LIF, leukemia inhibitory factor; MSP, macrophage stimulating factor; NGF, nerve growth factor; TGF-β, transforming growth factor.
Within the group of morphogenic factors (see Figure 1), Wnt-1, HGF, and TGF-β particularly standout due to their demonstrated ability to induce cancer initiation and progression [19–22]. The paracrine factor, Wnt1, when over expressed in fibroblasts, was found to promote transformation of C57MG mammary epithelial cells in culture with no accompanying transformation of the fibroblasts themselves [21]. More recently, human immortalized mammary fibroblasts transduced to ectopically express HGF or TGF-β1 alone or together were recombined with nontransformed human mammary epithelial organoids that were induced to form ductal carcinoma in situ, adenocarcinoma, and poorly differentiated carcinomas [23**]. In contrast mammary organoids recombined with control fibroblasts did not show evidence of neoplastic transformation. The expression of HGF-related ligands including the growth factor, MSP (macrophage stimulating protein), which can activate a heterodimeric receptor consisting of c-Met and the c-Met related receptor RON, is also known to promote carcinogenesis [24,25]. Alternatively, the over expression of c-Met, common in many cancers, can be associated with ligand independent activation [26] or increased sensitivity to normal physiologic levels of HGF [27].
The first genetic evidence for the TGF-β pathway being tumor suppressive in human tumors was the demonstration that the gene encoding the type II TGF-β receptor (TβRII), Tgfbr2, is very frequently inactivated in colon cancers with mismatch repair deficiency [28]. TβRII is required for signaling by all three TGF-β ligands [29]. A subsequent study of all types of mutations in Tgfbr2 in colorectal cancer lines demonstrated that most had mutations that would lead to loss of TβRII function [30]. However, there is often an increased expression of TGF-β in many carcinomas. The sources of TGF-β include the inflammatory cells, stromal fibroblasts as well as the carcinoma cells themselves in many cases [31]. Thus the prevailing paradigm has been that TGF-β has tumor suppressive role on normal epithelia, however transformed epithelia can overcome the growth inhibitory effects of TGF-β and take on a metastatic phenotype. Apart from the immuno-suppressive role of TGF-β on the host and its role stimulating a reactive tumor stromal environment little else is known regarding its effect on fibroblasts.
To study the role of TGF-β signaling in the fibroblasts, Cre-lox technology has been used to conditionally knockoutTgfbr2 gene expression. Selective knockout ofTgfbr2 in stromal fibroblasts was achieved by crossbreeding transgenic mice having floxedTgfbr2 (loxP sites flanking exon 2) [32] with FSP1-Cre (driven by the fibroblast specific protein 1 promoter) [33], termed Tgfbr2fspko mice [34**]. Interstitial fibroblasts of mature tissues throughout the mouse express FSP1 [35]. The Tgfbr2fspko mice exhibited preneoplastic lesions in the prostate (prostatic intraepithelial neoplasia) and invasive squamous cell carcinomas of the forestomach by six weeks of age with high penetrance [34**]. Cultured Tgfbr2fspko fibroblasts of the prostate and forestomach expressed elevated levels of HGF. Concurrent activation of the cognate receptor, c-Met, in the prostate and forestomach lesions in the Tgfbr2fspko mice suggested a paracrine signaling mechanism for stimulation of epithelial proliferation and transformation. The suppression of HGF expression by TGF-β signaling [36] in the fibroblasts was seemingly lost in the Tgfbr2fspKO mice [34**]. Thus alterations in TGF-β intercellular signaling in the fibroblasts can result in intercellular signaling that ultimately impact adjacent epithelial tumorigenesis in some tissues.
The lack of epithelial transformation in much of the other tissues in the Tgfbr2fspko mouse model was equally intriguing [34**]. One instance was the development of squamous cell carcinoma in the forestomach, while the adjacent squamous epithelia of the esophagus and the glandular stomach body were unchanged. Further, the carcinoma originating in the forestomach invaded the stomach body but not the esophagus. The traditional phenotypic markers for fibroblasts that include the expression of vimentin and FSP-1 are not sufficient to distinguish the differences in fibroblasts associated with the squamous epithelial forestomach and esophagus from the adjacent glandular epithelia of the stomach body. Since Tgfbr2 expression was conditionally knocked out in the fibroblasts of the esophagus, forestomach, and the glandular stomach of the Tgfbr2fspko mice, this suggests the possibility that there are may be as many types of stromal fibroblasts as there are epithelial types in the complex tissue architecture.
Conclusions
Recent data bring prominence to the tumor stromal environment in not only a supportive role, but also as a leading player in the initiation of carcinomas. Mutations in stromal cells specifically regulating paracrine growth factor expression have been shown to initiate epithelial cancers. Elevated expression of morphogens, anti-apoptotic, and proliferative factors in the microenvironment can be a potential cause of epithelial mutations that lead to transformation. The sensitivity of the epithelial cells to the microenvironment is partly tissue and cell type dependent. In turn the tissue specificity of the various cellular components of the microenvironment dictate the reciprocal sensitivity to the epithelially derived paracrine factors. Tumor development will be better understood as we uncover the intracellular cross talk within tissues.
Acknowledgments
This work was supported by a DOD USAMRMC grant (to NAB), NIH grants (to HLM), and the Vanderbilt-Ingram Cancer Center Support Grant.
References
* Of special interest
** Of outstanding interest
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park CC, Bissell MJ, Barcellos-Hoff MH. The influence of the microenvironment on the malignant phenotype. Mol Med Today. 2000;6:324–329. doi: 10.1016/s1357-4310(00)01756-1. [DOI] [PubMed] [Google Scholar]
- 3.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 4.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11:54–59. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 5.Fata JE, Werb Z, Bissell MJ. Regulation of mammary gland branching morphogenesis by the extracellular matrix and its remodeling enzymes. Breast Cancer Res. 2004;6:1–11. doi: 10.1186/bcr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werb Z, Sympson CJ, Alexander CM, Thomasset N, Lund LR, MacAuley A, Ashkenas J, Bissell MJ. Extracellular matrix remodeling and the regulation of epithelial-stromal interactions during differentiation and involution. Kidney Int Suppl. 1996;54:S68–74. [PMC free article] [PubMed] [Google Scholar]
- 7.Bissell MJ, Weaver VM, Lelievre SA, Wang F, Petersen OW, Schmeichel KL. Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res. 1999;59:1757–1763s. discussion 1763s–1764s. [PubMed] [Google Scholar]
- 8.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 9.Schittny JC, Yurchenco PD. Basement membranes: molecular organization and function in development and disease. Curr Opin Cell Biol. 1989;1:983–988. doi: 10.1016/0955-0674(89)90069-0. [DOI] [PubMed] [Google Scholar]
- 10.de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, Gotwals PJ, Lobb RR, Koteliansky VE. Regulation of inflammation by collagen-binding integrins alpha1beta1 and alpha2beta1 in models of hypersensitivity and arthritis. J Clin Invest. 2000;105:721–729. doi: 10.1172/JCI7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, Davis GE, Brooks PC, Yuen SM. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of beta1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. In a transgenic mouse model integrinβ1 expression was knocked out in mouse mammary epithelia. The data show that integrins that bind and signal through interaction with the ECM in the stroma can initiate mammary tumourigenesis and maintain the proliferative capacity of late-stage tumour cells. [DOI] [PubMed] [Google Scholar]
- 13.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 14**.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. Cell. Vol. 118. 2004. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer; pp. 285–296. Using a colitis-associated cancer model the authors deleted part of a major inflammatory pathway, through the conditional knockout of IKKbeta in myeloid cells. This resulted in a significant decrease in tumour incidence. [DOI] [PubMed] [Google Scholar]
- 15*.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–1260. This paper reports that irradiation-induced changes in the stromal cells can contribute to cancer progression in grafted non-tumourigenic mouse mammary epithelial cells. [PubMed] [Google Scholar]
- 16*.Ohuchida K, Mizumoto K, Murakami M, Qian LW, Sato N, Nagai E, Matsumoto K, Nakamura T, Tanaka M. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64:3215–3222. doi: 10.1158/0008-5472.can-03-2464. The data showed that radiation damage of stromal fibroblasts can promote aggressiveness of pancreatic cancer cells. [DOI] [PubMed] [Google Scholar]
- 17.Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561–573. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- 18.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 19.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Matsumoto K, Kiritoshi A, Tano Y. Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res. 1997;57:3305–3313. [PubMed] [Google Scholar]
- 21.Jue SF, Bradley RS, Rudnicki JA, Varmus HE, Brown AM. The mouse Wnt-1 gene can act via a paracrine mechanism in transformation of mammary epithelial cells. Mol Cell Biol. 1992;12:321–328. doi: 10.1128/mcb.12.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumont N, Arteaga CL. Transforming growth factor-beta and breast cancer: Tumor promoting effects of transforming growth factor-beta. Breast Cancer Res. 2000;2:125–132. doi: 10.1186/bcr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci U S A. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. Histopathologically normal human breast epithelial organoids from reduction mammoplasty samples were recombined in a cleared mammary fat pad xenograph with human mammary fibroblasts. When the fibroblasts over expressed HGF and/or TGF-β1, mammary tumorigenesis occurred. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angeloni D, Danilkovitch-Miagkova A, Miagkov A, Leonard EJ, Lerman MI. The soluble sema domain of the RON receptor inhibits macrophage-stimulating protein-induced receptor activation. J Biol Chem. 2004;279:3726–3732. doi: 10.1074/jbc.M309342200. [DOI] [PubMed] [Google Scholar]
- 25.Maggiora P, Lorenzato A, Fracchioli S, Costa B, Castagnaro M, Arisio R, Katsaros D, Massobrio M, Comoglio PM, Flavia Di Renzo M. The RON and MET oncogenes are co-expressed in human ovarian carcinomas and cooperate in activating invasiveness. Exp Cell Res. 2003;288:382–389. doi: 10.1016/s0014-4827(03)00250-7. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–361. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 28.Markowitz S, Wang J, Myeroff L, Parsons R, Sun L, Lutterbaugh J, Fan RS, Zborowska E, Kinzler KW, Vogelstein B, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 29.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 30.Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, Neumann A, Brattain MG, Chang J, Kim SJ, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 31.Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 1999;10:303–360. [PubMed] [Google Scholar]
- 32.Chytil A, Magnuson MA, Wright CV, Moses HL. Conditional inactivation of the TGF-beta type II receptor using Cre:Lox. Genesis. 2002;32:73–75. doi: 10.1002/gene.10046. [DOI] [PubMed] [Google Scholar]
- 33.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. This study targeted the deletion ofTgfbr2 gene expression in the fibroblasts of transgenic mice. The mice spontaneously developed preneoplastic lesions in the prostate and squamous cell carcinoma in the forestomach. [DOI] [PubMed] [Google Scholar]
- 35.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph H, Gorska AE, Sohn P, Moses HL, Serra R. Overexpression of a kinase-deficient transforming growth factor-beta type II receptor in mouse mammary stroma results in increased epithelial branching. Mol Biol Cell. 1999;10:1221–1234. doi: 10.1091/mbc.10.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 38.Barbero S, Bonavia R, Bajetto A, Porcile C, Pirani P, Ravetti JL, Zona GL, Spaziante R, Florio T, Schettini G. Stromal cell-derived factor 1alpha stimulates human glioblastoma cell growth through the activation of both extracellular signal-regulated kinases 1/2 and Akt. Cancer Res. 2003;63:1969–1974. [PubMed] [Google Scholar]
- 39.Russell PJ, Bennett S, Stricker P. Growth factor involvement in progression of prostate cancer. Clin Chem. 1998;44:705–723. [PubMed] [Google Scholar]
- 40.Lazar-Molnar E, Hegyesi H, Toth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine. 2000;12:547–554. doi: 10.1006/cyto.1999.0614. [DOI] [PubMed] [Google Scholar]